Local Administration of H2O2 Reduced Aspergilloma in a Patient with Chronic Granulomatous Disease: A Case Report
Article Information
Toyoki Nishimura1, Hideaki Nakamura2, Tomoyuki Mizukami1,3, Fumio Hidaka1, Makoto Matsukura4, Hiroshi Moritake1, Hiroyuki Nunoi1,5*
1Division of Pediatrics, Faculty of Medicine, University of Miyazaki, 5200 Kihara, Kiyotake-cho, Miyazaki-City, Miyazaki 889-1692, Japan
2Laboratory of Environmental Science and Technology, Faculty of Pharmaceutical Sciences, Sojo University, 4-22-1 Ikeda, Nishi-Ku, Kumamoto City 860-0082, Japan
3Department of Pediatrics, National Hospital Organization Kumamoto Medical Center, Kumamoto 1-5 Ninomaru, Chuo-ku, Kumamoto, 860-0008, Japan
4Laboratory of Clinical Pharmacology and Therapeutics, Faculty of Pharmaceutical Sciences, Sojo University, 4-22-1 Ikeda, Nishi-Ku, Kumamoto City 860-0082, Japan
5Aisenkai Nichinan Hospital, 3649-2 Kazeta, Nichinan-City, Miyazaki 887-0034, Japan
*Corresponding authors: Hiroyuki Nunoi, Division of Pediatrics, Faculty of Medicine, University of Miyazaki, 5200 Kihara, Kiyotake-cho, Miyazaki-City, Miyazaki 889-1692, Japan.
Received: 27 March 2024; Accepted: 02 April 2024; Published: 18 April 2024
Citation: Toyoki Nishimura, Hideaki Nakamura, Tomoyuki Mizukami, Fumio Hidaka, Makoto Matsukura, Hiroshi Moritake, Hiroyuki Nunoi. Local Administration of H2O2 Reduced Aspergilloma in a Patient with Chronic Granulomatous Disease: A Case Report. Journal of Biotechnology and Biomedicine. 7 (2024): 186-191.
View / Download Pdf Share at FacebookAbstract
Granulomas in patients with chronic granulomatous disease (CGD) are intractable to conventional treatments; they are sometimes inoperable and may even be lethal. A 2-year-old boy with X-linked CGD and a large aspergilloma was treated with an antibiotic and antifungal agent along with steroids and interferon-γ administration for 6 months, but the granuloma did not shrink. Slow local administration of 20-mM hydrogen peroxide to the granuloma lesion through the catheter reduced granuloma size within 1 month. One month later, the remaining aspergilloma was excised by right lower lobectomy. One year later, bone marrow transplantation was successfully performed, followed by two rounds of donor lymphocyte infusion and with rituximab, the latter owing to low chimerism of the donor type with autoantibodies. This case demonstrates the feasibility of local administration of H2O2 for treating granulomas in patients with CGD.
Keywords
Local administration; 20 mM Hydrogen Peroxide; Granuloma; CGD
Local administration articles; 20 mM Hydrogen Peroxide articles; Granuloma articles; CGD articles
Article Details
Abbreviations:
CGD: chronic granulomatous disease; NOX: nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex; TMP/SMX: trimethoprim-sulfamethoxazole; RIC: reduced-intensity conditioning; PEG-DAO: pegylated-D type amino acid oxidase
Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency characterized by the inability of phagocytes to produce reactive oxygen species (ROS), including O2-, H2O2, and HClO, owing to a defect in the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex (NOX). The superoxide anion radical O2- or H2O2 is initially generated in most cells by the mitochondria and NADPH oxidases and the related dual oxidase (NOX1-5, DUOX1-2) [1]. NOXs are primarily located on cell plasma membranes and typically release O2- outside the cell. Phagocytes (neutrophils, macrophages, monocytes, and eosinophils) and platelets produce O2- upon stimulation through receptor-mediated signaling. Clinically, most patients with CGD experience bacterial and fungal infections during childhood and recurring inflammatory disorders such as CGD-associated bowel inflammation and granulomas. The occurrence of fungal infections is a major determinant of survival in patients with CGD and the most common cause of death. Patients with X-linked disease generally have a more severe disease course than those with non-X-linked disease, and aspergillomas are frequently found in patients with low superoxide-producing gp91phox activity [2]. Therefore, improved treatment strategies for granulomas in this patient population are urgently needed. Antibiotics are pathogen-specific remedies. Potent pathogen-specific antibiotics or antifungal agents can kill pathogens. However, residual pathogenic components such as fungal cell wall β-glucan cause vital reactions in patients with CGD. Granulomas comprise macrophages and their derivatives, epithelioid cells, and complex multicellular tissue reactions induced by a cascade of interactive systems and chronic inflammatory tissue responses [3, 4, 5], involving many biological processes. Recently, ROS have been shown to regulate many biological processes. ROS and microbicidal proteases are essential in killing and digesting bacteria and fungi. During pyroptosis, activated caspase-1 is ROS-sensitive [6]. During NETosis, ROS activates peptidyl arginine deiminase 4 that undergoes histone modification, disintegrates its intracellular membranes, and releases decondensed chromatin (DNA/histones) and microbicidal granule proteins into the extracellular space to form neutrophil extracellular traps [7, 8]. During autophagy, ROS regulates engulfed Aspergillus conidia with microtubule-associated protein 1 light chain 3, recycling and eliminating damaged proteins and organelles [9, 10]. During apoptosis and efferocytosis, ROS promotes efferosome maturation and acidification. Apoptosis of inflammatory cells prevents secondary uncontrolled necrosis and reduces the overall levels of inflammatory cytokines [11]. However, it remains an open problem how H2O2 may induce many different cell death modalities. Additionally, there are other inflammatory cell death processes unrelated to ROS production [12]. Steroids [13], thalidomide [14], infliximab (a monoclonal antibody to tumor necrosis factor-alpha) [15], anakinra (an interleukin [IL]-1-receptor antagonist [16], and pioglitazone [17, 18], have been used to control inflammatory cell death processes in CGD. However, the effectiveness of local injection of ROS (H2O2) into granulomas has not been reported. We report the case of a patient with CGD with a large aspergilloma who was treated with local administration of H2O2 to reduce the size of the aspergilloma, followed by successful bone marrow transplantation. H2O2, a representative membrane-permeable oxidant and the most abundant ROS in cells, is considered a toxic molecule in human tissues [19]. However, it plays a crucial role in inhibiting the growth of microbes ingested by phagocytes, depending on its concentration. It is also indispensable for the above inflammasome signaling processes in inflamed tissues [20]. Therefore, this report also reviewed the basic functions of H2O2 in vivo and discussed the utility and drawbacks of H2O2 administration for treating granulomas with CGD.
Case Report
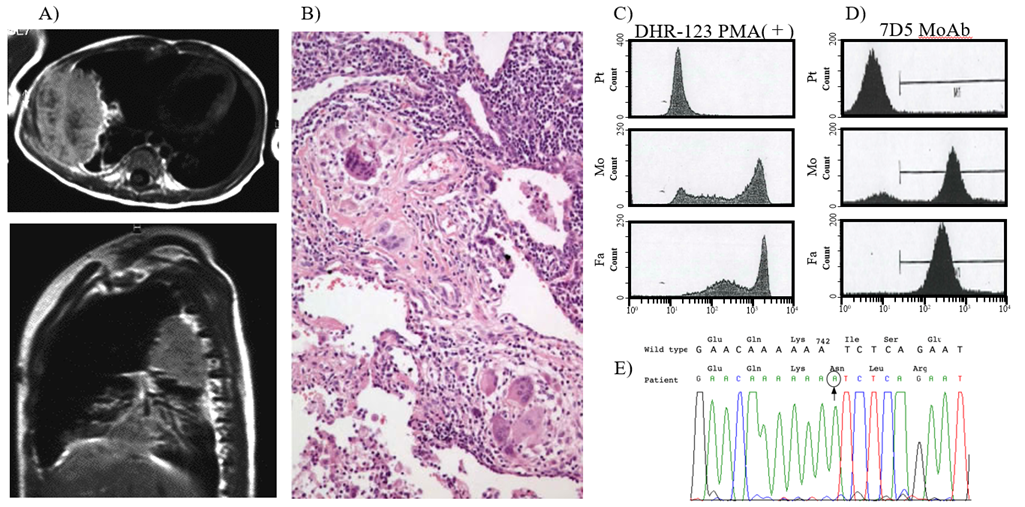
The male patient weighed 2730 g at a gestational age of 39 weeks without complications. He had an uncle who had died of pneumonia at the age of 1 month. Forty days after birth, the patient developed wheezing and was admitted to a nearby hospital with a diagnosis of bronchitis. He was transferred to the central hospital in Kumamoto and was referred to the University Hospital Kumamoto for investigation of primary immunodeficiency. Computed tomography (CT) and diagnostic lung biopsy revealed a right-sided granuloma with consolidation and disseminated multifocal aspergilloma (Figure 1A, B). The patient was diagnosed with Aspergillus infection with X-linked CGD at 4 months of age based on the results of pharyngeal culture, phagocyte dihydrorhodamine test, and 7D5 monoclonal antibody fluorescence-activated cell sorting analysis against gp91-phox (Figure 1C, D). Mutational analysis confirmed a c.742_743insA frameshift mutation (Figure 1E). Amphotericin B therapy was initiated at a dose of 0.25 mg/kg/d, followed by a daily dose increase of 0.25 mg/kg/d until the therapeutic dosage (1.0 mg/kg/d) was reached. After 8 months of amphotericin B and interferon-gamma (IFN-g) therapy, the granuloma diminished.
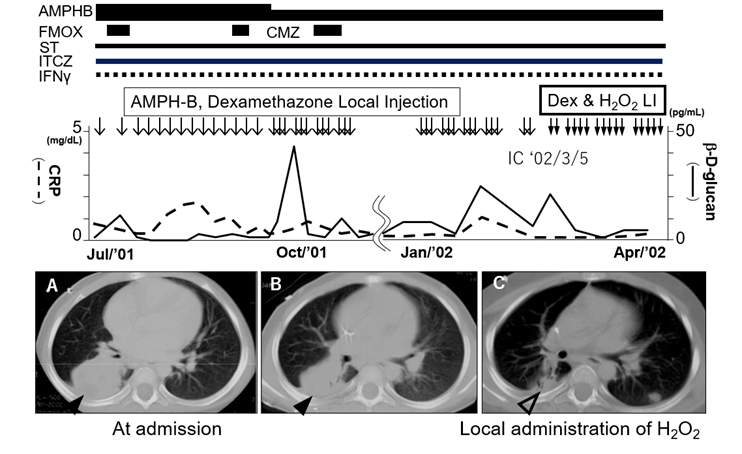
After hospital discharge, prophylactic therapy (trimethoprim-sulfamethoxazole) and amphotericin B or itraconazole and IFN-g were continued approximately for 1 year and 4 months. However, the patient developed a cough and rhinorrhea and was transferred to the Department of Pediatrics at Miyazaki University Hospital owing to the transfer of the attending physician. A CT scan revealed another large mass shadow in the right lower lung lobe (Figure 2A). The patient was treated with intravenous amphotericin B, which lessened the symptoms; however, the mass shadow grew with the involvement of the pulmonary arteries. Local injection of amphotericin B (5 mg) and dexamethasone (0.4 mg) administered through the catheter to the granuloma 6 months later led to a reduction in the mass size (Figure 2B); however, hypopotassemia and moon face (side effects of amphotericin B and steroids) appeared. With the ethical approval of the University of Miyazaki and parental informed consent, we administered a local injection of H2O2 (20 mM/1 mL syringe/2 h for 23 days) and dexamethasone through the catheter to the granuloma. The patient reported no itching or pain. After this treatment, the mass notably decreased, and the b-D-glucan and C-reactive protein (CRP) levels decreased 1 month later (Figure 2C). Right lower and upper partial lobectomy was performed at the Department of Surgery at Fukuoka University Hospital at 3.5 years of age. As the cumulative usage of amphotericin B was nearly 4.0 g and the patient showed subtle renal dysfunction as a side effect, the antifungal agent was changed to miconazole when the patient was 3 years and 8 months old. The CRP (1.2 mg/dL) and b-D glucan (16.9 pg/mL) levels remained high. He had bloody stool 1 month later, and a colonoscopy revealed submucosal granuloma lesions (leopard sign), which were relieved by miconazole administration through central venous catheter within 1 month. At this stage, continuous intravenous administration of miconazole or bone marrow transplantation might be necessary to maintain his condition. One year later, at the age of 4 years and 10 months, he received hematopoietic cell transplantation (HCT) from an unrelated human leukocyte antigen matched donor with the reduced-intensity conditioning regimen (fludarabine, cyclophosphamide, rabbit antithymocyte globulin and 3 Gy total body irradiation), followed by graft versus host disease (GVHD) prophylaxis (tacrolimus + short-term methotrexate) at Kyoto University Hospital. Although GVHD (erythema, lymphopenia, chronic diarrhea, thrombocytopenia, and neutropenia) and low chimerism of the donor type with autoantibodies were observed, donor lymphocyte infusion (DLI) with rituximab alleviated all the symptoms. The treatment and its time course have been described elsewhere [21]. The patient is now 25 years old with no complaints; he is a working professional.
Discussion
Since the late 1990s, HCT treatment has been restricted to patients with high-risk diseases and matched donors. HCT was first reported as a potential curative treatment for intractable CGD in 2001 and 2002 [22, 23]. Since then, HCT has been applied to patients with CGD, including new techniques such as cord blood transplantation, RIC, DLI, and post-transplantation cyclophosphamide administration for GVHD prophylaxis. In 2003, our patient was treated with antifungal agents and conventional prophylaxis (steroids and IFN-g), and he experienced the side effects of cumulative amphotericin B administration; however, these treatments were not effective enough. The administration of HCT was impossible owing to the persistent large granuloma involving the pulmonary artery. In this case, preexisting infections and inflammation would have influenced the transplant approach and patient outcomes. Therefore, we attempted local administration of H2O2 to reduce the size of the granuloma.
Given the following biological functions of H2O2, it was injected locally at 1 mL/2 h without any reported pain or side effects:
First, the use of a low concentration of H2O2, such as 20 mM, can prevent the potential adverse effects associated with high concentrations, such as 3% (975 mM) (e.g., delayed wound healing [24], inflammation, embolism [25], hemolysis, and renal damage [20, 26]). This low concentration seems to strike a balance between effectiveness and safety. Second, the role of H2O2 as a signaling molecule that regulates various biological processes, such as cell proliferation, differentiation, and migration, through alterations of membrane potential, generation of new molecules, and changes in intracellular redox balance, is well established [20, 27]. H2O2 acts as a signaling molecule regulating various biological processes at low concentrations (<10 μM) [28] and exhibits antimicrobial and anticancer properties at high concentrations (>100 μM) [29]. While the H2O2 level in human blood ranges from 0.25 μM to 5 μM, a high concentration of 30–50 μM has been reported in certain disease states or during chronic inflammation [30]. Therefore, the concentration of 20 mM that we selected for our patient falls within the effective range for bactericidal purposes while potentially facilitating signaling mechanisms. Third, H2O2 is a cellular membrane-permeable transporter; thus, it is passively transported to intracellular locations to act as a signaling molecule or bactericidal agent [26]. Therefore, the external administration of H2O2 can replace the action of naturally occurring H2O2 inside the cells. However, at high concentrations (>100 uM), H2O2 is degraded rapidly by catalases (Vmax=53400 μmoles/min/mg; Km=80 mM of H2O2), while at physiological concentrations (tens of μM), H2O2 is degraded slowly by glutathione peroxidase (Vmax=0.52mmoles/min/mg, Km= 20 mM of H2O2) [31, 32]. Therefore, the 20 mM of H2O2 injected into the lesion likely resulted in a concentration gradient, with an exponential decrease from 20 mM to several tens of μM further from the catheter injection site.
Fourth, the estimated H2O2 production by neutrophils in full activation by Escherichia coli or ConA + cytochalasin D is 20–30 mM/104 neutrophils/minute [33]. Polyethylene glycol-conjugated D-amino acid oxidase with D-type amino acids, which was recently proposed as a candidate drug for enzyme replacement therapy in patients with CGD [34, 35], produces approximately the same amount of H2O2 (5–50 mM/mg/minute), suggesting that the injected H2O2 concentration is physiologically relevant and aligns with the concentration involved in natural processes. Although the direct, local administration of 20 mM H2O2 to the granuloma effectively reduced the granuloma’s size in our patient, this approach appears to be well-supported by the current understanding of the role of H2O2 in biological systems. Continued monitoring and further research in CGD patients can provide additional insights into the therapeutic potential of H2O2 in various contexts.
Conclusion
Based on our experience, we propose slow local administration of 20 mM H2O2 as a candidate treatment for intractable granulomas in patients with CGD.
Acknowledgments
We thank Emeritus Professor Tatsutoshi Nakahata, Professor Souichi Adachi, and their staff members at the Department of Pediatrics at Kyoto University for their comprehensive HCT and anti-graft failure treatments over an extended period. We also thank Naoto Adachi of the Department of Pediatrics of Kumamoto University Hospital and Yoshio Itai of Kumamoto Rosai Hospital for patient follow-up in Kumamoto. Finally, we thank Emeritus Professor Takayuki Shiraushima and his staff members at the Department of Surgery, Division of Thoracic, Endocrine, and Pediatric Surgery at Fukuoka University School of Medicine for the lobectomy.
Conflict of Interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Ethics approval and consent to participate
The treatment strategy of local administration of H2O2 was reviewed and approved by the Ethics Committee of Miyazaki University. Written informed consent for the publication of this report was obtained from the patient’s legal guardians.
Consent for publication
Consent for publication was obtained from the parents and the patient.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was supported by a grant from the Japan Ministry of Health, Labor, and Welfare (201911006 B).
Author’s Contributions
TN and HN analyzed the data and drafted the manuscript. TN, HN, and HM performed the study and provided clinical data. TM analyzed the gene mutations. TN, HN, and MM collaborated on PEG-DAO and provided important ideas. All authors contributed to the manuscript and approved the submitted version.
References
- Panday A, Sahoo MK, Osorio D, et al. NADPH Oxidases: An Overview from Structure To Innate Immunity-Associated Pathologies. Cellular & Molecular Immunology 12 (2015): 5-23.
- Marciano BE, Spalding C, Fitzgerald A, et al. Common Severe Infections in Chronic Granulomatous Disease. Clinical Infectious Diseases 60 (2015): 1176-1183.
- Gideon HP, Hughes TK, Tzouanas CN, et al. Multimodal Profiling of Lung Granulomas in Macaques Reveals Cellular Correlates of Tuberculosis Control. Immunity 55 (2022): 827-846.
- Endo D, Fujimoto K, Hirose R, et al. Genetic Phagocyte NADPH Oxidase Deficiency Enhances Nonviable Candida albicans–Induced Inflammation in Mouse Lungs. Inflammation 40 (2017): 123-135.
- Gideon HP, Phuah J, Junecko BA, et al. Neutrophils Express Pro- and Anti-Inflammatory Cytokines in Granulomas from Mycobacterium Tuberculosis-Infected Cynomolgus Macaques. Mucosal Immunology 12 (2019): 1370-1381.
- Guo H, Callaway J, Ting JY. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nature Medicine 21 (2015): 677-687.
- Stoiber W, Obermayer A, Steinbacher P, et al. The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans. Biomolecules 5 (2015): 702-723
- Seger RA. Chronic Granulomatous Disease 2018: Advances in Pathophysiology and Clinical Management. LymphoSign Journal 6 (2019): 1-16.
- Mehta P, Henault J, Kolbeck R, et al. Noncanonicalautophagy: One Small Step for LC3, a Giant Leap for Immunity. Current Opinion in Immunology 26 (2014): 69-75.
- Akoumianaki T, Kyrmizi I, Valsecchi I, et al. Aspergillus Cell Wall Melanin Blocks LC3-associated Phagocytosis to Promote Pathogenicity. Cell Host & Microbe 19 (2016): 79-90.
- Bagaitkar J, Huang J, Zeng MY, et al. NADPH Oxidase Activation Regulates Apoptotic Neutrophil Clearance by Murine Macrophages. Blood 13 (2018): 2367-2378.
- Tang D, Kang R, Berghe TV, et al. The Molecular Machinery of Regulated Cell Death. Cell Research 29 (2019): 347-364.
- Nakazawa Y, Kawai T, Arai K, et al. Fecal Calprotectin Rise in Chronic Granulomatous Disease-Associated Colitis. Journal of Clinical Immunology 37 (2017): 741-743.
- Noel N, Mahlaoui N, Blanche S, et al. Efficacy and Safety of Thalidomide in Patients with Inflammatory Manifestations of Chronic Granulomatous Disease: A Retrospective Case Series. The Journal of Allergy and Clinical Immunology 132 (2013): 997-1000.e4.
- Uzel G, Orange JS, Poliak N, et al. Complications of Tumor Necrosis Factor-α Blockade in Chronic Granulomatous Disease-related Colitis. Clinical Infectious Diseases 51 (2010): 1429-1434.
- Hahn KJ, Ho N, Yockey L, et al. Treatment with Anakinra, a Recombinant IL-1 Receptor Antagonist, Unlikely to Induce Lasting Remission in Patients with CGD Colitis. The American Journal of Gastroenterology 110 (2015): 938-939.
- Fernandez-Boyanapalli RF, Frasch SC, Thomas SM, et al. Pioglitazone Restores Phagocyte Mitochondrial Oxidants and Bactericidal Capacity in Chronic Granulomatous Disease. The Journal of Allergy and Clinical Immunology 135 (2015b): 517-527.e12.
- Migliavacca M, Assanelli A, Ferrua F, et al. Pioglitazone as a Novel Therapeutic Approach in Chronic Granulomatous Disease. The Journal of Allergy and Clinical Immunology 137 (2016): 1913-1915.e2.
- European Union Risk Assessment Report: Hydrogen peroxide CAS No: 7722-84-1. 2nd Priority List, Volume 38, 2003EURAR V38: hydrogen peroxide http://esis.jrc.ec.europa.eu/doc/existingchemicals/risk assessment/REPORT/hydrogenperoxidereport022.pdf
- Rhee SG. Cell Signaling. H2O2, a Necessary Evil for Cell Signaling. Science 312 (2006): 1882-1883.
- Kato I, Umeda K, Awaya T, et al. Successful Treatment of Refractory Donor Lymphocyte Infusion-Induced Immune-Mediated Pancytopenia with Rituximab. Pediatric Blood & Cancer 54 (2010): 329-331.
- Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of Chronic Granulomatous Disease with Nonmyeloablative Conditioning and a T-Cell–Depleted Hematopoietic Allograft. The New England Journal of Medicine 334 (2001): 881-888.
- Seger RA, Gungor T, Belohradsky BH, et al. Treatment of Chronic Granulomatous Disease with Myeloablative Conditioning and an Unmodified Hemopoietic Allograft: A Survey of the European Experience, 1985–2000. Blood 100 (2002): 4344-4350.
- Zhu G, Wang Q, Lu S, et al. Hydrogen Peroxide: A Potential Wound Therapeutic Target? Medical Principles and Practice 26 (2017): 301-308.
- Giorgio M, Trinei M, Migliaccio E, et al. Hydrogen Peroxide: A Metabolic By-product or a Common Mediator of Ageing Signals? Nature Reviews Molecular Cell Biology 8 (2007): 722-728.
- Watt BE, Proudfoot AT, Vale JA. Hydrogen Peroxide Poisoning. Toxicological Reviews 231 (2004): 51-57.
- Song JJ, Lim HW, Kim K, et al. Effect of Caffeic Acid Phenethyl Ester (CAPE) on H2O2 Induced Oxidative and Inflammatory Responses in Human Middle Ear Epithelial Cells. International Journal of Pediatric Otorhinolaryngology 76 (2012): 675-679.
- Park WH. Hydrogen Peroxide Inhibits the Growth of Lung Cancer Cells Via the Induction of Cell Death and G1-Phase Arrest. Oncology Reports 40 (2018): 1787-1794.
- SD Varma, PS Devamanoharan. H202 in Human Blood. Free Radical Research Communications 14 (1991): 125-131.
- Forman HJ, Bernardo A, Davies KJA. What is the Concentration of Hydrogen Peroxide in Blood and Plasma? Archives of Biochemistry and Biophysics 603 (2016): 48-53.
- Information on EC 1.11.1.6 - catalase - BRENDA Enzyme Database (brenda-enzymes.org)
- Information on EC 1.11.1.9 - glutathione peroxidase - BRENDA Enzyme Database (brenda-enzymes.org)
- Takeshige K, Matsumoto T, Shibata T, et al. Simple and Rapid Method for the Diagnosis of Chronic Granulomatous Disease, Measuring Hydrogen Peroxide and Superoxide Anions Released from Leukocytes in Whole Blood. Clinica Chimica Acta 91 (1979): 329-335.
- Hiroshi Maeda. The 35th Anniversary of the Discovery of EPR Effect: A New Wave of Nanomedicines for Tumor-Targeted Drug Delivery—Personal Remarks and Future Prospects Reprinted from: Journal of Personalized Medicine 229 (2021): 229.
- Nunoi H, Xie P, Nakamura H, et al. Treatment with Polyethylene Glycol–Conjugated Fungal D-Amino Acid Oxidase Reduces Lung Inflammation in a Mouse Model of Chronic Granulomatous Disease. Inflammation 45 (2022): 1668-1679.