Lead-Induced Peripheral Neuropathy in a CKD Patient on Maintenance Hemodialysis
Article Information
Muhammad Humayoun Rashid1, Syeda Neelam Yamin Bukhari2, Henna Pervaiz3, Sheheryar Sharif4, Ghoza Chaudhery5, Ayesha Ali6
1Department of Neurology, Mukhtar A. Sheikh Hospital, Pakistan
2Department of Medicine, Nishtar Hospital Multan, Pakistan
3Department of Medicine, Ayub Teaching Hospital, Pakistan
4Department of Medicine, Lady Reading Hospital, Pakistan
5Department of Pediatric Surgery, Children Hospital Lahore, Pakistan
6Department of Medicine, Avicenna Hospital Lahore, Pakistan
*Corresponding Author: Muhammad Humayoun Rashid, Mukhtar A. Sheikh Hospital, Pakistan
Received: 24 August 2021; Accepted: 07 September 2021; Published: 20 September 2021
Citation: Muhammad Humayoun Rashid, Syeda Neelam Yamin Bukhari, Henna Pervaiz, Sheheryar Sharif, Ghoza Chaudhery, Ayesha Ali. Lead-Induced Peripheral Neuropathy in a CKD Patient on Maintenance Hemodialysis. Archives of Clinical and Medical Case Reports 5 (2021): 649-655.
View / Download Pdf Share at FacebookAbstract
Peripheral neuropathy due to lead intoxication is a common presentation but secondary to maintenance hemodialysis is a rare find, especially with the international guidelines in place about the dialysate fluid required for hemodialysis. We report herein a 26-year-old Asian man who developed end-stage renal failure, followed by bilateral foot drop, in whom the maximal total blood lead was 34 µg/dL. Measurements of lead in his tibia and calcaneus by K-x-ray fluorescence, however, showed markedly elevated values. The foot drop cleared after few months of treatment with oral succimer and subsequent lead levels were below 10 µg/dL. The Lead source was investigated and found to be high in the dialysate used for maintenance hemodialysis. Succimer therapy along with dialysate meeting the international standards were used which resulted in improvement of the symptoms.
Keywords
Lead toxicity; Peripheral neuropathy; Dialysis; Chronic kidney disease; Foot drop
Lead toxicity articles; Peripheral neuropathy articles; Dialysis articles; Chronic kidney disease articles; Foot drop articles
Lead toxicity articles Lead toxicity Research articles Lead toxicity review articles Lead toxicity PubMed articles Lead toxicity PubMed Central articles Lead toxicity 2023 articles Lead toxicity 2024 articles Lead toxicity Scopus articles Lead toxicity impact factor journals Lead toxicity Scopus journals Lead toxicity PubMed journals Lead toxicity medical journals Lead toxicity free journals Lead toxicity best journals Lead toxicity top journals Lead toxicity free medical journals Lead toxicity famous journals Lead toxicity Google Scholar indexed journals Peripheral neuropathy articles Peripheral neuropathy Research articles Peripheral neuropathy review articles Peripheral neuropathy PubMed articles Peripheral neuropathy PubMed Central articles Peripheral neuropathy 2023 articles Peripheral neuropathy 2024 articles Peripheral neuropathy Scopus articles Peripheral neuropathy impact factor journals Peripheral neuropathy Scopus journals Peripheral neuropathy PubMed journals Peripheral neuropathy medical journals Peripheral neuropathy free journals Peripheral neuropathy best journals Peripheral neuropathy top journals Peripheral neuropathy free medical journals Peripheral neuropathy famous journals Peripheral neuropathy Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Dialysis articles Dialysis Research articles Dialysis review articles Dialysis PubMed articles Dialysis PubMed Central articles Dialysis 2023 articles Dialysis 2024 articles Dialysis Scopus articles Dialysis impact factor journals Dialysis Scopus journals Dialysis PubMed journals Dialysis medical journals Dialysis free journals Dialysis best journals Dialysis top journals Dialysis free medical journals Dialysis famous journals Dialysis Google Scholar indexed journals Chronic kidney disease articles Chronic kidney disease Research articles Chronic kidney disease review articles Chronic kidney disease PubMed articles Chronic kidney disease PubMed Central articles Chronic kidney disease 2023 articles Chronic kidney disease 2024 articles Chronic kidney disease Scopus articles Chronic kidney disease impact factor journals Chronic kidney disease Scopus journals Chronic kidney disease PubMed journals Chronic kidney disease medical journals Chronic kidney disease free journals Chronic kidney disease best journals Chronic kidney disease top journals Chronic kidney disease free medical journals Chronic kidney disease famous journals Chronic kidney disease Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Genotype articles Genotype Research articles Genotype review articles Genotype PubMed articles Genotype PubMed Central articles Genotype 2023 articles Genotype 2024 articles Genotype Scopus articles Genotype impact factor journals Genotype Scopus journals Genotype PubMed journals Genotype medical journals Genotype free journals Genotype best journals Genotype top journals Genotype free medical journals Genotype famous journals Genotype Google Scholar indexed journals Tuberculomas articles Tuberculomas Research articles Tuberculomas review articles Tuberculomas PubMed articles Tuberculomas PubMed Central articles Tuberculomas 2023 articles Tuberculomas 2024 articles Tuberculomas Scopus articles Tuberculomas impact factor journals Tuberculomas Scopus journals Tuberculomas PubMed journals Tuberculomas medical journals Tuberculomas free journals Tuberculomas best journals Tuberculomas top journals Tuberculomas free medical journals Tuberculomas famous journals Tuberculomas Google Scholar indexed journals Foot drop articles Foot drop Research articles Foot drop review articles Foot drop PubMed articles Foot drop PubMed Central articles Foot drop 2023 articles Foot drop 2024 articles Foot drop Scopus articles Foot drop impact factor journals Foot drop Scopus journals Foot drop PubMed journals Foot drop medical journals Foot drop free journals Foot drop best journals Foot drop top journals Foot drop free medical journals Foot drop famous journals Foot drop Google Scholar indexed journals
Article Details
1. Introduction
Lead (Pb) is a well-known source of toxicity. Among the toxic manifestations of lead are peripheral neuropathy (usually motor), nephropathy, encephalopathy, abdominal colic, anemia, polyarthralgia (linked to gout), and hypertension. Lead accumulation in the body can affect both the central nervous system and peripheral nervous system. Peripheral neuropathy may cause reduced motor activity causing muscular weakness, fatigue, and lack of muscular coordination [1]. Lead present in the bloodstream is either excreted in urine or bile at a clearance rate of 1 to 3 mL/min or is distributed to different soft tissues in the body and eventually accumulates in bone. The half-life of blood lead is 30 days but that in bone is 20 to 30 years. Therefore, blood lead levels are more reflective of acute exposure, whereas bone lead levels better reflect cumulative exposure over time.
Patients with chronic renal failure requiring treatment by hemodialysis are at risk of developing trace element imbalances. One of these disturbances is raised lead (Pb) levels which have been demonstrated in various studies. A study on trace element status of hemodialyzed patients saw that the concentration of blood lead was greater than normal [2]. Another study measuring blood lead in end-stage renal disease (ESRD) patients who were on maintenance dialysis (MHD) showed that the blood lead level was higher in the ESRD patients who were on MHD than in healthy adults. The blood lead concentration was found to increase with the duration of the MHD [3]. Similarly, a study was done to measure serum trace elements in the blood of children with ESRD [4]. It found out that serum lead levels were higher in children on hemodialysis than in healthy children or those with ESRD but managed conservatively. Lead can cause damage to the nervous system through various mechanisms both directly and indirectly. Direct effects include disruption of synapse formation, interference with neuronal migration and differentiation, interference with neurotransmitter release, inhibition of NMDA-ion channel, and inhibition of Na-K-ATPase pump [5]. Lead also disrupts calcium release from mitochondria, resulting in increased reactive oxygen species formation and self-destruction of mitochondria [6]. Indirect effects on the nervous system result from the increasing risk of numerous conditions that may have adverse effects on nervous system functions, including hypertension, impaired renal function, and impaired thyroid function.
Here we discuss a case report of lead-induced peripheral neuropathy in a chronic kidney disease patient on maintenance hemodialysis.
2. Case Report
A 26-year-old male first presented with hypertension (160/100) in February 2018. Urinalysis on initial presentation showed 2+ proteins. Sediments were negative for RBC and white blood cells (WBC). There were no RBC or WBC casts. Hematocrit was 17.3%, and hemoglobin was 6.3 g/dL. WBC was 6,800 g/dl and platelet count, 276,000 g/dl. Venereal Disease Research Laboratory test results were negative. Calcium was 5.9 mg/dL and phosphorus 6.6 mg/dL. Sodium was 130 mEq/L; potassium, 4.7 mEq/L; chloride, 104 mEq/L; carbon dioxide, 12 mEq/L. A presumptive diagnosis of end-stage renal disease secondary to hypertensive nephrosclerosis was made. However, no renal biopsy was performed to confirm the diagnosis, and a concrete underlying cause was not found. The patient was then placed on a three times weekly hemodialysis.
In September 2020 he presented with difficulty going up the stairs, pain and numbness in the legs, and difficulty getting up from the floor. These symptoms developed progressively over the previous three months. During this time, he also noticed progressive muscle loss in the legs. He had been wheelchair-bound for a month. There was no history of weakness or muscle loss in the arms. He also didn’t have any visual changes, shortness of breath, or difficulty swallowing. His urinary output was zero due to chronic kidney disease and was on hemodialysis (AVF in the left arm). He had lost 27 kg weight in 2 years. There was no family history of such a disease.
On physical examination, he was thin, lean, and pale. His attention, concentration, orientation memory, language, and fund of knowledge were within normal limits. He had bilateral foot drop (Figure 1) and mildly weak flexion in both knees. There was muscle loss in the thigh and calf region of both legs. Deep tendon reflexes were reduced in both lower extremities and plantar reflexes were absent. Vibratory sensations were reduced in the toes. Muscle strength in all other body parts was normal. There were no cranial nerve deficits. He had normal upper and lower limb muscle tone.
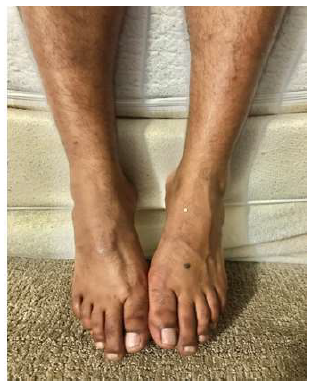
Figure 1: Bilateral Footdrop.
His blood workup (Table 1) at the time showed Hemoglobin 10 g/dl, Urea 18mg/dl, Creatinine 7.11mg/dl, Potassium 2.9mmol/L, and Sodium 134mmol/L. Erythrocyte sedimentation 12 mm/hr and C-reactive protein 1mg/L. He had negative results for ANA, ASMA, AMA, ENA, and ANCA. C3, C4, Thyroid hormones, PTH and vitamin B12 levels were also normal. On Echocardiogram he had a dilated right atrium, concentric left ventricular hypertrophy, pericardial effusion, and an ejection fraction of 65%. On lumbosacral spine MRI there were some changes in L4-L5, but there was no evidence of right (L2-5, S1) and left (L4-5) lumbosacral radiculopathy. Nerve conduction study and Electromyography (EMG) were abnormal with electrodiagnostic evidence of chronic predominantly moderate to severe axonal motor polyneuropathy with some demyelinating features.
|
CBC |
NORMAL |
PATIENT’S |
|
WBC count (10^3/ul) |
4-11 |
8.2 |
|
RBC count (10^3/ul) |
4.2 – 5.4 |
3.6 |
|
Hemoglobin% g/dl |
11 |
10 |
|
HCT (PCV) % |
26 – 50 |
20 |
|
MCV (fl) |
77-96 |
77 |
|
Platelets (10^3/ul) |
150-400 |
184,000 |
|
Neutrophils % |
40-75 |
55 |
|
Lymphocytes % |
20-45 |
35 |
|
LIVER FUNCTION TESTS |
||
|
Bilirubin total (mg/dl) |
0.1 – 1 |
0.6 |
|
Alanine aminotransferase (ALT) U/L |
< 34 |
28 |
|
Aspartate aminotransferase (AST) U/L |
< 35 |
25 |
|
Alkaline phosphatase (U/L) |
46 - 122 |
54 |
|
Gamma glutamyl transferase (U/L) |
< 38 |
32 |
|
RENAL FUNCTION TESTS |
||
|
Serum creatinine (mg/dl) |
0.6 – 1.1 |
7.11 |
|
Urea (mg/dl) |
10 – 50 |
18 |
|
Blood urea nitrogen (mg/dl) |
8 - 22 |
25 |
|
URINE EXAMINATION |
||
|
PH |
4.5 – 7.8 |
5 |
|
Sugar |
Nil |
Nil |
|
Protein |
Nil |
2+ |
|
Ketone |
Nil |
Nil |
|
Pus cells /HPF |
Nil |
Nil |
|
RBCs /HPF |
Nil |
Nil |
|
Nitrite |
Nil |
Nil |
Table 1: Baseline Investigations.
Because of the progression of her neuropathy, a blood lead level was checked on September 12, 2020, and returned high at 34 µg/dL. Measurements of lead in her tibia and calcaneus by K-x-ray fluorescence, however, showed markedly elevated values. The Lead source was investigated and found to be high in the dialysate used for maintenance hemodialysis. Dialysate meeting the international standards was used thereafter and Succimer therapy was begun. The follow-up blood lead levels were 22 µg/dL and 15 µg/dL and 8 µg/dL. His footdrop resolved at that time and hemoglobin improved. Subsequent blood lead levels were never above 10 µg/dL. A kidney transplant was recommended for long-term management.
3. Discussion
Peripheral neuropathy is a disorder related to the peripheral nervous system. It has various causes and diverse presentations. Causes include metabolic disorders, traumatic injuries, nutritional deficiencies, various toxicities, and inherited disorders. Metabolic disorders are one of the most common and treatable causes of peripheral neuropathy and include diabetes, hypothyroidism, and nutritional deficiencies. Workup to evaluate a patient with peripheral neuropathy includes complete blood count, serum electrolytes, ESR, CRP, thyroid-stimulating hormone (TSH), blood sugar, serum vitamin B12 levels, and serum trace mineral studies [7]. Patients with end-stage renal disease managed with maintenance dialysis are prone to suffer from trace element irregularities.
Hemodialysis is a very common treatment modality for end-stage renal disease patients but is associated with many complications such as accelerated cardiovascular disease, infections, and trace element disturbances. However, the possibility that trace elements may accumulate in patients with kidney failure and causes unrecognized toxicity has received surprisingly little attention. Lead accumulation in the patient discussed above, treated with maintenance dialysis may be due to one of the following mechanisms.
- Hemodialysis uses a semipermeable membrane to exchange toxins between blood and dialysate. Dialysate contains all the essential electrolytes such as potassium, sodium, calcium, bicarbonate, and chloride so that their concentration in the blood remains constant. But the concentration of trace elements is not taken into consideration; hence, disturbances in blood concentration of these elements can occur. Hemodialysis patients are exposed to very high volumes of dialysate (>300ml/week); therefore, even minute levels of trace elements in the dialysate can lead to concentration differences with blood and chronic exposure to these can lead to clinically significant toxicities. Thus, patients of chronic hemodialysis are at increased risk of both accumulation or deficiency of trace elements depending upon the dialysis fluid used, their dietary intake, and the residual kidney function [8].
- The other mechanism that can play a role is that uremic plasma contains an active transport inhibitor that inhibits the transport of sodium ions out of RBCs. Uremic toxins competitively inhibit transport ATPase resulting in the accumulation of intracellular sodium and ATP [9]. This can affect lead transport as well. Thus, inhibition of sodium transport out of RBCs and lead transport into RBCs can cause an increase in plasma lead levels. This chronically increased plasma lead levels can then result in such clinical manifestations as polyneuropathy which was specifically motor axonal neuropathy in our case.
Clinical manifestation of lead neuropathy depends upon the time duration and extent of exposure. Subacute exposure to lead is associated with classic motor manifestations involving weakness of extensors of wrist and fingers which gradually move on to involve other muscle groups. Rarely, it starts from distal lower limb muscles involved in ankle dorsiflexion and toe extension which is very much similar to the presentation of our patient. Occasionally, if the intoxication is very severe it can progress to quadriplegia. The weakness is accompanied by muscle atrophy but fasciculations are not reported. Chronic exposure can lead to severe polyneuropathy involving both large and small nerve fibers damaging not only motor control but also the sensory modalities such as pain, vibration, pinprick, and temperature sensations [10].
Electromyography and nerve conduction studies can be used to evaluate the extent of damage done by lead. Electromyography done in our patient showed that he had moderate to severe axonal motor polyneuropathy mainly affecting the distal muscles of the lower limb. Muscles present in the extensor and flexor compartment of lower legs were affected resulting in bilateral weakness of ankle movement. The prognosis of recovery is good, once the exposure has been reduced, depending upon and the extent of weakness. If the extent of damage is severe then the recovery will be slow and incomplete.
4. Conclusion
Peripheral neuropathy due to lead intoxication is a common presentation but secondary to maintenance hemodialysis is a rare find, especially with the international guidelines in place about the dialysate fluid required for hemodialysis. This case shows that still strict monitoring and implementation of these
guidelines is required and negligence can have drastic consequences.
References
- Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdisciplinary toxicology 5 (2012): 47-58.
- Krachler M, Wirnsberger G, Irgolic KJ. Trace element status of hemodialyzed patients. Biological trace element research 58 (1997): 209.
- Subha Palaneeswari M, Rajan PA, Silambanan Santhi J. Blood Lead in End-Stage Renal Disease (ESRD) Patients who were on Maintainence Haemodialysis. Journal of Clinical and Diagnostic Research: JCDR 6 (2012): 1633.
- Esmaeili M, Rakhshanizadeh F. Serum trace elements in children with end-stage renal disease. Journal of renal nutrition. 29 (2019): 48-54.
- Silbergeld EK. Mechanisms of lead neurotoxicity, or looking beyond the lamppost. The FASEB journal 6 (1992): 3201-3206.
- Simons TJ. Cellular interactions between lead and calcium. British medical bulletin 42 (1986): 431-434.
- Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. American family physician 81 (2010): 887-892.
- D'haese PC, De Broe ME. Adequacy of dialysis: trace elements in dialysis fluids. Nephrology Dialysis Transplantation 11 (1996): 92-97.
- Kramer HJ, Gospodinov D, Krück F. Functional and metabolic studies on red blood cell sodium transport in chronic uremia. Nephron 16 (1976): 344-358.
- Thomson RM, Parry GJ. Neuropathies associated with excessive exposure to lead. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 33 (2006): 732-741.
