Intramuscular Racemic Ketamine Antidepressant Response and Reduced Length of Admission in Psychiatric Inpatients with Treatment Resistant Depression and Borderline Personality
Article Information
Nathan Carter1*
1Novant Health Presbyterian Medical Center - Charlotte, NC
*Corresponding Author: Nathan Carter, Novant Health Presbyterian Medical Center - Charlotte, NC
Received: 03 July 2023; Accepted: 11 July 2023; Published: 21 July 2023
Citation: Nathan Carter. Intramuscular Racemic Ketamine Antidepressant Response and Reduced Length of Admission in Psychiatric Inpatients with Treatment Resistant Depression and Borderline Personality. Journal of Psychiatry and Psychiatric Disorders. 7 (2023): 89-97.
View / Download Pdf Share at FacebookAbstract
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, the largest study of MDD conducted in the United States, showed that even with enriched resources devoted to treatment, recovery with the first selected SSRI occurred only about 30% of the time. In fact, after initiating antidepressants, the time for an expected response is longer than the typical inpatient psychiatric unit length of stay. Ketamine constitutes a novel, rapid and efficacious treatment choice for patients suffering from moderate to severe treatment resistant depression and exhibits rapid and significant antidepressant and anti-suicidal effects. Intramuscular racemic ketamine, in subanesthetic doses, is an effective, safe, off-label treatment for severe/moderate treatment refractory depression and rapid stabilization of suicidal behaviors/ideations, in under 36 hours, post injection. No consultations warranted from other medical specialties. Larger, heterogeneous studies are needed, which provide historical data of continued response/remission following ketamine, whether from intramuscular or intravenous administration. Comparative studies are needed comparing efficacy between racemic, R-ketamine, and S-ketamine.
Keywords
Intramuscular racemic ketamine; BDNF (Brain-derived neurotrophic factor); Glutamate; GABA (gamma-aminobutyric acid); NMDA (N-Methyl D-Aspartate) Antagonist
Intramuscular racemic ketamine articles; BDNF (Brain-derived neurotrophic factor) articles; Glutamate articles; GABA (gamma-aminobutyric acid) articles; NMDA (N-Methyl D-Aspartate) Antagonist articles
Intramuscular racemic ketamine articles Intramuscular racemic ketamine Research articles Intramuscular racemic ketamine review articles Intramuscular racemic ketamine PubMed articles Intramuscular racemic ketamine PubMed Central articles Intramuscular racemic ketamine 2023 articles Intramuscular racemic ketamine 2024 articles Intramuscular racemic ketamine Scopus articles Intramuscular racemic ketamine impact factor journals Intramuscular racemic ketamine Scopus journals Intramuscular racemic ketamine PubMed journals Intramuscular racemic ketamine medical journals Intramuscular racemic ketamine free journals Intramuscular racemic ketamine best journals Intramuscular racemic ketamine top journals Intramuscular racemic ketamine free medical journals Intramuscular racemic ketamine famous journals Intramuscular racemic ketamine Google Scholar indexed journals Brain-derived neurotrophic factor articles Brain-derived neurotrophic factor Research articles Brain-derived neurotrophic factor review articles Brain-derived neurotrophic factor PubMed articles Brain-derived neurotrophic factor PubMed Central articles Brain-derived neurotrophic factor 2023 articles Brain-derived neurotrophic factor 2024 articles Brain-derived neurotrophic factor Scopus articles Brain-derived neurotrophic factor impact factor journals Brain-derived neurotrophic factor Scopus journals Brain-derived neurotrophic factor PubMed journals Brain-derived neurotrophic factor medical journals Brain-derived neurotrophic factor free journals Brain-derived neurotrophic factor best journals Brain-derived neurotrophic factor top journals Brain-derived neurotrophic factor free medical journals Brain-derived neurotrophic factor famous journals Brain-derived neurotrophic factor Google Scholar indexed journals Glutamate articles Glutamate Research articles Glutamate review articles Glutamate PubMed articles Glutamate PubMed Central articles Glutamate 2023 articles Glutamate 2024 articles Glutamate Scopus articles Glutamate impact factor journals Glutamate Scopus journals Glutamate PubMed journals Glutamate medical journals Glutamate free journals Glutamate best journals Glutamate top journals Glutamate free medical journals Glutamate famous journals Glutamate Google Scholar indexed journals gamma-aminobutyric acid articles gamma-aminobutyric acid Research articles gamma-aminobutyric acid review articles gamma-aminobutyric acid PubMed articles gamma-aminobutyric acid PubMed Central articles gamma-aminobutyric acid 2023 articles gamma-aminobutyric acid 2024 articles gamma-aminobutyric acid Scopus articles gamma-aminobutyric acid impact factor journals gamma-aminobutyric acid Scopus journals gamma-aminobutyric acid PubMed journals gamma-aminobutyric acid medical journals gamma-aminobutyric acid free journals gamma-aminobutyric acid best journals gamma-aminobutyric acid top journals gamma-aminobutyric acid free medical journals gamma-aminobutyric acid famous journals gamma-aminobutyric acid Google Scholar indexed journals N-Methyl D-Aspartate articles N-Methyl D-Aspartate Research articles N-Methyl D-Aspartate review articles N-Methyl D-Aspartate PubMed articles N-Methyl D-Aspartate PubMed Central articles N-Methyl D-Aspartate 2023 articles N-Methyl D-Aspartate 2024 articles N-Methyl D-Aspartate Scopus articles N-Methyl D-Aspartate impact factor journals N-Methyl D-Aspartate Scopus journals N-Methyl D-Aspartate PubMed journals N-Methyl D-Aspartate medical journals N-Methyl D-Aspartate free journals N-Methyl D-Aspartate best journals N-Methyl D-Aspartate top journals N-Methyl D-Aspartate free medical journals N-Methyl D-Aspartate famous journals N-Methyl D-Aspartate Google Scholar indexed journals Antagonist articles Antagonist Research articles Antagonist review articles Antagonist PubMed articles Antagonist PubMed Central articles Antagonist 2023 articles Antagonist 2024 articles Antagonist Scopus articles Antagonist impact factor journals Antagonist Scopus journals Antagonist PubMed journals Antagonist medical journals Antagonist free journals Antagonist best journals Antagonist top journals Antagonist free medical journals Antagonist famous journals Antagonist Google Scholar indexed journals Borderline Personality Disorder articles Borderline Personality Disorder Research articles Borderline Personality Disorder review articles Borderline Personality Disorder PubMed articles Borderline Personality Disorder PubMed Central articles Borderline Personality Disorder 2023 articles Borderline Personality Disorder 2024 articles Borderline Personality Disorder Scopus articles Borderline Personality Disorder impact factor journals Borderline Personality Disorder Scopus journals Borderline Personality Disorder PubMed journals Borderline Personality Disorder medical journals Borderline Personality Disorder free journals Borderline Personality Disorder best journals Borderline Personality Disorder top journals Borderline Personality Disorder free medical journals Borderline Personality Disorder famous journals Borderline Personality Disorder Google Scholar indexed journals phosphorylation articles phosphorylation Research articles phosphorylation review articles phosphorylation PubMed articles phosphorylation PubMed Central articles phosphorylation 2023 articles phosphorylation 2024 articles phosphorylation Scopus articles phosphorylation impact factor journals phosphorylation Scopus journals phosphorylation PubMed journals phosphorylation medical journals phosphorylation free journals phosphorylation best journals phosphorylation top journals phosphorylation free medical journals phosphorylation famous journals phosphorylation Google Scholar indexed journals primary care provider articles primary care provider Research articles primary care provider review articles primary care provider PubMed articles primary care provider PubMed Central articles primary care provider 2023 articles primary care provider 2024 articles primary care provider Scopus articles primary care provider impact factor journals primary care provider Scopus journals primary care provider PubMed journals primary care provider medical journals primary care provider free journals primary care provider best journals primary care provider top journals primary care provider free medical journals primary care provider famous journals primary care provider Google Scholar indexed journals depression articles depression Research articles depression review articles depression PubMed articles depression PubMed Central articles depression 2023 articles depression 2024 articles depression Scopus articles depression impact factor journals depression Scopus journals depression PubMed journals depression medical journals depression free journals depression best journals depression top journals depression free medical journals depression famous journals depression Google Scholar indexed journals
Article Details
1. Introduction
In 2005, a single dose of ketamine was reported to rapidly decrease depression. “Whereas the intravenous route is predominantly employed route of administration, safety and efficacy have been with other routes [1]. Many studies highlighted intravenous infusion of ketamine, which is not only cumbersome, but poses safety concerns on an inpatient psychiatric unit. In the Indian Journal of Psychiatry, intramuscular racemic ketamine was administered to two patients with severe depression [2]. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, the largest study of MDD conducted in the United States, showed that even with enriched resources devoted to treatment, recovery with the first selected SSRI occurred only about 30% of the time [3]. In fact, after initiating antidepressants, the time for an expected response is longer than the typical inpatient psychiatric unit length of stay. In order to determine whether a medication will lead to response (≥50% reduction in depressive symptoms) or remission (nearly complete resolution of symptoms), it is recommended that a physician wait to see if it will be effective [4]. “The proposed explanation of SSRI/SNRI’s mechanism of action does not explain the temporal relationship between depressive crises and improvement of mood, occurring after the antidepressant initiation” [5]. Traditionally, patients suffering from Borderline Personality Disorder (BPD), have limited options for psychotropic medications; symptoms may worsen to include increased impulsivity and dangerous behaviors/cutting/suicide attempts. “Despite the common use of pharmacotherapies for patients with BPD, the available evidence does not support the efficacy of pharmacotherapies alone to reduce the severity of BPD” [6]. Borderline Personality Disorder associated impulsivity often, “results from disturbed inhibitory control, a function mainly regulated by GABA levels in the Anterior Cingulate Cortex (ACC), and the frontostriatal system” [7]. Ketamine constitutes a novel, rapid and efficacious treatment choice for patients suffering from moderate to severe treatment resistant depression and exhibits rapid and significant antidepressant and anti-suicidal effects. “Intravenous ketamine (0.5 mg/kg) produces robust, rapid and long-lasting antidepressant effects, but is unpractical” [8]. Brain-derived neurotrophic factor (BDNF) is a growth factor that regulates neurite outgrowth, functional neuronal connections, synapse formation and synaptic plasticity in the central nervous system [9]. Moreover, classical antidepressants induce BDNF-related changes following several weeks of administration. In contrast, ketamine administration rapidly (within 30 min of administration) increases the phosphorylation (activation) of hippocampal TrkB9.
New administration routes might serve as alternative to intravenous regimes for potential usage in outpatient settings [8]. A middle- aged male with severe depression, scoring >50 on MADRS, was initially amenable to Electroconvulsive Therapy) ECT. After watching ECT video, he rescinded. He was currently taking two different antidepressants, also on augmentation therapy; ECT being next step in treatment, urgently needed, based on significant depression. In reviewing some evidence-based literature, studies highlighted intravenous infusion of ketamine, which is not only cumbersome, but poses safety concerns on an inpatient psychiatric unit. As per Dr. Kenneth Certa, “psychiatric nursing staff may be uncomfortable with many “medical”, interventions and medical needs are frequently unable to be met due to safety and licensure requirements [10]. “There have also been reports of repeated, spaced dosing of IM ketamine to maintain the antidepressant benefits, with repeated doses in some instances continued at 3–4-day intervals for months. [11]. Our client was counseled that a new indication for use of ketamine to help with severe depression, with him being amenable to trial, in lieu of ECT. Intramuscular injections are not uncommon on our inpatient unit, IV infusions were scarce, and the initial trial of intramuscular ketamine, as an antidepressant was administered by psychiatric nursing, at practicing hospital in 2019.
2. Case Presentations
2.1 Patient Initials Replaced by K1, K2, and K3. Legend with Corresponding Dates Of Signed Informed Consent Following
K1 is a 66 y/o female with past psychiatric history significant for MDD, severe, w/ psychotic features, with suicidal ideations and plan to commit suicide by drowning. At time of referral, she was admitted to the inpatient psychiatric unit at an affiliate hospital; She was fully compliant with her regimen consisting of bupropion 450 mg daily, loxapine mg TID, mirtazapine 15 mg HS, lamotrigine 100 mg bid, zolpidem 5 mg HS. At outside hospital ECT consultation completed, however ultimately patient refused, due to past adverse effects; she cited significant memory deficits. At >60 days length of stay, without significant improvement, referral made by psychiatrist, with acceptance of transfer. She was discharged from affiliate facility, transported to author’s facility, admitted and bedded. (Presbyterian Medical Center (PMC) on 8/18/2021). On the second day of hospitalization, (8/19/2021), she was assessed and informed consent obtained. Denied auditory/visual hallucinations; was not responding to internal stimuli on exam. Baseline MADRS 41. Vital signs at baseline recorded with administration of intramuscular ketamine 25 mg IM x 1 (0.5 mg/kg) administered on day1; with continuation of oral psychotropics. K2 is a 56 y/o married Caucasian male with past psychiatric history significant for MDD, recurrent, severe without psychotic features. Chronic course of depressive episodes with two previous suicide attempts. Over the past two months, neurovegetative symptoms worsened and suicidal ideations which have increased. Endorsed plan to check into a hotel, then commit suicide with a handgun after his family goes out of town. K2 and his psychiatrist discussed resuming maintenance ECT, or potentially a ketamine trial, with K2 opting for ketamine trial. On 6/12/2019, K2 was admitted to the psychiatric unit with ketamine injection scheduled the next day. No modifications in patient’s home medication regimen – nortriptyline 150 mg QHS, lurasidone 120 mg daily, lithium 300 mg BID, levothyroxine 75 mcg daily, pantoprazole 40 mg daily, and atorvastatin 20 mg daily continued. Baseline MADRS: 21. 6/13/19: Informed consent obtained. Patient weight 126.1 kg. Intramuscular injection of 63 mg racemic ketamine (0.5 mg/kg) administered. K3 is a single, 19 year old female with past psychiatric history significant for Post-Traumatic Stress Disorder (PTSD), Severe Anorexia, and Borderline Personality Disorder, admitted on 9/28/21, to the inpatient psychiatric unit after she reported suicidal ideations. History of four suicide attempts by medication overdose. Medical history significant for irregular menstruation and had been believed to have Irritable Bowel Syndrome (IBS), after reporting gastrointestinal discomfort to her primary care provider (PCP). PCP reported her symptoms were not IBS, but more a consequence of restrictive eating, excessive laxative ingestion and self-induced vomiting. K3 admitted to increased purging and reported drinking a 296 ml bottle of magnesium citrate daily. She was assessed 9/27/21 by her outpatient psychiatrist with continuation of aripiprazole 400 mg IM every 30 days, and mirtazapine 15 mg QHS. Previous antidepressants including, but not limited to, escitalopram, sertraline, fluoxetine, duloxetine, venlafaxine XR, olanzapine, lurasidone. K3 reported crisis after sexual assault weeks prior to admission. She was informed regarding racemic ketamine injection with goal of improving her mood/decreasing suicidal ideations and she consented.
On 9/29/21 K3 received racemic ketamine 22 mg IM. Baseline MADRS score of 39.
2.2 Methods
Exclusion criteria: Ketamine contraindicated in patients with allergies to ketamine, history of aortic dissection/stenosis, uncontrolled HTN, Myocardial infarction or aneurysms.
Informed consent obtained of benefits, risks, side effects, obtained for each patient. Baseline and post-treatment MADRS obtained. Patients NPO for one hour prior to ketamine injection; two hours following. Dose corresponding to patient weight, (0.5 mg/kg), drawn by nursing, with verification by physician. Blood pressure and heart rate obtained at baseline and every 20 minutes thereafter, for 120 minutes. Patient were administered deltoid intramuscular racemic ketamine for treatment resistant depression; Patients agreed to sit for the monitoring period, in addition to being NPO for two hours after ketamine injection.
MADRS score calculated using www.MDCalc.com
3. Results
Common side effects reported dizziness, disorientation, and dissociation. No episodes of hemodynamic instability or other significant adverse effect.
Rapid improvement in depression or suicidal behavior measured by MADRS (Montgomery- Aberg Depression Rating Scale) score reduction over 36 hours, with patients meeting criteria for treatment response, if not remission (Based on MADRS).
Following ketamine injections, patients continued hospitalization on inpatient unit, while routine discharge planning ongoing. No consults warranted from other medical specialties:
- K1 - MADRS day 1 post-injection was 9, reduced by 78%, meeting criteria for remission. MADRS score on next 3 days was 4. She was monitored on the inpatient unit, with no relapse of depression or adverse effects from her regimen. She was discharged to her skilled nursing facility on day 5 of hospitalization. (8/23/2021).
- K2 - 6/14/19: Patient voiced wanting to discharge and wife voiced no concerns, as she would be there to observe for safety. MADRS at DC purported to be 6-10 (MADRS not completed but nursing and patient report). Per patient, “The first fifteen minutes was like a roller coaster. I felt nauseous for a few minutes; I was altered, but not too bad. Then at about thirty minutes I felt an explosion in my brain. I went to sleep, slept through the night, and I woke up rested.”
- K3 – MADRS score 1 day after ketamine injection decreased from 39 to 19. Most notable was improvement in score for reduced appetite. MADRS two days after ketamine was 11, however increased to 20 on day three; She reported indigestion, however she had been limiting water/fluid intake. Treatment team encouraging increased fluid intake; She was given IV normal saline for dehydration however no further medical intervention was required. Last day writer assessed K3 was on day 5; She was reported by nursing to be eating 100% of meals with no purging observed. K3 was discharged on 10/7/21, with referral to eating disorder facility.
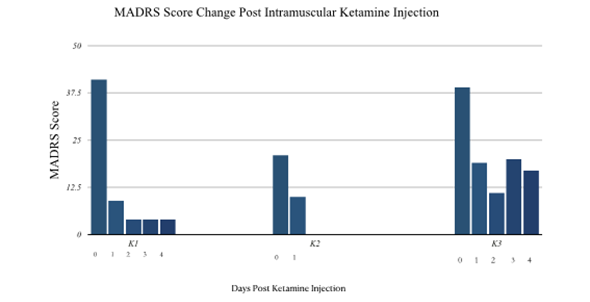
Figure 1: MADRS Score Change Post Intramuscular Ketamine Injection
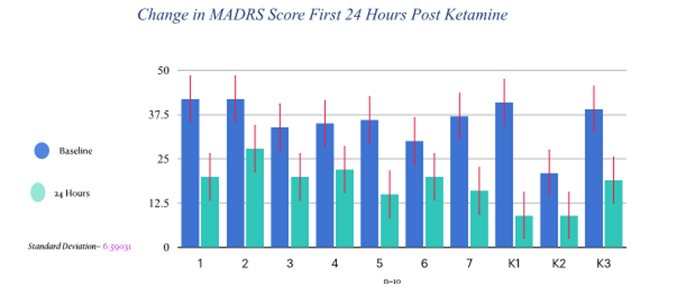
Figure 2: Change in MADRS Score First 24 Hours Post Ketamine
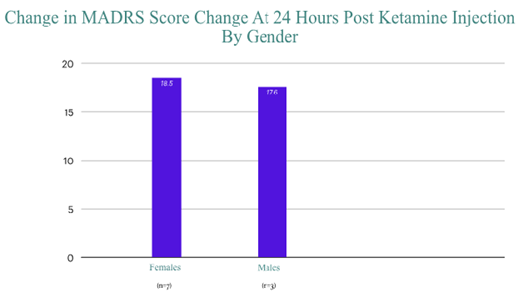
Figure 3: Change in MADRS Score Change at 24 Hours Post Ketamine Injection By Gender
4. Discussion
Ketamine has shown rapid, robust, reproducible improvement of depression, with no significant adverse effects. As of this writing, I have treated greater than 20 patients with intramuscular racemic ketamine on the inpatient psychiatric unit. Differences in pharmacokinetics of intramuscular vs intravenous administration of racemic ketamine is not known, however future studies may offer additional insight regarding ketamine metabolism post injection. There was no significant advantage seen with gender or age, in regards to antidepressant response following ketamine injection.
“Individual-level meaningful change for the PHQ-9 and MADRS was effectively quantified using a clinical anchor to interpret efficacy from patients with TRD and their treating clinicians. The most appropriate MCT for the MADRS was -10 points” [12].
4.1 Clinically significant response from intramuscular racemic ketamine measured by greater than 10 point reduction of MADRS score:
In 2020, “the Missouri University Psychiatric Center, formed a ketamine infusion team composed solely of mental health clinicians and staff to investigate the use of ketamine infusions by a psychiatric team.” [13] The use of IM ketamine does not require employment of other departments, with limited interruption of inpatient treatment plan, as patients return to baseline cognition by 120 minutes after injection.
FDA approved esketamine, the S-enantiomer of ketamine. In terms of chirality of ketamine, According to a 2021 study, “negatively experienced psychopathology with S-ketamine”, and “antidepressant effect of ketamine might depend on a pleasant experience of altered consciousness. The ideal ketamine composition to treat depression should include R-ketamine” [14]. It is plausible racemic ketamine supplies both enantiomers, however specific enantiomer qualities/characteristics have not been well characterized. Whether the stereoisomer precludes or limits antidepressant effects of R-ketamine or racemic ketamine?
Ketamine increased GABAergic neurotransmission, with stimulation of BDNF expression, is thought to result in increased neuroplasticity8; this may quickly palliate acute crises suffered by patients with BPD. Borderline Personality Disorder associated impulsivity often, “results from disturbed inhibitory control, a function mainly regulated by GABA levels in the Anterior Cingulate Cortex (ACC), and the frontostriatal system” [15].
Screening candidates, excluding patients with contraindications, significant recent substance use, ongoing psychotic crises, should be involved with prescribing of ketamine.
5. Conclusion
Intramuscular racemic ketamine, in subanesthetic doses, is an effective, safe, off-label treatment for severe/moderate treatment refractory depression and rapid stabilization of suicidal behaviors/ideations, in under 36 hours, post injection. No consultations warranted from other medical specialties. Although these findings are promising, larger, heterogeneous studies are needed, which provide historical data of continued response/remission following ketamine, whether from intramuscular or intravenous administration. Comparative studies are needed comparing efficacy between racemic, R-ketamine, and S-ketamine.
Author Contributions
The author of this paper managed data collection, data interpretation, and drafting the manuscript.
Conflict of Interest
The author declares that he has no financial, or any other conflicts of interest.
References
- Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63 (2006): 856-64.
- Harihar C, Dasari P, Srinivas JS. Intramuscular ketamine in acute depression: a report on two cases. Indian J Psychiatry 55 (2013): 186-188.
- Rush AJ. STAR*D: what have we learned?. Am J Psych 164 (2007): 201–204.
- Fochtmann LJ, Gelenberg AJ. 2nd ed. Washington, DC: American Psychiatric Association; Guideline Watch: Practice Guideline for the Treatment of Patients with Major Depressive Disorder (2005).
- Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63 (2006): 856-64.
- Wang GY, van Eijk J, Demirakca T, et al. ACC GABA levels are associated with functional activation and connectivity in the fronto-striatal network during interference inhibition in patients with borderline personality disorder. Neuroimage 147 (2017): 164-174.
- Gartlehner G, Crotty K, Kennedy S, et al. Pharmacological Treatments for Borderline Personality Disorder: A Systematic Review and Meta-Analysis. CNS Drugs 35 (2021): 1053-1067.
- Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63 (2006): 856-64.
- Andrade C. Ketamine for Depression, 4: In What Dose, at What Rate, by What Route, for How Long, and at What Frequency? J Clin Psychiatry 78 (2017): 852-857.
- Certa K. Medically and Psychiatrically Complicated Patients. Psychiatric Times. 2017 34 (5).
- Cusin C, Hilton GQ, Nierenberg AA, et al. Long-term maintenance with intramuscular ketamine for treatment-resistant bipolar II depression. Am J Psychiatry 169 (2012): 868-869.
- Hudgens S, Floden L, Blackowicz M, et al. Meaningful Change in Depression Symptoms Assessed with the Patient Health Questionnaire (PHQ-9) and Montgomery-Åsberg Depression Rating Scale (MADRS) Among Patients with Treatment Resistant Depression in Two, Randomized, Double-blind, Active-controlled Trials of Esketamine Nasal Spray Combined With a New Oral Antidepressant. J Affect Disord 15 (2021): 767-775.
- Ithman M, Sobule R, Kundert C, et al. Ketamine Infusions Administered Solely by Psychiatric Staff. Mo Med 119 (2022): 164-166.
- Passie T, Adams HA, Logemann F, et al. Comparative effects of (S)-ketamine and racemic (R/S)-ketamine on psychopathology, state of consciousness and neurocognitive performance in healthy volunteers. Eur Neuropsychopharmacol (2021).
- Loo CK, Gálvez V, O'Keefe E, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134 (2016): 48-56.
