Lack of Benefit of Routine Serum Laboratory Control Samples during Treatment of Diabetic Foot Infection
Article Information
Pascal R. Furrer1, Madlaina Schöni1, Felix WA. Waibel1, Martin C. Berli1, Benjamin A. Lipsky4, Ilker Uçkay1, 2, 3
1Department of Orthopedic Surgery, Balgrist University Hospital, Forchstrasse 340, 8008 Zurich / Switzerland
2Infectiology, Balgrist University Hospital, Zurich / Switzerland
3Unit for Applied and Clinical Research, Balgrist University Hospital, University of Zurich, Switzerland
4Department of Medicine, University of Washington, Seattle, WA, USA
*Corresponding author: Pascal R. Furrer, Balgrist University Hospital, Forchstrasse 340, 8008 Zurich /Switzerland
Received: 15 February 2022; Accepted: 24 February 2022; Published: 02 March 2022
Citation: Pascal R. Furrer, Madlaina Schöni, Felix WA. Waibel, Martin C. Berli, Benjamin A. Lipsky, İlker Uçkay. Lack of Benefit of Routine Serum Laboratory Control Samples during Treatment of Diabetic Foot Infection. Archives of Microbiology and Immunology 6 (2022): 115-122
View / Download Pdf Share at FacebookAbstract
Purpose: Clinicians often routinely order measurements of serum inflammatory and other laboratory markers to assess the response to treatment of diabetic foot infections (DFI). Scientific evidence supporting this widespread practice is very limited and unclear. We sought to determine if the levels (or their dynamic change) of these markers predict important future outcomes, including recurrence of infection and development of foot ischemia
Methods: At Balgrist University Hospital we keep a registry of patients treated for diabetic foot diseases. This allowed us to compare, at study enrolment and at the end of treatment of a DFI, the patient’s blood levels of: C-reactive protein; leukocytes; thrombocytes; and, hemoglobin.
Results: Among 1,013 DFI episodes (patients median age 67 years, 78% male), 882 (87%) of which involved bone and the other 131 only soft tissues. Overall, we noted remission in 758 DFIs (75%), while combined surgical and medical therapy failed to control infection in the remaining 225. The incidence of infection recurrence with a microbiologically-identical strain was 5%. In crude comparisons, as well as by case-mix adjustment using a multivariate Cox regression analysis, none of the laboratory parameters was predictive of the likelihood of overall clinical failure or microbiological recurrence. The relative decrease in the level of each parameter was similar between DFI episodes with clinical remission or failure, with the exception of a moderate increase in the final thrombocyte counts among failing episodes.
Conclusions: This retrospective review of our results suggests that routine blood sampling of infection markers during DFI therapy is not beneficial for assessing key clinical outcomes.
Keywords
1. serum laboratory parameters 2. cohort study 3. diabetic foot infection 4. routine control sampling 5. outcomes
1. serum laboratory parameters 2. cohort study 3. diabetic foot infection 4. routine control sampling 5. outcomes
Article Details
1. Introduction
In a diabetic person presenting with a foot infection, obtaining a blood test to determine the leukocyte count is an important part of assessing the severity of the infection. Many clinicians also routinely order additional blood tests to measure inflammatory or other laboratory markers during the therapy of diabetic foot infections (DFI) with the belief that these results will help predict the likelihood of therapeutic success [1-4]. There is, however, only limited and unclear scientific evidence to support this widespread practice. As obtaining additional blood specimens after the initial assessments is associated with some patient discomfort, healthcare staff time and financial cost [5, 6], it is important to assess the value of such testing. These adverse effects can be further exaggerated if a surprising (and often spurious) result leads to additional blood or other diagnostic tests.
In a previous prospective study conducted in Geneva, we showed that monitoring the course of the serum C-reactive protein (CRP) does not predict treatment success in DFIs [7]. While CRP is perhaps the most frequently ordered serum inflammatory marker, it is not the only one routinely ordered by clinicians. To assess the potential value of these blood tests on predicting important clinical outcomes when treating DFIs, we conducted a retrospective follow-up study in a center in Zurich to compare eventual future outcomes (including recurrence of infection or development of foot ischemia) based on the value of blood tests for CRP, leukocyte count, thrombocytes and hemoglobin counts at enrolment and at the end of therapy.
2. Methods
The Balgrist University Hospital in Zurich, Switzerland, has kept a registry of patients treated for diabetic feet diseases since 2014. We record specified clinical and laboratory data on all surgically managed patients with DFI, including diabetic foot osteomyelitis (DFO), using the definitions and criteria of the International Working Group on the Diabetic Foot (IWGDF) [8]. For this study we limited our review of blood test laboratory markers to CRP levels, leukocyte counts, thrombocyte counts and hemoglobin levels at the initial assessment (enrolment date, +/- 2 days) and at the end of therapy (+/- 3 days). We selected these four markers as they are the most commonly ordered by clinicians and were available for the majority of treatment episodes. Our primary outcome of interest was “clinical failure” after DFI therapy, which we defined as any clinical problem requiring surgical revision, further antibiotic or other antimicrobial therapy or other infection-related intervention within twelve months for the same anatomical location. This could be a recurrent (specimen with same pathogen and antibiogram) or new (different pathogen/antibiogram) infection, new onset of clinically significant ischemia, wound dehiscence, development of a major hematoma, or other severe wound problems. We defined “microbiological recurrence” as a clinically infected wound from which a specimen for culture revealed at least 50% of the identical pathogens found at the site at presentation with the index infection. All blood samples were processed by the Zentral Labor Zurich (ZLZ; www.zlz.ch), and specimens for microbiological culture were processed at Institute of Medical Microbiology, University of Zurich; neither of which participated in the academic part of the study.
2.2 Statistical analyses
The primary outcome was "clinical failure," which we correlated with the four blood parameters under investigation. For crude group comparisons, we used the Pearson-χ2 or the Wilcoxon-ranksum-test, as appropriate. To adjust for the large case-mix, we performed a multivariate Cox regression analysis with the outcome “clinical failure” and excluded a significant effect modification by interaction terms. We analysed our data using STATA™ software (15.0; College Station, USA) assigning P-values <0.05 as statistically significant.
3. Results
3.1 Study population
We included 1,013 DFI episodes among 586 different adult patients seen as inpatients in our medical center from month, year to month, year. The median age of the patients was 67 years, and 78% were men. Overall, 882 DFIs (87%) involved bone and with the remaining 131 involving only the soft tissues. Among these patients we noted remission of infection noted in 758 (75%), while despite our combined surgical and medical therapy clinical failure was noted in 225 cases (25%), which occurred after a median delay of 355 days after the end of the initial treatment. A microbiologically-identical recurrence occurred in 47 cases, for an incidence of 5%. The median medical follow-up among these patients was 7.7 years.
In addition to surgical treatments (a median of 1 intervention/case) and systemic antibiotic therapy (for a median duration 21 days, of which a median of 4 days was administered intravenously), all of the patients received professional wound care, pressure offloading methods and casting and, if needed, lower extremity angioplasty or vascular bypass procedures. Culture results (by standard, not molecular methods) identified 99 different microbiological constellations; among these two pathogen groups dominated: Staphylococcus aureus (n=177; 17%), and coagulase-negative staphylococci (n=79; 8%). Polymicrobial infection (i.e., more than one isolate) was identified in samples submitted in 324 cases (32%). Table 1 summarizes key characteristics of patients in the study population. Table 2 compares clinical parameters in patients who had infection remission versus those who were clinical failures.
Table 1: Characteristics of 1,013 adult patients with a diabetic foot infection
|
n = 1,013 |
|
|
Male sex |
794 (78%) |
|
Median age (years) |
67 (range, 25-96 years) |
|
Osteomyelitis present (%) |
882(87%) |
|
Soft tissue infection only (%) |
131 (13%) |
|
Death (any cause) during the time of study (%) |
290 (29%) |
|
Clinical failures* |
255 (25%) |
|
Microbiological recurrences+ |
47 (5%) |
*Ischemia, new infection episodes, non-infection major complications
+ Infection recurrence with at least one pathogen among the intraoperative samples identical in this, versus compared to the index episode
Table 2: Clinical comparison between patients classified as infection remission vs. clinical failure
|
Remission |
Failure |
p-value * |
|
|
Male sex |
591 (78%) |
203 (80%) |
0.582 |
|
Median age |
68 years |
65 years |
0.089 |
|
Osteomyelitis |
669 (88%) |
213 (83%) |
0.052 |
|
Peripheral arterial disease |
559 (74%) |
205 (80) |
0.033 |
|
- Underwent bypass or angioplasty |
408 (54%) |
164 (64) |
0.003 |
* Pearson-χ² or Wilcoxon-ranksum-tests. Significant results are displayed in bold and italics
3.2 Associations with blood samples values
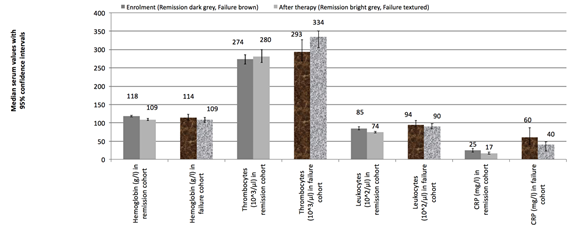
Figure 1 shows the median values for all blood tests of interest, stratifying each of the four single laboratory parameters between values in patients who had remission of infection and those who were clinical failures, as well as between values obtain at “enrollment” and “a at the end of the initial treatment”. As expected, most values were higher at enrolment than after treatment for the DFI. At most, few values fully normalized at the end of the treatment: 23% of all hemoglobulin levels, 70% of the thrombocyte counts, 81% of the leukocytes, and 15% of the serum CRP levels. In absolute proportions, values for leukocytes and thrombocytes were the ones that most commonly returned to normal levels. Relatively speaking, the median hemoglobulin values dropped by 5% in the time lag between enrolment and end of treatment. This change was +1% for thrombocytes, -12% for leukocytes, and -34% for CRP. The relative changes in absolute values from enrolment to end of treatment did not discriminate between cases with infection remission and those with clinical failure. Only the final value of thrombocytes was more elevated among the failures than remissions. Neither the relative drop of the initial leukocyte count, nor that of the CRP showed a significant difference between cases with infection remission and clinical failures (Figure 1).
3.3 Multivariate adjustment
In the multivariate results targeting the outcome clinical failure (Table 3), the only factor that paralleled the number of failures (confounding by indication) was the number of surgical interventions. None of the other variables were statistically significant. Thus, the result of "enrolment" and “after treatment” values were not associated with the risk of future failure. The relative drop of the each of the four serum parameters was not statistically significant with one exception: the relatively high increase of thrombocytes at the end of treatment was associated with clinical failure (odds ratio 2.8, 95% confidence interval 1.5-5.1; Table 3). Of note, the receiver-operating-curve (ROC) value of our final model was 0.74 (95% confidence interval 0.7-0.89), highlighting an acceptable accuracy of our multivariate model.
Table 3: Factors associated with “clinical failure" by univariate and multivariate Cox regression analyses
|
Clinical failures |
Univariate |
Multivariate |
|
Binomial or continuous variables |
Hazard Ratio, 95% CI |
Hazard Ratio, 95% CI |
|
Age |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
Osteomyelitis |
1.1, 0.8-1.5 |
1.4, 0.7-2.6 |
|
Symptomatic peripheral arterial disease |
1.1, 0.8-1.5 |
0.9, 0.6-1.6 |
|
Hemoglobin at enrolment |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- Hemoglobin after therapy |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- Relative drop of hemoglobulin |
0.5, 0.6-4.4 |
- |
|
Thrombocyte count preoperative |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
Thrombocyte count at enrolment |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- Thrombocytes after therapy |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- Relative increase of thrombocytes |
2.8, 1.5-5.1 |
- |
|
Leukocyte count at enrolment |
1.0, 1.0-1.1 |
1.0, 0.9-1.0 |
|
- Leukocyte count after therapy |
1.1, 1.1-1.2 |
1.1, 0.9-1.2 |
|
- Relative drop of leukocytes |
1.0, 0.7-1.3 |
0.8, 0.5-1.3 |
|
C-reactive protein at enrolment |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- C-reactive protein after therapy |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- Relative drop of C-reactive protein |
1.0, 0.9-1.0 |
1.0, 1.0-1.0 |
|
Peripheral angioplasty or surgical bypass |
1.2, 0.9-1.5 |
0.9, 0.5-1.5 |
|
Number of surgical interventions |
1.6, 1.4-1.9 |
2.3, 1.6-3.2 |
|
Duration of antibiotic therapy |
1.0, 1.0-1.0 |
1.0, 1.0-1.0 |
|
- Administered intravenous therapy |
1.0, 1.0-1.0 |
- |
* Statistically significant results are displayed in bold and italic, “ - “ = not done / included in the final model
4. Discussion
In our single-center, retrospective cohort study, we found that none of the frequently sampled blood parameters (hemoglobulin, thrombocytes, CRP and leukocytes) was significantly associated with (future) clinical failure (or microbiological recurrences) after the combined surgical and antibiotic therapy for DFI, including for DFO. These serum parameters certainly appear to be helpful in the diagnosis and primary evaluation of patients suspected of having a DFI. They could also potentially help in recognizing adverse events or new clinical problems occurring during ongoing treatment of DFI. However, as with the few other published studies of this issue, we found no evidence of their additional value solely for the monitoring of the effectiveness of an established treatment regimen [7, 9, 10]. We thus confirm previous studies in the field of DFI that found the iterative sampling of CRP or leukocyte measurements lacked predictive power for clinical outcomes [7]. Blood samples and inflammatory markers are influenced by many different pathologies, not just infection itself.9 Thus, adverse consequences of ordering these unhelpful tests is not only the unnecessary patient discomfort, staff time and financial costs, but also the potential for false (premature) assurance. Usually, these adverse events are detected clinically. We think the widespread use of these laboratory parameters relies on believing in an apparently logical approach, along accepting traditional teaching of clinicians during their medical education. Clinicians in resource-rich settings like to support their clinical impressions with the results of laboratory tests. However, in our current era of increasing antibiotic resistance which necessitates antimicrobial stewardship, we think this practice should be abandoned for routine surveillance during treatment of clinically stable patients with localized DFI.
The strengths of our study are that it is based on a study of over 1000 DFI episodes, for which there was a long median follow-up of up of eight years. Furthermore, all data were collected in a specialized, academic diabetic foot unit where treatment was conducted by evidenced-based guidelines. The main limitation is that this study was retrospective, based on registry data. However, this is our second study confirming this finding, the first of which was based on a prospectively collected database of two randomized-controlled trials [7]. In both study centers, Geneva and Zurich, we found no benefit for following serial inflammatory markers in DFI episodes, making a hospital bias unlikely.
In conclusion, when treating DFI patients, there appears to be little if any benefit to ordering repeated serum inflammatory markers to follow the course of treatment. We suggest abandoning this widespread practice, especially in clinically stable patients who appear to be responding to treatment.
Statements and Declarations
Acknowledgments
We thank all the teams of the Balgrist Hospital and of the Zentral Labor Zurich who assisted us in the care of our DFI patients. We are indebted to the UCAR (Unit for Clinical and Applied Research) study nurses: Kati Sairanen, Sabrina Catanzaro and Nathalie Kühne.
Potential conflict of interests
None of the authors have any financial or other conflicts of interest with this work.
Funding
There was no funding for this work.
Ethical issues
This research is one of a series of retrospective DFI investigations (DF-MANAG studies) we have designed that are aimed at streamlining DFI therapies, is in line with the principles of the Declaration of Helsinki and were approved by the Ethical Committee of Zurich (BASEC 2019-01994).
References
- Aragón-Sánchez J, Lipsky BA. Modern management of diabetic foot osteomyelitis. The when, how and why of conservative approaches. Expert Rev Anti Infect Ther 16 (2018): 35-50.
- Lázaro Martínez JL, García Álvarez Y, Tardáguila-García A, García Morales E. Optimal management of diabetic foot osteomyelitis: challenges and solutions. Diabetes Metab Syndr Obes 12 (2019): 947-59.
- Uçkay I, Gariani K, Pataky Z, Lipsky BA. Diabetic foot infections: state-of-the-art. Diabetes Obes Metab 16 (2014): 305-16.
- Lipsky BA, Uçkay ?. Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina (Kaunas) 57 (2021).
- Van Asten SA, Nichols A, La Fontaine J, Bhavan K, Peters EJ, Lavery LA. The value of inflammatory markers to diagnose and monitor diabetic foot osteomyelitis. Int Wound J 14 (2017): 40-5.
- Michail M, Jude E, Liaskos C, Karamagiolis S, Makrilakis K, Dimitroulis D, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds 12 (2013): 94-9.
- Pham TT, Wetzel O, Gariani K, Kressmann B, Jornayvaz FR, Lipsky BA, et al. Is routine measurement of the serum C-reactive protein level helpful during antibiotic therapy for diabetic foot infection? Diabetes Obes Metab 23 (2021): 637-41.
- Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 36 (2020): e3280.
- Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol 9 (2018): 754.
- Gans SL, Atema JJ, Stoker J, Toorenvliet BR, Laurell H, Boermeester MA. C-reactive protein and white blood cell count as triage test between urgent and nonurgent conditions in 2961 patients with acute abdominal pain. Medicine (Baltimore) 94 (2015): e569.