Left Atrial Dissection During Percutaneous Coronary Intervention: Case Report and Review of Literature
Article Information
Sanjeev Vaderah1*, Rishi Parmar1, Nainika Vaderah2, Alex Parrish1, Sameer Gafoor3
1Skagit Regional Health, 1400 E Kincaid St, Mount Vernon, WA 98274, United States
2University of Washington, Seattle, WA, United States
3Swedish Medical Center, First Hill Campus, Seattle, United States
*Corresponding Author: Sanjeev Vaderah, Skagit Regional Health, 1400 E Kincaid St, Mount Vernon, WA 98274, United States
Received: 13 December 2022; Accepted: 21 December 2022; Published: 03 January 2023
Citation: Sanjeev Vaderah, Rishi Parmar, Nainika Vaderah, Alex Parrish, Sameer Gafoor. Left Atrial Dissection During Percutaneous Coronary Intervention: Case Report and Review of Literature. Journal of Surgery and Research 6 (2023): 645-647.
View / Download Pdf Share at FacebookKeywords
Obstructive coronary artery disease, Left atrial dissection, Coronary intervention
Research Article articles
Obstructive coronary artery disease articles Obstructive coronary artery disease Research articles Obstructive coronary artery disease review articles Obstructive coronary artery disease PubMed articles Obstructive coronary artery disease PubMed Central articles Obstructive coronary artery disease 2023 articles Obstructive coronary artery disease 2024 articles Obstructive coronary artery disease Scopus articles Obstructive coronary artery disease impact factor journals Obstructive coronary artery disease Scopus journals Obstructive coronary artery disease PubMed journals Obstructive coronary artery disease medical journals Obstructive coronary artery disease free journals Obstructive coronary artery disease best journals Obstructive coronary artery disease top journals Obstructive coronary artery disease free medical journals Obstructive coronary artery disease famous journals Obstructive coronary artery disease Google Scholar indexed journals Left atrial dissection articles Left atrial dissection Research articles Left atrial dissection review articles Left atrial dissection PubMed articles Left atrial dissection PubMed Central articles Left atrial dissection 2023 articles Left atrial dissection 2024 articles Left atrial dissection Scopus articles Left atrial dissection impact factor journals Left atrial dissection Scopus journals Left atrial dissection PubMed journals Left atrial dissection medical journals Left atrial dissection free journals Left atrial dissection best journals Left atrial dissection top journals Left atrial dissection free medical journals Left atrial dissection famous journals Left atrial dissection Google Scholar indexed journals Coronary intervention articles Coronary intervention Research articles Coronary intervention review articles Coronary intervention PubMed articles Coronary intervention PubMed Central articles Coronary intervention 2023 articles Coronary intervention 2024 articles Coronary intervention Scopus articles Coronary intervention impact factor journals Coronary intervention Scopus journals Coronary intervention PubMed journals Coronary intervention medical journals Coronary intervention free journals Coronary intervention best journals Coronary intervention top journals Coronary intervention free medical journals Coronary intervention famous journals Coronary intervention Google Scholar indexed journals Swedish Medical Center articles Swedish Medical Center Research articles Swedish Medical Center review articles Swedish Medical Center PubMed articles Swedish Medical Center PubMed Central articles Swedish Medical Center 2023 articles Swedish Medical Center 2024 articles Swedish Medical Center Scopus articles Swedish Medical Center impact factor journals Swedish Medical Center Scopus journals Swedish Medical Center PubMed journals Swedish Medical Center medical journals Swedish Medical Center free journals Swedish Medical Center best journals Swedish Medical Center top journals Swedish Medical Center free medical journals Swedish Medical Center famous journals Swedish Medical Center Google Scholar indexed journals percutaneous cardiac procedures articles percutaneous cardiac procedures Research articles percutaneous cardiac procedures review articles percutaneous cardiac procedures PubMed articles percutaneous cardiac procedures PubMed Central articles percutaneous cardiac procedures 2023 articles percutaneous cardiac procedures 2024 articles percutaneous cardiac procedures Scopus articles percutaneous cardiac procedures impact factor journals percutaneous cardiac procedures Scopus journals percutaneous cardiac procedures PubMed journals percutaneous cardiac procedures medical journals percutaneous cardiac procedures free journals percutaneous cardiac procedures best journals percutaneous cardiac procedures top journals percutaneous cardiac procedures free medical journals percutaneous cardiac procedures famous journals percutaneous cardiac procedures Google Scholar indexed journals lethal complication articles lethal complication Research articles lethal complication review articles lethal complication PubMed articles lethal complication PubMed Central articles lethal complication 2023 articles lethal complication 2024 articles lethal complication Scopus articles lethal complication impact factor journals lethal complication Scopus journals lethal complication PubMed journals lethal complication medical journals lethal complication free journals lethal complication best journals lethal complication top journals lethal complication free medical journals lethal complication famous journals lethal complication Google Scholar indexed journals balloon angioplasty articles balloon angioplasty Research articles balloon angioplasty review articles balloon angioplasty PubMed articles balloon angioplasty PubMed Central articles balloon angioplasty 2023 articles balloon angioplasty 2024 articles balloon angioplasty Scopus articles balloon angioplasty impact factor journals balloon angioplasty Scopus journals balloon angioplasty PubMed journals balloon angioplasty medical journals balloon angioplasty free journals balloon angioplasty best journals balloon angioplasty top journals balloon angioplasty free medical journals balloon angioplasty famous journals balloon angioplasty Google Scholar indexed journals transthoracic echocardiography articles transthoracic echocardiography Research articles transthoracic echocardiography review articles transthoracic echocardiography PubMed articles transthoracic echocardiography PubMed Central articles transthoracic echocardiography 2023 articles transthoracic echocardiography 2024 articles transthoracic echocardiography Scopus articles transthoracic echocardiography impact factor journals transthoracic echocardiography Scopus journals transthoracic echocardiography PubMed journals transthoracic echocardiography medical journals transthoracic echocardiography free journals transthoracic echocardiography best journals transthoracic echocardiography top journals transthoracic echocardiography free medical journals transthoracic echocardiography famous journals transthoracic echocardiography Google Scholar indexed journals Surgical technique articles Surgical technique Research articles Surgical technique review articles Surgical technique PubMed articles Surgical technique PubMed Central articles Surgical technique 2023 articles Surgical technique 2024 articles Surgical technique Scopus articles Surgical technique impact factor journals Surgical technique Scopus journals Surgical technique PubMed journals Surgical technique medical journals Surgical technique free journals Surgical technique best journals Surgical technique top journals Surgical technique free medical journals Surgical technique famous journals Surgical technique Google Scholar indexed journals
Article Details
Introduction
Left atrial dissection is a rare complication of cardiac surgery and percutaneous cardiac procedures. It is defined as a false blood-filled cavity or lumen from mitral annular area to the left atrial free wall or into the atrial septum thereby creating a new chamber with or without communications with the true left atrium [1]. It has been most commonly described following mitral valve surgery [1].
Cases of left atrial dissection have also been reported following aortic valve surgery chest, CABG, trauma acute MI, infective endocarditis, radiofrequency ablation for atrial fibrillation and rarely following percutaneous coronary interventions [2]. The case we describe here occurred in the setting of a percutaneous coronary intervention, such occurrence is less common. The purpose of our case report is to increase recognition of this rare but potentially lethal complication and to review management strategies.
Case Presentation
A 77-year-old man with known obstructive coronary artery disease presented to the cath lab for coronary intervention of his left circumflex artery. This was a trifurcation lesion involving the 2nd and 3rd obtuse marginal branch as well as the ongoing AV groove circumflex. Due to significant vessel overlap the guide wires had to be repositioned multiple times, wires were placed in the OM branch and the ongoing Left circumflex. During the procedure one of the wires was inadvertently advanced into the AV circumflex branch. Initial balloon angioplasty was then done with a 2.0 balloon (Euphora, Medtronic) ) A 2.75 x 15 mm stent (Resolute Onyx, Medtronic) was deployed in the obtuse marginal branch and 3.0 x 23 mm stent(Resolute Onyx, Medtronic) was delivered in the proximal left circumflex. Kissing balloon inflations in the two obtuse marginal branches were performed. Following that, the proximal circumflex was dilated with a 3.0 noncompliant balloon. Final angiographic results were good. Review of the films post procedure suggested that there may have been faint contrast staining from wire in the left atrial circumflex branch.
About an hour following the procedure the patient developed chest discomfort and became hypotensive with a blood pressure of 78/57 mmHg. He was successfully resuscitated with iv fluids. Repeat EKG did not show any significant changes. An urgent echocardiogram was performed. The echocardiogram revealed an independently mobile linear structure in the left atrium. It extended the perimeter of the left atrium and terminated in the mitral and lateral annulus.
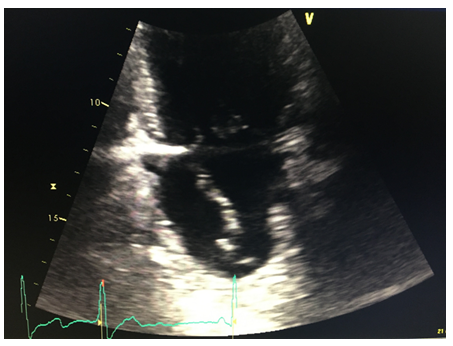
Figure 1: LA dissection 2C view
A CT angiogram of the chest was performed subsequently. It revealed a low density mass along the posterior aspect of the left atrium measuring approximately 8.7 x 7.6 x 5.2 cm, within the mass, high density material concerning for continued hemorrhage and active expansion was suspected (Figures 3,4). A repeat echocardiogram was then immediately performed. The echocardiogram revealed that a dissection was had enlarged and was filled with thrombus.
Obstruction of pulmonary venous inflow and mitral flow can result in CHF and low output syndrome. This is a dreaded complication. In view of CT scan suggesting ongoing bleeding a decision was made to transfer the patient to a tertiary care hospital. At the tertiary care facility the patient remained hemodynamically stable. It was decided to observe the patient and perform serial imaging. The patient's intra atrial hematoma remained stable and did not enlarge further. He was discharged on the 10th postop day.
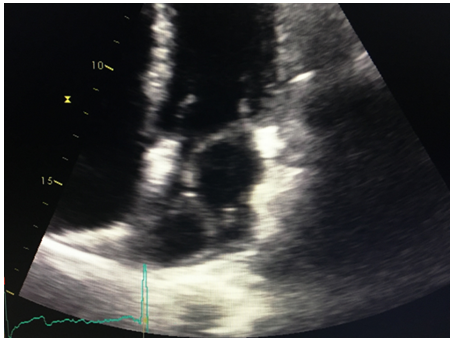
Figure 2: LA dissection 4C view
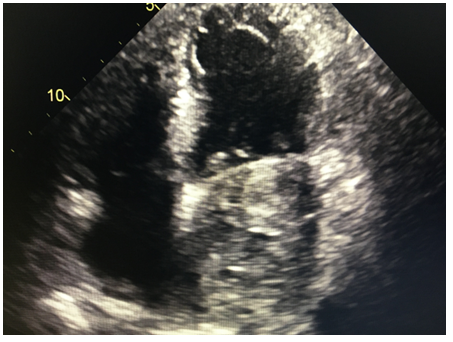
Figure 3: LA filled with thrombus
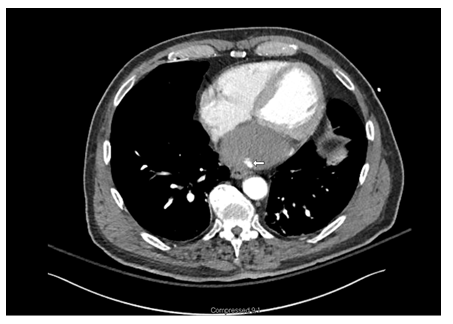
Figure 4: LA thrombus with active leak
Discussion
Incidence of left atrial dissection is low and is reported to be 0.16% following mitral valve surgery and 0.02% following coronary artery bypass grafting [3]. This condition is being increasingly recognized following surgery because of increased use of TEE since 1990 [4]. Left atrial dissection following PCI is less common and literature search is limited to sporadic case reports, it is only after 2000 that case reports of LatD as a complication of percutaneous procedures started appearing in literature [5,6].
Left atrial dissection has a variable clinical course. Cases described have ranged from self-limiting stable disease to lethal outcomes. Mortality rate of up to 13.8% has been described in surgical literature [3]. Left atrial dissection presents immediately and majority of the cases, though it has been reported to occur months or even years after the procedure [4]. Dyspnea is the commonest presenting symptom and present in about a quarter of the patients. Chest pain and arrhythmias have been reported, about 10% of the patients are asymptomatic [1].
Our case was readily recognized by transthoracic echocardiography. Duplication of left atrial free wall is the most common abnormality seen on echocardiography. Left atrial dissection, especially if there is thrombus in the false lumen, can mimic left atrial mass. Cardiac tamponade, hiatal hernia, plueropericardial cysts causing extrinsic compression of the left atrium can also mimic left atrial dissection [4]. Multimodality imaging has been recommended to confirm diagnosis of this rare condition. In our case CT scanning coupled with TTE corroborated the diagnosis.
Pathogenesis of left atrial dissection is varied. Majority of the cases arise from injury along the AV junction which results in separation of the endocardium of the left atrium causing the dissection cavity. Other entry points are also possible and likely explain LatD arising from aortic valve replacement, percutaneous coronary interventions and pulmonary vein cannulation [4]. Similar to the cases reported by Solzbach and Cresce et al, we too believe that a distal perforation from guide wire manipulation resulted in left atrial injury [5].
Indication for surgery should be based on clinical presentation [2] in patients with hemodynamic instability prompt surgical approach is warranted. Surgical technique involves obliteration of the false cavity and addressing the entry point if possible. Medical management or non-operative approach, supported by serial imaging is reasonable in a stable patient. Reversal of anticoagulation when feasible should also be considered.
References
- Fukuhara S, Dimitrova K, Geller C, et al. Left atrial dissection: And almost unknown entity. Interactive cardiovascular and thoracic surgery 20 (2015): 96-100.
- Cereda A, Luca F, Lanzone A. Case report and systemic review of iatrogenic left atrial dissection and different cardiovascular specialities: A common treatment for an uncommon complication?. Catheterizations and cardiovascular interventions 95 (2020): e30-37.
- Fukuhara S, Dimitrova K, Geller D. et al. Left atrial dissection: Etiology and treatment. Annals of thoracic surgery 95 (2013): 1557-1562.
- Tsukui H, Iwasa S, Yamazaki K. Left atrial dissection. General thoracic and cardiovascular surgery 63 (2015): 434-445.
- Solzbach U, Beuter M, Haas H. Left atrial hematoma after percutaneous intervention. International Journal of cardiology 141 (2010): e37-38.
- Sardar R, Kaddissi G, Sabir S et al. First case of left atrial dissection after transcatheter aortic valve replacement. Cardiovascular revascularization medicine 17 (2016): 279-281.
