Livedoid Vasculopathy Associated with Cryoglobulinemia Revealing Multiple Myeloma: A Case Report
Article Information
Madiha El Jazouly1*, Khalqui Slamti1, Ghita Basri1, Kenza Oqbani2, Soumya Chiheb1
1Dermatology Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI Health Science University
2Pathology Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI Health Science University
*Corresponding author: El jazouly Madiha, Dermatology Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI Health Science University, Casablanca, Morocco.
Received: 31 August 2023; Accepted: 12 September 2023; Published: 24 November 2023.
Citation: Madiha El Jazouly, Khalqui Slamti, Ghita Basri, Kenza Oqbani, Soumya Chiheb. Livedoid Vasculopathy Associated with Cryoglobulinemia Revealing Multiple Myeloma: A Case Report. Archives of Clinical and Biomedical Research. 7 (2023): 586-589.
View / Download Pdf Share at FacebookAbstract
Livedoid vasculopathy is a thrombotic occlusive vascular disease. The diagnosis of the disease can be a challenge for clinicians because of the overlap in clinical and histological features with other skin conditions mainly vasculitis. It is characterized by recurrent painful ulcers of the lower extremities. The treatment is also difficult. Several therapies based on anticoagulant effects have been described. We report an unusual case of an extensive livedoid vasculopathy associated with cryoglobulinemia successfully treated with rivaroxaban and revealed multiple myeloma, an association rarely reported in the literature, especially with monoclonal gammapathy of undetermined significance (MGUS).
Keywords
Livedoid vasculopathy; Rivaroxaban; Cryoglobulinemia; Cutaneous necrosis; Thrombotic vasculopathy
Article Details
1. Introduction
Livedoid vasculopathy is a thrombotic occlusive vascular disease. Initially described by Milian in 1929 as white atrophy (atrophie blanche) and reported in the 1950s by Feldaker as a coagulation disorder called livedo reticularis with summer ulcerations. Then the definitions succeeded one another, segmental hyalinizing vasculitis which corresponds to a histological definition of the disease. The inappropriate term livedo vasculitis was also used as well as the English acronym PPURPLE (painful purpuric ulcers with a reticular pattern of the lower extremities [1,2]. It reflects an evolving understanding of its pathogenesis which is related to coagulation disorders and often associated with autoimmune disease. The diagnosis and treatment of the disease can be a challenge for clinicians. Diagnosis is difficult because clinical and histological features overlap with other skin conditions, mainly vasculitis and thrombotic occlusive vasculopathy [3]. We report an interesting case of an extensive severe livedoid vasculopathy associated with cryoglobulinemia successfully treated with rivaroxaban and reveling multiple myeloma, an association rarely reported in the literature, especially with monoclonal gammapathy of undetermined significance (MGUS).
2. Case report:
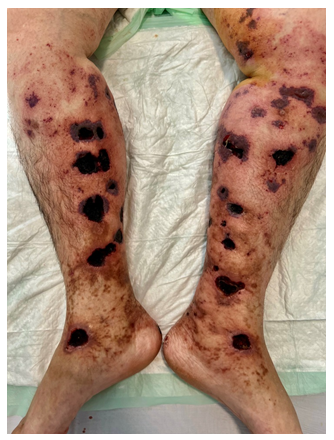
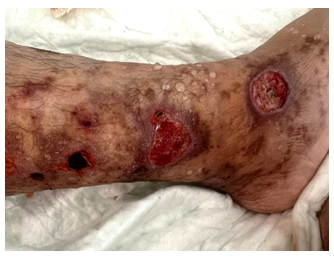
A 70-year-old female patient with a pathological history of venous insufficiency and recurrent peri malleolar ulcerations of the legs treated with antiplatelet medication for 1 year, was hospitalized at our department for painful necrotic and annular ulcers, with brown livedo involving symmetrically the lower limbs [Figure1A]. Skin examination noted also multiple white scars in the peri malleolar region in both feet with no cyanosis or edema [Figure 1B].
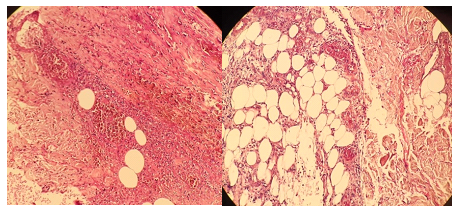
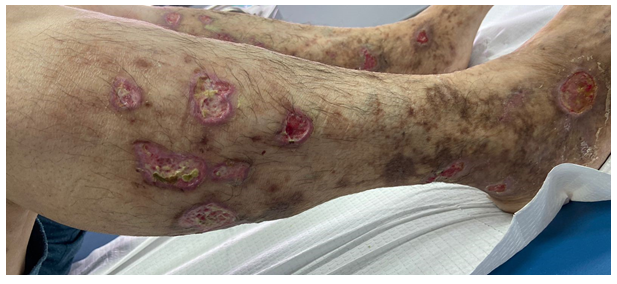
Pulses were equal and palpable in all four extremities. The patient denied fever, arthralgias, or fatigue. An arterial echo-doppler and an angiography scanner were performed and returned negative. Routine tests and thrombophilia investigation were normal. Other complementary laboratory blood tests including antinuclear (ANA) antibodies, lupus anticoagulant, anticardiolipin antibodies, venereal disease research laboratory (VDRL) test, and hepatitis Band C serologies were negative, except for the presence of type 1 cryoglobulin and IgG Kappa isotype in electrophoretic protein gram with immunoglobulin fractions. Anatomopathological examination showed that the superficial and deep dermis vessels were congestive, showing inflammatory damage to their walls with numerous fibrinoid thrombi obstructing their lumen, with endothelial proliferation [Figure 2], a feature compatible with a hyalinizing thrombotic vasculopathy. Standard cytogenetic and FISH studies were in accordance with a multiple myeloma type (MGUS): monoclonal gammopathy of undetermined significance. The therapeutic management consisted of corticosteroid therapy and Rivaroxaban at 10 mg daily. In week 2, we suspended steroids and adjusted the Rivaroxaban dose to 20 mg per day for 6 months. A good evolution was noted with regression and the lesions' healing and the pain's disappearance within one month [Figure 3]. We recommend the use of compression stockings after complete healing. The patient is referred to the hematology department for complementary assessment. At follow-up 1 year later, the patient remained in remission and there was no recurrence of skin.
Discussion:
Our observation is particular by its clinical presentation, the association with cryoglobulinemia, multiple myeloma, and the favorable evolution under rivaroxaban treatment. The diagnosis of LV remains clinical and according to some authors, the histopathologic findings in LV are not essential for the diagnosis and direct immunofluorescence features are not specific to LV. The typical manifestation of LV is a triad composed of a livedo racemosa of the skin and painful episodic ulcerations of the distal face of the legs followed by white-porcelain scars called "white atrophy" [4]. Our patient presented with very extensive necrotic feature lesions reflecting the severity of the involvement and the association with coagulopathy and venous insufficiency. Numerous publications have reported associations between livedoid vasculopathy and thrombophilias such as antiphospholipid syndrome, hyperhomocysteinemia, protein C, S, antithrombin deficiency, and Factor V Leiden and prothrombin II gene mutations. More recently, the role of the plasminogen activator inhibitor (PAI-1) in the development of VL has been established [5,6,7]. The association with cryoglobulinemia type 1 is rarely reported. Cryoglobulinemia type I is also a type of occlusive nonvasculitic vasculopathy characterized by a single monoclonal Ig, an IgM or IgG precipitate in cold temperature involved in a thrombus of the small vessel explaining the clinical manifestations [8]. Cutaneous manifestations include palpable purpura with necrosis and ulceration, acrocyanosis, and livedo reticularis. In our case, the diagnosis clinically suspected is confirmed by biological tests and histological analysis. The literature contains reports of various histopathologic findings. Noninflammatory hyaline thrombosis usually was observed in patients with high circulating levels of type I cryoglobulins and less often leukocytoclastic vasculitis [8,9]. Confirmation can be provided by complementary IgM immunostaining and the periodic acide-Schiff (PAS) [10]. It is associated with lymphoproliferative disorders (10%–15%) such as myeloma or B-cell lymphoma or less frequently with monoclonal gammopathy of undetermined significance (MGUS) as our patient [11]. According to some authors, the treatment of type 1 cryoglobulinemia focuses on the underlying hematological malignancy, that requires combination chemotherapy, whereas rituximab or plasmapheresis therapy is generally preferred for IgM MGUS [9,12]. Anticoagulation is important for the improvement of cutaneous features of thrombotic vasculopathy. However, various treatments have been studied such as anabolic steroids, intravenous immunoglobulins, and antiplatelet agents, and have shown good clinical results [13]. Many studies reported the beneficial effects of treating LV with rivaroxaban [14,15]. Our case supports rivaroxaban's potential utility in treating occlusive vasculopathy and underlines the need to consider the diagnosis of cryoglobulinemia in the presence of extensive and severe necrosis, and the necessity of long-term follow-up to detect associated disease. Therefore, the first step to achieving an accurate diagnosis of occlusive vasculopathy is a histopathologic study. Although it is crucial to correlate skin biopsy findings with clinical history, and physical examination, and to complete exhaustive laboratory tests to reach a final diagnosis.
Conflicts of interest:
None
Financial support and sponsorship:
Nil
References:
- Eswaran H, Googe P, Vedak P, et al. Livedoid vasculopathy: A review with focus on terminology and pathogenesis. Vascular medicine (London, England) 27 (2022): 593-603.
- Feldaker m, hines e A jr, and kierland r R. Livedo reticularis with summer ulcerations. A.M.A. archives of dermatology 72 (1955): 31-42.
- Criado P R, Rivitti E A, Sotto M N, et al. Livedoid vasculopathy: an intringuing cutaneous disease. Anais brasileiros de dermatologia 86 (2011): 961-977.
- Kerk N, and Goerge T. Livedoid vasculopathy - current aspects of diagnosis and treatment of cutaneous infarction. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology: JDDG 11 (2013): 407-410.
- Castillo-Martínez C, Moncada B, Valdés-Rodríguez R, et al. Livedoid vasculopathy (LV) associated with sticky platelets syndrome type 3 (SPS type 3) and enhanced activity of plasminogen activator inhibitor (PAI-1) anomalies. International journal of dermatology 53 (2014): 1495-1497.
- Acland K M, Darvay A, Wakelin S H, et al. Livedoid vasculitis: a manifestation of the antiphospholipid syndrome?. The British journal of dermatology 140 (1999): 131-135.
- Feng S Y, Jin P Y, and Shao C G. The significance of anticardiolipin antibody and immunologic abnormality in livedoid vasculitis. International journal of dermatology 50 (2011): 21-23.
- Desbois A C, Cacoub P, and Saadoun D. Cryoglobulinemia: An update in 2019. Joint bone spine 86 (2019): 707-713.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive Nonvasculitic Vasculopathy. The American Journal of dermatopathology 39 (2017): 637-662.
- Murphy A, Kilian A, Lowe L, et al. Severe cutaneous necrosis associated with type I cryoglobulinemia. JAAD case reports 5 (2019): 736-738.
- Runge J S, Pearson T L, Keren D F, et al. (2021). Multiple myeloma presenting as cryoglobulinemic vasculitis. JAAD case reports 11 (2021): 81-83.
- Harel S, Mohr M, Jahn I, et al. Clinico-biological characteristics and treatment of type I monoclonal cryoglobulinaemia: a study of 64 cases. British journal of haematology 168 (2015): 671-678.
- Ratzinger G, Zelger B G, and Zelger, B. W. Bar code reader - an algorithmic approach to cutaneous occluding vasculopathies? Part I: small vessel vasculopathies. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG 17 (2019): 895-904.
- Franco Marques G, Criado P R, Alves Batista Morita T C, et al. The management of livedoid vasculopathy focused on direct oral anticoagulants (DOACs): four case reports successfully treated with rivaroxaban. International journal of dermatology 57 (2018): 732-741.
- Kerk N, Drabik A, Luger T A, et al. Rivaroxaban prevents painful cutaneous infarctions in livedoid vasculopathy. The British journal of dermatology 168 (2013): 898-899.