Laboratory Parameters Suggesting a Potential Diagnosis of COVID-19
Article Information
Naivy SAnchez1, Iliana Rodríguez1, Patricia Lorenzo-Luaces2, Carmen Valenzuela2, Lizet Sanchez2, Tania Crombet2, Zaima Mazorra2, Carlos Hidalgo1, Danay Saavedra2*
1Manuel Fajardo Universitary Hospital, Santa Clara, Villa Clara, Cuba
2Center of Molecular Immunology, Havana, Cuba
*Corresponding Author: Danay Saavedra, Center of Molecular Immunology, Havana, Cuba
Received: 10 December 2020; Accepted: 23 December 2020; Published: 20 May 2022
Citation: Naivy SAnchez, Iliana Rodríguez, Patricia Lorenzo-Luaces, Carmen Valenzuela, Lizet Sanchez, Tania Crombet, Zaima Mazorra, Carlos Hidalgo, Danay Saavedra. Laboratory Parameters Suggesting A Potential Diagnosis of COVID-19. Archives of Clinical and Medical Case Reports 6 (2022): 428-436.
View / Download Pdf Share at FacebookAbstract
COVID-19 presents with a broad clinical spectrum. In symptomatic patients, the clinical manifestations could be similar with those observed in other respiratory tract infections. Hematological and biochemical parameters are different between patients with COVID-19 disease and non- COVID-19 patients. To recognize which laboratory parameter could be useful for identifying COVID-19 patients, we applied receiver operator characteristic curves, logistic regression and represented a nomogram. We found that the number of white blood cells (WBC), lymphocytes and neutrophils were significantly lower in COVID-19 than in non-COVID-19 patients, whereas platelets to lymphocyte ratio, Gamma-Glutamyl Transferase (GGT) and creatinine were higher in COVID-19 patients. Based on receiver operating characteristic curves and logistic regression were selected lymphocytes <1.95 x109/L, GGT>46.5 U/L and WBC <6.6 x109/L for predicting the presence of COVID- 19. The use of the nomogram as a practical application can predict the presence of COVID-19 among the symptomatic patients.
Keywords
Biomarkers; COVID-19; Diagnosis Laboratory; parameters
Biomarkers articles; COVID-19 articles; Diagnosis Laboratory articles; parameters articles
Biomarkers articles Biomarkers Research articles Biomarkers review articles Biomarkers PubMed articles Biomarkers PubMed Central articles Biomarkers 2023 articles Biomarkers 2024 articles Biomarkers Scopus articles Biomarkers impact factor journals Biomarkers Scopus journals Biomarkers PubMed journals Biomarkers medical journals Biomarkers free journals Biomarkers best journals Biomarkers top journals Biomarkers free medical journals Biomarkers famous journals Biomarkers Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Diagnosis Laboratory articles Diagnosis Laboratory Research articles Diagnosis Laboratory review articles Diagnosis Laboratory PubMed articles Diagnosis Laboratory PubMed Central articles Diagnosis Laboratory 2023 articles Diagnosis Laboratory 2024 articles Diagnosis Laboratory Scopus articles Diagnosis Laboratory impact factor journals Diagnosis Laboratory Scopus journals Diagnosis Laboratory PubMed journals Diagnosis Laboratory medical journals Diagnosis Laboratory free journals Diagnosis Laboratory best journals Diagnosis Laboratory top journals Diagnosis Laboratory free medical journals Diagnosis Laboratory famous journals Diagnosis Laboratory Google Scholar indexed journals polyangiitis articles polyangiitis Research articles polyangiitis review articles polyangiitis PubMed articles polyangiitis PubMed Central articles polyangiitis 2023 articles polyangiitis 2024 articles polyangiitis Scopus articles polyangiitis impact factor journals polyangiitis Scopus journals polyangiitis PubMed journals polyangiitis medical journals polyangiitis free journals polyangiitis best journals polyangiitis top journals polyangiitis free medical journals polyangiitis famous journals polyangiitis Google Scholar indexed journals parameters articles parameters Research articles parameters review articles parameters PubMed articles parameters PubMed Central articles parameters 2023 articles parameters 2024 articles parameters Scopus articles parameters impact factor journals parameters Scopus journals parameters PubMed journals parameters medical journals parameters free journals parameters best journals parameters top journals parameters free medical journals parameters famous journals parameters Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Xerostomia articles Xerostomia Research articles Xerostomia review articles Xerostomia PubMed articles Xerostomia PubMed Central articles Xerostomia 2023 articles Xerostomia 2024 articles Xerostomia Scopus articles Xerostomia impact factor journals Xerostomia Scopus journals Xerostomia PubMed journals Xerostomia medical journals Xerostomia free journals Xerostomia best journals Xerostomia top journals Xerostomia free medical journals Xerostomia famous journals Xerostomia Google Scholar indexed journals Smirnov test articles Smirnov test Research articles Smirnov test review articles Smirnov test PubMed articles Smirnov test PubMed Central articles Smirnov test 2023 articles Smirnov test 2024 articles Smirnov test Scopus articles Smirnov test impact factor journals Smirnov test Scopus journals Smirnov test PubMed journals Smirnov test medical journals Smirnov test free journals Smirnov test best journals Smirnov test top journals Smirnov test free medical journals Smirnov test famous journals Smirnov test Google Scholar indexed journals
Article Details
1. Introduction
World Health Organization (WHO) declared the COVID-19 epidemic as a public health emergency of international concern on January 30, 2020 and as a pandemic on March 11, 2020 [1]. In Cuba, the first case was reported on March 11 [2]. COVID-19 presents with a broad clinical spectrum and also with a large number of asymptomatics [3]. The majority of patients manifests a mild or subclinical disease, especially in the early phase [4]. In symptomatic patients, the clinical manifestations of the disease consisting of fever, cough, nasal congestion, fatigue, sore throat, among others that could be similar with other Respiratory Tract Infections (RTI). Therefore, it may be challenging for healthcare workers in the primary care or emergency room setting to determine individuals who are more likely to have COVID-19, and testing is needed to confirm the diagnosis [5]. Currently, patient’s identification depends on the performance of the real time reverse transcriptase-polymerase chain reaction (RT-PCR) testing, which may not be available if criteria for testing are overly expansive or in resource-limited settings [6]. Previous works showed that hematological and biochemical parameters are clearly different between patients with COVID-19 infection and non-COVID-19 patients [7-9]. Abnormal liver function tests are frequently reported in COVID-19 patients [10], as well as decreased leukocyte, lymphocyte and neutrophil counts [11]. Consequently, blood tests could play an important role in the identification and early diagnosis of patients this disease. Here we show demographic, clinical and hemochemical laboratory characteristics of COVID-19 and non-COVID-19 patients with mild-moderate symptoms admitted in Manuel Fajardo Hospital, one of the Cuban institutions devoted to the attention of COVID-19 patients. In this study, we aimed to recognize which laboratory parameters could be useful for allowing the identification of COVID-19 patients.
2. Materials and Methods
2.1. Patients
In this retrospective study, patients diagnosed with mild-moderate Respiratory Tract Infections (RTIs) from October 2019, to May 2020, and patients diagnosed with mild-moderate COVID-19 from March 2020 to May 2020, were enrolled. All of them were admitted in Manuel Fajardo Hospital in Santa Clara, Villa Clara, Cuba. The ethics committee of the institution approved the study.
2.2. Samples collection and processing
Blood samples were collected the same day of the hospital admission. All biochemical parameters were measured on a Chemistry Analyzer Spin 200E (SPINREACT, Girona, Spain). Hematological parameters and leukocyte formula were measured on a hematology analyzer Spincell 3 (SPINREACT, Girona, Spain). Nasopharyngeal swab samples were collected for COVID-19 test on admission.
2.3. Data Collection and Statistical Analysis
Clinical symptoms, comorbidities and laboratory data were collected from patients’ medical records. Kolmogorov-Smirnov test was used to assess the normality of variables. For the comparison between groups, Likelihood ratio or Fisher exact test were applied for qualitative variables and U Mann Whitney for quantitative ones. Cutpoints for laboratory parameters (for classifying patients with COVID-19) were found using Receiver operating characteristic curves (ROC). In order to select the more relevant parameters for classifying COVID-19 patients, a logistic regression was used. As an easier and practical application, the selected logistic model was represented as a nomogram. All statistical analyses were performed using SPSS v. 21 and R 3.5 softwares. The statistical data were considered significant if p<0.05.
3. Results
3.1. Demographic and clinical characteristics of the patients
One hundred 75 patients with symptoms of mild-moderate respiratory tract infections were evaluated from October 2019 to May 2020 in the Manuel Fajardo Hospital in Santa Clara, Villa Clara, Cuba. From March 2020, all the patients with this kind of symptomatology were tested for SARS-CoV-2. Among them, 79 were diagnosed with COVID-19. The group of positive patients was composed of 47 females and 32 males, whereas the non-COVID-19 group (n=96) was composed of 46 females and 50 males. Both groups were similar regarding sex (p=0.126 Likelihood ratio, Table 1) and age distribution (p=0.153 Mann Whitney test, Table 1). At admission, both COVID-19 and non-COVID-19 patients presented with cough, fever, rhinorrhea, sore throat, dyspnea, headache and expectoration. However, COVID-19 group had lower frequency of patients with expectoration than non-COVID-19 group did (p=0.01 Likelihood ratio, Table 1). We found hypertension, diabetes and cardiovascular disease among the most frequent comorbidities in both groups. COVID-19 patients had less chronic obstructive pulmonary disease (COPD) than non-COVID-19 patients (p=0.01 Likelihood ratio, Table 1).
|
COVID-19 |
Non-COVID-19 |
P-value |
|
|
patients |
patients |
||
|
Age (years, mean) |
51.71 |
47.02 |
0.153 |
|
(min-max) |
(18-100) |
(18-90) |
|
|
Sex |
|||
|
Female |
47 (59.49%) |
46 (47.91%) |
0.126 |
|
Male |
32 (40.51%) |
50 (52.09%) |
|
|
Signs and symptoms at admission |
|||
|
Cough |
46 (58.2%) |
55 (57.3%) |
0.9 |
|
Fever |
26 (32.9%) |
42 (43.8%) |
0.14 |
|
Rhinorrhea |
21 (26.6%) |
26 (27.1%) |
0.94 |
|
Sore throat |
20 (25.3%) |
21 (21.9%) |
0.59 |
|
Dyspnea |
17 (21.5%) |
23 (24.0%) |
0.7 |
|
Headache |
16 (20.3%) |
15 (15.6%) |
0.43 |
|
Expectoration |
11 (13.9%) |
29 (30.2%) |
0.01 |
|
Comorbidities |
|||
|
Hypertension |
28 (35.4%) |
39 (40.6%) |
0.48 |
|
Diabetes |
7 (8.9%) |
4 (4.2%) |
0.23f |
|
Cardiovascular disease |
6 (7.6%) |
7 (7.3%) |
0.94 |
|
COPD |
1 (1.3%) |
10 (10.4%) |
0.01 |
|
P-value indicates differences between COVID-19 and non-COVID-19 patients, p<0.05 was considered statistically significant (highlighted in bold), Likelihood ratio or Fisher exact test f; COPD: chronic obstructive pulmonary disease. |
|||
Table 1: Demographic and clinical characteristics of the study population.
3.2. Laboratory parameters
Comparison of the hematological parameters of COVID-19 and non-COVID-19 patients showed significant differences in the white blood counts (WBC) (p=0.000 Mann Whitney test, Table 2), lymphocyte (p=0.000 Mann Whitney test, Table 2) and neutrophil count (p=0.002 Mann Whitney test, Table 2). All the values were lower in the COVID-19 group than in non-COVID-19 patients. The platelet to lymphocyte ratio were also different between both groups (PLR, p=0.001 Mann Whitney test, Table 2). In this case, the higher values corresponded to COVID-19 patients. Regarding the evaluation of the biochemical parameters, patients with COVID-19 had significantly higher gamma-glutamyl transferase (GGT, p=0.045 Mann Whitney test, Table 2), cholesterol (p=0.019 Mann Whitney test, Table 2) and creatinine (p=0.003 Mann Whitney test, Table 2) levels in serum.
|
Parameters |
COVID-19 |
Non-COVID-19 |
p value |
|
patients |
patients |
Mann Whitney |
|
|
(n=79) |
(n=96) |
||
|
Hemoglobin (g/l) |
127.0 ± 21.1 |
131.0 ± 32.0 |
0.09 |
|
WBC (×109/L) |
6.2 ± 2.8 |
8.0 ± 4.2 |
0 |
|
Neutrophils (×109/L) |
3.8 ± 2.3 |
4.9 ± 3.7 |
0.002 |
|
Lymphocytes (×109/L) |
1.7 ± 1.1 |
2.3 ± 1.1 |
0 |
|
Platelets (×109/L) |
188.0 ± 72.0 |
199.5 ± 72.5 |
0.083 |
|
NLR |
2.2 ± 1.8 |
2.1 ± 1.8 |
0.761 |
|
PLR |
114.2 ± 92.2 |
90.8 ± 58.7 |
0.001 |
|
Cholesterol (mmol/L) |
4.3 ± 1.7 |
3.5 ± 1.3 |
0.019 |
|
ALT (U/L) |
27.0 ± 17.2 |
26.0 ± 17.0 |
0.889 |
|
AST (U/L) |
22.0 ± 22.0 |
40.0 ± 26.8 |
0.724 |
|
GGT (U/L) |
58.0 ± 24.0 |
22.0 ± 9.5 |
0.045 |
|
Creatinine (μmol/L) |
103.0 ± 28,2 |
84.0 ± 22,5 |
0.003 |
|
ALP (U/L) |
182.0 ± 58,2 |
193.0 ± 103.0 |
0.956 |
|
LDH (U/L) |
346.0 ± 142,5 |
302.0 ± 122.0 |
0.248 |
|
P-value indicates differences between COVID-19 and non-COVID-19 patients, p<0.05 was considered statistically significant (highlighted in bold), Mann Whitney test; WBC: white blood cells, NLR: neutrophils to lymphocyte ratio; PLR: platelets to lymphocyte ratio; ALT alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; ALP: alkaline phosphatase; LDH: lactate dehydrogenase. |
|||
Table 2: Laboratory parameters in COVID-19 and non-COVID-19 patients.
3.3. Predictive parameters for COVID-19
ROC curves of studied parameters were employed for classifying patients with COVID-19. WBC lower than 6.6 x109/L, neutrophils count lower than 4.35 x109/L, lymphocytes number lower than 1.95x 109/L and PLR higher than 95.8, serum creatinine higher than 91.5 μmol/L and GGT higher than 46.5 U/L could be cut offs for selecting patients with COVID-19 among the patients with mild-moderate symptoms (Table 3). For other biomarkers, the probability of correct classification or prediction was not high. With ROC curve selected variables, it was adjusted a binary logistic regression to determine the correct parameters for predicting the presence of COVID-19. The logistic regression model including WBC, lymphocytes and GGT reached a good classification, 90.9% of COVID-19 patients were correctly classified (Table 3). Therefore, the patients with mild-moderate cough, fever, rhinorrhea, sore throat, dyspnea or headache, with lymphocytes <1.95 x109/L, GGT>46.5 U/L and WBC <6.6 x109/L had a very high probability of being diagnosed with COVID-19 (Table 3).
|
ROC curves |
||||||||
|
Parameter |
Cut-off |
AUC |
Sig. |
95% CI |
Sensitivity |
Specificity |
||
|
WBC(×109/L) |
< 6.6* |
0.669 |
0 |
0.589 |
0.75 |
0.595 |
0.705 |
|
|
Neutrophils(×109/L) |
< 4.35* |
0.638 |
0.002 |
0.556 |
0.721 |
0.582 |
0.653 |
|
|
Lymphocytes(×109/L) |
< 1.95* |
0.693 |
0 |
0.614 |
0.773 |
0.62 |
0.653 |
|
|
PLR |
≥ 95.8 |
0.651 |
0.001 |
0.567 |
0.735 |
0.711 |
0.533 |
|
|
Creatinine |
≥ 91.5 |
0.696 |
0.003 |
0.581 |
0.811 |
0.725 |
0.606 |
|
|
GGT |
≥ 46.5 |
0.737 |
0.045 |
0.538 |
0.937 |
0.818 |
0.687 |
|
|
Logistic regression |
||||||||
|
Parameter |
Coeff |
Sig. |
OR (exp Coeff) |
95% C.I. (OR) |
||||
|
LL |
UL |
|||||||
|
Lymphocytes <1.95 |
3.257 |
0.045 |
25.97 |
1,079 |
625,116 |
|||
|
GGT > 46.5 |
3.987 |
0.014 |
53.876 |
2,237 |
1,297,681 |
|||
|
WBC < 6.6 |
3.493 |
0.022 |
32.872 |
1,652 |
654,271 |
|||
|
Constant |
-5.696 |
0.011 |
0.003 |
|||||
|
ROC: receiver operating characteristic; AUC: Area under curve; Sig: significance, CI: confidence interval; Coeff: coefficient; OR: odds ratio; WBC: white blood cell; PLR: platelets to lymphocyte ratio, GGT: gamma-glutamyl transferase. *The reference category was non-COVID-19 patients |
||||||||
Table 3: Predictive values of laboratory parameters for COVID-19 diagnosis according the ROC curves and the binary logistic regression.
Based on the logistic regression model, it was built a nomogram (Figure 1) to classify the patients with higher probability of being diagnosed with COVID-19. To determine a cut point in the total points line, it was adjusted a ROC curve. The Area Under curve (AUC) was 0.91, the sensitivity 100%, the specificity 66.6% and the cut point in the total point’s line was 84. In the present series, the totality of patients with COVID-19 received more than 84 points. Consequently, the probability of correct classification was high.
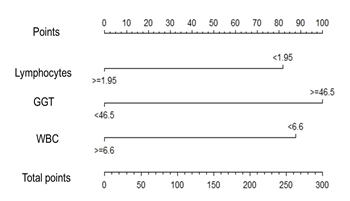
Figure 1: Nomogram to estimate the probability of COVID-19 diagnosis in patients with mild-moderate respiratory symptoms. GGT: gamma-glutamyl transferase; WBC: white blood cell.
4. Discussion
COVID-19 is an extremely contagious disease that represents a serious threat to public health [6]. Despite the world efforts in controlling the virus, there is still a dangerous spreading of the SARS-CoV-2 and more than 39 million patients have confirmed globally. In order to warrant optimal patient care and to optimize medical resources, it is essential to recognize the disease from other RTI. Blood test analysis might be very useful for identifying possible COVID-19 patients [6, 12]. Based on the findings of this study WBC, lymphocytes and serum GGT have very good accuracy in predicting cases with COVID-19. Patients of the two studied groups (COVID-19 and non-COVID-19) had no significant difference in age, sex, symptoms and chronic disease status, except expectoration and COPD, more frequent in non-COVID-19 patients. In contrast, a study performed by Zhou and colleagues reported higher frequency of patients with expectoration and COPD in patients with SARS-CoV-2-negative community-acquired pneumonia [13]. We found that the number of WBC, lymphocytes and neutrophils were significantly lower in COVID-19 than in non-COVID-19 patients, whereas PLR, GGT and creatinine were higher in COVID-19 patients. Previous studies described lower lymphocyte and WBC counts in COVID-19 patients, which is in line with our work [8,14]. The number of circulating neutrophils progressively increase in COVID-19 patients, principally in patients with symptoms of severe disease [15]. Interestingly, in our series, non-COVID-19 patients showed higher neutrophils count than COVID-19, suggesting that neutrophils might not be affected in the initial phase of COVID-19 disease.
On the other hand, it has been reported that a high PLR may indicate a pro-inflammatory cytokine release due to enhanced platelet activation [14]. Cytokine release syndrome (CRS) was found to be a major cause of morbidity in patients with severe COVID-19. This manifestation is characterized by a pro-inflammatory response with increase of inflammatory cytokines such as IL-6, IL-1, IFNγ and TNFα [16,17]. It has also been indicated that patients with COVID-19 had liver comorbidities and abnormal liver enzymes levels during COVID-19 disease. However, liver damage in mild cases of COVID-19 is often transient and can return to normal without any special treatment [18]. In the present study, ROC curves were adjusted to analyze the specificity and sensitivity of different laboratory parameters in COVID-19 and non-COVID-19 patients. The AUC of WBC, neutrophils, lymphocytes, PLR and serum creatinine and GGT indicated that they could be used to predict the presence of COVID-19 disease. Moreover, a binary logistic regression was adjusted to determine the most relevant variables for predicting the presence of COVID-19 in our series. The model selected, included lymphocytes <1.95 x109/L, GGT>46.5 U/L and WBC <6.6 x109/L. Based on the logistic regression model, it was designed a nomogram to support the identification of patients with higher probability of being affected with COVID-19 disease. Previous works have proposed different strategies for the identification of COVID-19 patients prior to the performance of RT-PCR, such us the application of empirical thresholds of biochemical parameters like LDH and AST [6] or using ROC curve to select laboratory parameters [12]. In our study, applying the logistic regression derived nomogram to our series, all COVID-19 patients were correctly identified, suggesting that the use of the nomogram could be an effective tool for the identification of COVID-19 patients with high accuracy. Due to the high infectivity of SARS-CoV-2, the early detection and diagnosis of COVID-19 patients results very relevant in order to control the transmission and the effects of the disease. The definitive diagnosis is made by demonstrating a viral presence through the performance of RT-PCR. Some frequent situations such us high number of samples or insufficient lab capacities could enlarge the time it takes to have a diagnosis. Hence, the early and simple identification of symptomatic patients, potentially affected by this illness, based on laboratory parameters is greatly recommended. This study has some limitations. First, it has a limited number of patients. Second, it has been performed in only one hospital. Multi-center studies with more patients are needed for further evaluation.
- Conclusions
Our results showed that patients with mild-moderate COVID-19 had lower leukocyte, lymphocyte and neutrophil counts, whereas higher PLR, serum GGT and creatinine than non-COVID-19 patients. Based on the results of total points in the linear regression-based nomogram we can predict the presence of the disease by using the routine laboratory tests leukocytes count, lymphocytes count and serum GGT.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, NS, CH, Z.M, T.C. and D.S.; methodology, NS and DS.; formal analysis, NS, DS, P.L_L., C.V., L.S.; investigation, N.S., I.R.; data curation, N.S, D.S, P.L-L.; writing—original draft preparation, N.S, D.S.; writing—review and editing, N.S, Z.M, T.C, C.H. and D.S; visualization, N.S, D.S.; project administration, N.S., D.S., All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are extremely thankful to our patients and their relatives and to the research teams of Manuel Fajardo Hospital and the Center of Molecular Immunology.
Conflicts of Interest
The authors declare no conflict of interest
References
- Guo T, Shen Q, Guo W, et al. Clinical Characteristics of Elderly Patients with COVID-19 in Hunan Province, China: A Multicenter, Retrospective Study. Gerontology 66 (2020):467-475.
- Diaz Y, Ramos-Suzarte M, Martin Y, et al. Use of a Humanized Anti-CD6 Monoclonal Antibody (Itolizumab) in Elderly Patients with Moderate COVID-19. Gerontology (2020): 1-9.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395 (2020):507-513.
- Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol 112 (2020):553-559.
- Sun Y, Koh V, Marimuthu K, et al. Epidemiological and Clinical Predictors of COVID-19. Clin Infect Dis 71 (2020): 786-792.
- Ferrari D, Motta A, Strollo M, et al. Routine blood tests as a potential diagnostic tool for COVID-19. Clin Chem Lab Med 58 (2020):1095-1099.
- Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID-19 infection. Am J Hematol 95 (2020): 131-134.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 58 (2020): 1021-1028.
- Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 58 (2020):1131-1134.
- Bertolini A, van de Peppel IP, Bodewes F, et al. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology (2020).
- Usul E, San I, Bekgoz B, et al. Role of hematological parameters in COVID-19 patients in the emergency room. Biomark Med 14 (2020):1207-1205.
- Mardani R, Ahmadi Vasmehjani A, Zali F, et al. Laboratory Parameters in Detection of COVID-19 Patients with Positive RT-PCR; a Diagnostic Accuracy Study. Arch Acad Emerg Med 8 (2020): 43.
- Zhou Y, Guo S, He Y, et al. COVID-19 Is Distinct From SARS-CoV-2-Negative Community-Acquired Pneumonia. Front Cell Infect Microbiol 10 (2020): 322.
- Qu R, Ling Y, Zhang YH, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol (2020).
- Wang J, Jiang M, Chen X, et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 108 (2020):17-41.
- Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 34 (2020).
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis (2020).
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 5 (2020): 428-430.
