Intracranial Multiple Myeloma After Chemotherapy
Article Information
Michael Kogan MD PhD1,3 and Adnan H. Siddiqui MD PhD1-5*
1Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York
2Department of Radiology, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York
3Department of Neurosurgery, Gates Vascular Institute at Kaleida Health, New York, USA
4Toshiba Stroke and Vascular Research Center, University at Buffalo, State University of New York
5Jacobs Institute, Buffalo, New York, USA
*Corresponding Author: Adnan H. Siddiqui MD, University at Buffalo Neurosurgery, 100 High Street, Suite B4
Buffalo, NY 14203 USA, Tel: 716 218 1000; Fax: 716 859 7479;
Received: 08 August 2017; Accepted: 15 August 2017; Published: 18 August 2017
View / Download Pdf Share at FacebookKeywords
Central nervous system, Chemotherapy, Intracranial, Multiple myeloma, Bortezomib,Leptomeningeal, Pachymeningeal, Stem-cell transplant
Central nervous system articles Central nervous system Research articles Central nervous system review articles Central nervous system PubMed articles Central nervous system PubMed Central articles Central nervous system 2023 articles Central nervous system 2024 articles Central nervous system Scopus articles Central nervous system impact factor journals Central nervous system Scopus journals Central nervous system PubMed journals Central nervous system medical journals Central nervous system free journals Central nervous system best journals Central nervous system top journals Central nervous system free medical journals Central nervous system famous journals Central nervous system Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals Intracranial articles Intracranial Research articles Intracranial review articles Intracranial PubMed articles Intracranial PubMed Central articles Intracranial 2023 articles Intracranial 2024 articles Intracranial Scopus articles Intracranial impact factor journals Intracranial Scopus journals Intracranial PubMed journals Intracranial medical journals Intracranial free journals Intracranial best journals Intracranial top journals Intracranial free medical journals Intracranial famous journals Intracranial Google Scholar indexed journals Multiple myeloma articles Multiple myeloma Research articles Multiple myeloma review articles Multiple myeloma PubMed articles Multiple myeloma PubMed Central articles Multiple myeloma 2023 articles Multiple myeloma 2024 articles Multiple myeloma Scopus articles Multiple myeloma impact factor journals Multiple myeloma Scopus journals Multiple myeloma PubMed journals Multiple myeloma medical journals Multiple myeloma free journals Multiple myeloma best journals Multiple myeloma top journals Multiple myeloma free medical journals Multiple myeloma famous journals Multiple myeloma Google Scholar indexed journals Bortezomib articles Bortezomib Research articles Bortezomib review articles Bortezomib PubMed articles Bortezomib PubMed Central articles Bortezomib 2023 articles Bortezomib 2024 articles Bortezomib Scopus articles Bortezomib impact factor journals Bortezomib Scopus journals Bortezomib PubMed journals Bortezomib medical journals Bortezomib free journals Bortezomib best journals Bortezomib top journals Bortezomib free medical journals Bortezomib famous journals Bortezomib Google Scholar indexed journals Leptomeningeal articles Leptomeningeal Research articles Leptomeningeal review articles Leptomeningeal PubMed articles Leptomeningeal PubMed Central articles Leptomeningeal 2023 articles Leptomeningeal 2024 articles Leptomeningeal Scopus articles Leptomeningeal impact factor journals Leptomeningeal Scopus journals Leptomeningeal PubMed journals Leptomeningeal medical journals Leptomeningeal free journals Leptomeningeal best journals Leptomeningeal top journals Leptomeningeal free medical journals Leptomeningeal famous journals Leptomeningeal Google Scholar indexed journals Pachymeningeal articles Pachymeningeal Research articles Pachymeningeal review articles Pachymeningeal PubMed articles Pachymeningeal PubMed Central articles Pachymeningeal 2023 articles Pachymeningeal 2024 articles Pachymeningeal Scopus articles Pachymeningeal impact factor journals Pachymeningeal Scopus journals Pachymeningeal PubMed journals Pachymeningeal medical journals Pachymeningeal free journals Pachymeningeal best journals Pachymeningeal top journals Pachymeningeal free medical journals Pachymeningeal famous journals Pachymeningeal Google Scholar indexed journals Stem-cell transplant articles Stem-cell transplant Research articles Stem-cell transplant review articles Stem-cell transplant PubMed articles Stem-cell transplant PubMed Central articles Stem-cell transplant 2023 articles Stem-cell transplant 2024 articles Stem-cell transplant Scopus articles Stem-cell transplant impact factor journals Stem-cell transplant Scopus journals Stem-cell transplant PubMed journals Stem-cell transplant medical journals Stem-cell transplant free journals Stem-cell transplant best journals Stem-cell transplant top journals Stem-cell transplant free medical journals Stem-cell transplant famous journals Stem-cell transplant Google Scholar indexed journals manifestation articles manifestation Research articles manifestation review articles manifestation PubMed articles manifestation PubMed Central articles manifestation 2023 articles manifestation 2024 articles manifestation Scopus articles manifestation impact factor journals manifestation Scopus journals manifestation PubMed journals manifestation medical journals manifestation free journals manifestation best journals manifestation top journals manifestation free medical journals manifestation famous journals manifestation Google Scholar indexed journals Myeloma articles Myeloma Research articles Myeloma review articles Myeloma PubMed articles Myeloma PubMed Central articles Myeloma 2023 articles Myeloma 2024 articles Myeloma Scopus articles Myeloma impact factor journals Myeloma Scopus journals Myeloma PubMed journals Myeloma medical journals Myeloma free journals Myeloma best journals Myeloma top journals Myeloma free medical journals Myeloma famous journals Myeloma Google Scholar indexed journals
Article Details
1. Introduction
Intracranial localization is reported in <1% of cases of multiple myeloma [1], with pachymeningeal and leptomeningeal lesions associated with hematogenous spread [2]. Interestingly, the incidence of central nervous system (CNS) myeloma has been increasing, which may be reflective of new chemotherapeutic agents used to treat the primary manifestation [3].
2. Case Report
A 68-year-old African-American woman with a history of multiple myeloma in remission presented with slurred speech. Computed tomography of the head showed multiple hemorrhagic lesions (Figure 1A and 1B). Brain magnetic resonance imaging confirmed pachymeningeal involvement (Figure 1C and 1D). A diagnosis of monoclonal gammopathy had been made 7 years prior to the present admission. She developed multiple myeloma 1? years prior. She was beta-2-macroglobulin positive and underwent induction chemotherapy with lenalidomide, bortezomib, and dexamethasone. A bone marrow biopsy performed 4 months prior to the present admission was negative. Bortezomib therapy alone was maintained. No stem-cell transplant was performed.
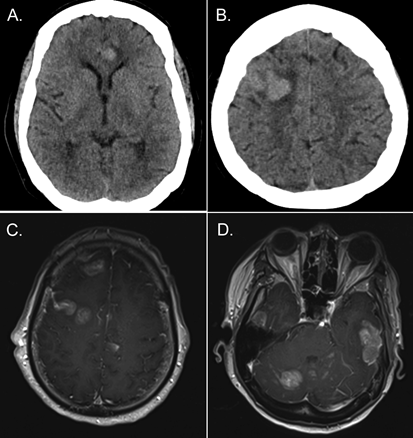
Figure 1: Computed tomographic scans obtained at the time of admission, with evidence of hemorrhagic tumor growth (A-B). Admission magnetic resonance T1-weighted images with contrast enhancement showing multiple pachymeningeal lesions (C-D).
Our workup revealed no lesions other than a questionable T2 spinal process lesion. We performed a right-sided excisional biopsy of a superficial temporal lesion to establish the diagnosis, which confirmed the presence of plasmacytoid cells.
3. DiscussionIn a recent review, 7 patients who underwent stem cell transplant and treatment with novel agents for multiple myeloma developed intracranial disease [3]. Our case is an unusual presentation of isolated intracranial disease in conjunction with complete remission on sole bortezomib therapy (subsequent to induction combination chemotherapy) and no stem-cell transplant.
4. Conclusion
This case highlights the possibility that CNS manifestation may be related to potential lack of blood-brain barrier penetrance of novel agents [1].
5. Author Contributions
Conception and design: both authors. Acquisition of data: both authors. Analysis and interpretation of data: both authors. Drafting the manuscript: Kogan. Critically revising the article: both authors. Reviewed submitted version of manuscript: both authors.
6. Financial Relationships/Potential Conflicts of Interest (none directly related to this work)
Kogan: None. Siddiqui: Financial interests:Buffalo Technology Partners Inc., Cardinal, International Medical Distribution Partners; Medina Medical Systems, Neuro technology Investors, StimSox, Valor Medical; Consultant:Amnis Therapeutics Ltd., Cerebrotech Medical Systems Inc., CereVasc LLC, Codman, Corindus Inc., Covidien (acquired by Medtronic), GuidePoint Global Consulting, Lazarus (acquired by Medtronic), Medina Medical (acquired by Medtronic), Medtronic, MicroVention, Neuravi, Penumbra, Pulsar Vascular, Rapid Medical, Rebound Medical, Reverse Medical (acquired by Medtronic), Silk Road Medical Inc., Stryker, The Stroke Project Inc., Three Rivers Medical Inc., W.L. Gore & Associates; Principal Investigator/National Steering Committee:Covidien SWIFT PRIME Trial, LARGE Trial, Medtronic SWIFT DIRECT, MicroVention CONFIDENCE Study, MicroVention FRED Trial, Penumbra 3D Separator Trial, Penumbra COMPASS Trial, Penumbra INVEST Trial, POSITIVE Trial; Board Member:Intersocietal Accreditation Committee.
References
- Fassas AB, Muwalla F, Berryman T, et al. Myeloma of the central nervous system: association with high-risk chromosomal abnormalities, plasmablastic morphology and extramedullary manifestations. Br J Haematol 117 (2002): 103-108.
- Moulopoulos LA, Granfield CA, Dimopoulos MA, et al. Extraosseous multiple myeloma: imaging features. AJR Am J Roentgenol 161 (1993): 1083-1087.
- Gangatharan SA, Carney DA, Prince HM, et al. Emergence of central nervous system myeloma in the era of novel agents. Hematol Oncol 30 (2012): 170-174.
