Insect Venoms and their Bioactive Components: A Novel Therapeutic Approach in Chronic Diseases and Cancer
Article Information
Deepshikha Moran2, Uma Dutta1*, Ajaikumar B Kunnumakkara3, Enush Daimari1, Bhabesh Deka4
1Cell and Molecular Biology Laboratory, Department of Zoology, Cotton University, Guwahati-781001, Assam, India
2Department of Zoology, Dibru College, Dibrugarh-786003, Assam, India
3Department of Biosciences and Bioengineering, Indian Institute of Technology, Guwahati-781039, Assam, India
4Department of Entomology, Tea Research Association, North Bengal Regional R&D Centre, Jalpaiguri-735225, West Bengal, India
*Corresponding Author: Uma Dutta, HOD and Associate professor, Cell and Molecular Biology Laboratory, Department of Zoology, Cotton University, Guwahati- 781001, Assam, India.
Received: 11 August 2022; Accepted: 07 September 2022; Published: 14 October 2022
Citation: Deepshikha Moran, Uma Dutta, Ajaikumar B Kunnumakkara, Enush Daimari, Bhabesh Deka. Insect Venoms and their Bioactive Components: A Novel Therapeutic Approach in Chronic Diseases and Cancer. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 360-382.
View / Download Pdf Share at FacebookAbstract
To counteract the growing burden of chronic diseases, discovery of highly selective target specific drugs is of utmost importance in present scenario. Various advanced therapeutic procedures and modern drugs have been developed and approved in last three decades for treating these disorders. The very first limitation of these therapies is their side effects, which are severe and long term. Also, these treatments are a costly affair and of limited therapeutic advantages. To overcome it, exploration and mining of natural products is much necessary. Phytotherapy is already well-established in the field of drug discovery. Focus should also be provided on zoo-therapy, as it is loaded with paramount of possibilities regarding disease treatment. Insect venoms are cocktail of bioactive components with different physiological actions that have undergone evolutionary refinement through a long time-scale. This evolutionary selection over time makes them more suitable candidate for target specific drug discovery. In this review we are trying to throw some light on some significant insect venom components with their mechanism of patho-physiological actions, relevance in the field of advanced drug discovery targeting chronic diseases including cancer.
Keywords
Bioactive Components; Cancer; Chronic Diseases; Insect Venom; Therapeutics
Bioactive Components articles; Cancer articles; Chronic Diseases articles; Insect Venom articles; Therapeutics articles
Bioactive Components articles Bioactive Components Research articles Bioactive Components review articles Bioactive Components PubMed articles Bioactive Components PubMed Central articles Bioactive Components 2023 articles Bioactive Components 2024 articles Bioactive Components Scopus articles Bioactive Components impact factor journals Bioactive Components Scopus journals Bioactive Components PubMed journals Bioactive Components medical journals Bioactive Components free journals Bioactive Components best journals Bioactive Components top journals Bioactive Components free medical journals Bioactive Components famous journals Bioactive Components Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Chronic Diseases articles Chronic Diseases Research articles Chronic Diseases review articles Chronic Diseases PubMed articles Chronic Diseases PubMed Central articles Chronic Diseases 2023 articles Chronic Diseases 2024 articles Chronic Diseases Scopus articles Chronic Diseases impact factor journals Chronic Diseases Scopus journals Chronic Diseases PubMed journals Chronic Diseases medical journals Chronic Diseases free journals Chronic Diseases best journals Chronic Diseases top journals Chronic Diseases free medical journals Chronic Diseases famous journals Chronic Diseases Google Scholar indexed journals Insect Venom articles Insect Venom Research articles Insect Venom review articles Insect Venom PubMed articles Insect Venom PubMed Central articles Insect Venom 2023 articles Insect Venom 2024 articles Insect Venom Scopus articles Insect Venom impact factor journals Insect Venom Scopus journals Insect Venom PubMed journals Insect Venom medical journals Insect Venom free journals Insect Venom best journals Insect Venom top journals Insect Venom free medical journals Insect Venom famous journals Insect Venom Google Scholar indexed journals Therapeutics articles Therapeutics Research articles Therapeutics review articles Therapeutics PubMed articles Therapeutics PubMed Central articles Therapeutics 2023 articles Therapeutics 2024 articles Therapeutics Scopus articles Therapeutics impact factor journals Therapeutics Scopus journals Therapeutics PubMed journals Therapeutics medical journals Therapeutics free journals Therapeutics best journals Therapeutics top journals Therapeutics free medical journals Therapeutics famous journals Therapeutics Google Scholar indexed journals Mastoparan articles Mastoparan Research articles Mastoparan review articles Mastoparan PubMed articles Mastoparan PubMed Central articles Mastoparan 2023 articles Mastoparan 2024 articles Mastoparan Scopus articles Mastoparan impact factor journals Mastoparan Scopus journals Mastoparan PubMed journals Mastoparan medical journals Mastoparan free journals Mastoparan best journals Mastoparan top journals Mastoparan free medical journals Mastoparan famous journals Mastoparan Google Scholar indexed journals Cytochrome P450 articles Cytochrome P450 Research articles Cytochrome P450 review articles Cytochrome P450 PubMed articles Cytochrome P450 PubMed Central articles Cytochrome P450 2023 articles Cytochrome P450 2024 articles Cytochrome P450 Scopus articles Cytochrome P450 impact factor journals Cytochrome P450 Scopus journals Cytochrome P450 PubMed journals Cytochrome P450 medical journals Cytochrome P450 free journals Cytochrome P450 best journals Cytochrome P450 top journals Cytochrome P450 free medical journals Cytochrome P450 famous journals Cytochrome P450 Google Scholar indexed journals Lipooxygenase articles Lipooxygenase Research articles Lipooxygenase review articles Lipooxygenase PubMed articles Lipooxygenase PubMed Central articles Lipooxygenase 2023 articles Lipooxygenase 2024 articles Lipooxygenase Scopus articles Lipooxygenase impact factor journals Lipooxygenase Scopus journals Lipooxygenase PubMed journals Lipooxygenase medical journals Lipooxygenase free journals Lipooxygenase best journals Lipooxygenase top journals Lipooxygenase free medical journals Lipooxygenase famous journals Lipooxygenase Google Scholar indexed journals Omega-Ctenitoxin-Cs1a articles Omega-Ctenitoxin-Cs1a Research articles Omega-Ctenitoxin-Cs1a review articles Omega-Ctenitoxin-Cs1a PubMed articles Omega-Ctenitoxin-Cs1a PubMed Central articles Omega-Ctenitoxin-Cs1a 2023 articles Omega-Ctenitoxin-Cs1a 2024 articles Omega-Ctenitoxin-Cs1a Scopus articles Omega-Ctenitoxin-Cs1a impact factor journals Omega-Ctenitoxin-Cs1a Scopus journals Omega-Ctenitoxin-Cs1a PubMed journals Omega-Ctenitoxin-Cs1a medical journals Omega-Ctenitoxin-Cs1a free journals Omega-Ctenitoxin-Cs1a best journals Omega-Ctenitoxin-Cs1a top journals Omega-Ctenitoxin-Cs1a free medical journals Omega-Ctenitoxin-Cs1a famous journals Omega-Ctenitoxin-Cs1a Google Scholar indexed journals Vascular Endothelial Growth Factor articles Vascular Endothelial Growth Factor Research articles Vascular Endothelial Growth Factor review articles Vascular Endothelial Growth Factor PubMed articles Vascular Endothelial Growth Factor PubMed Central articles Vascular Endothelial Growth Factor 2023 articles Vascular Endothelial Growth Factor 2024 articles Vascular Endothelial Growth Factor Scopus articles Vascular Endothelial Growth Factor impact factor journals Vascular Endothelial Growth Factor Scopus journals Vascular Endothelial Growth Factor PubMed journals Vascular Endothelial Growth Factor medical journals Vascular Endothelial Growth Factor free journals Vascular Endothelial Growth Factor best journals Vascular Endothelial Growth Factor top journals Vascular Endothelial Growth Factor free medical journals Vascular Endothelial Growth Factor famous journals Vascular Endothelial Growth Factor Google Scholar indexed journals
Article Details
Abbreviations:
MPN – Mastoparan; PLA2 - Phospholipase A2; Cav - Voltage Gated Calcium Channel; Clv - Voltage Gated Chloride Channels; Nav - Voltage Gated Sodium Channels; Kv - Voltage Gated Potassium Channels; COX – Cyclooxygenase; CYP450 - Cytochrome P450; LOX – Lipooxygenase; GPCR - G-Protein Coupled Receptors; sPLA2 - Secretory Phospholipase A2; SK channels - Small Conductance Channels; IgE – Immunoglobulin E; IFN-g - Interferon g; MCD - Mast Cell Degranulating Peptide; AaH IT - Beta-Insect Excitatory Toxin 1; CSTX-1 - Omega-Ctenitoxin-Cs1a; VEGFR-2 – Vascular Endothelial Growth Factor; PI3K – Phosphoinositide 3-Kinases; HT 29 – Human Colorectal Adenocarcinoma Cell Line; MAPK- Mitogen-Activated Protein Kinase; CD44 – Cluster of Differentiation 44; BmK AGAP – Buthus martensii Karsch Antitumor Analgesic Peptide; BmK CT – Buthus martensii Karsch chlorotoxin?like Toxin; Bcl-2 – B-Cell Lymphoma 2; NFkB – Nuclear Factor k B; CD4 – Cluster of Differentiation 4; IL – Interleukin; KCa – Ca activated Potassium Channel; MPTP – 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; AChE – Acetylcholinesterase; TNFa - Tumor Necrosis Factor a; PARP – Poly (ADP-ribose) Polymerase; PEG – Polyethylene Glycol; TXA2 - Thromboxane A2; ERK/MAP - Extracellular Signal-Regulated Kinases / Mitogen-Activated Protein; EGF - Epidermal Growth Factor; MMP 9 - Matrix Metallopeptidase 9; PI3K/Akt/mTOR - Phosphatidylinositol 3-Kinase / Protein Kinase B / Mammalian Target of the Rapamycin; FAK - Focal Adhesion Kinase; STAT - Signal Transducer and Activator of Transcription; HYAL – Hyaluronidase; TGF-β - Transforming Growth Factor-β; WWOX - WW Domain-Containing Oxidoreductase; GTP - Guanosine Triphosphate; Erα - Estrogen Receptor-α; LBD - Ligand Binding Domain; VSAP - Vasodilator Stimulated Phosphoprotein
1. Introduction
Phylum Arthropoda is the largest of all existing animal phyla occupying about 80% of the total all the living animal species. A number of species of this phylum have been a part of human life from time immemorial through many aspects viz. as food and nutritional component, as medicine as well as household usages such as in preparation of dyes, ink, fabrics, ornaments, wax etc. Insects have been a part of traditional medicine as well as cuisine from ancient era in countries like India, China, Africa, Laos, Japan, Papua New Guinea etc [1-5]. For example, entomophagy culture of China, where fried scorpions are enjoyed as delicacies. Dried whole body of the scorpion is also being used as a cure for epilepsy and pain reliever agent in China [6,7]. In Japan larvae of yellow jacket wasps (common name Hebo) are marketed as canned products and consumed for their nutritional values and taste [3,8]. The eggs of red ants are fried and consumed in India and it is also associated with cultural emotion of one of the states, Assam, India. The winged termites of the family Macrotermitinae, merging from termite hills at the end of dry season are captured and consumed in many parts of the world. Queen termites are so rich in fatty acids (linoleic acid), proteins and other micronutrients that the fried or sun dried termite queens are fed to malnourished children in some countries like Uganda and Zambia [9-13]. Development of new therapeutical procedures in the medical field has led to find cures to some extent, of many chronic disorders like rheumatoid arthritis, diabetes, cystic fibrosis etc. Many therapies viz. radiotherapy, chemotherapy etc. are also developed and approved in the field of cancer therapy in past decades. However, these therapies come along with long term side effects and a limited therapeutic advantage. In some cases, the patient acquires resistance towards the therapies (such as radio-resistance, chemoresistance) [14-16]. Moreover, the cost of these expensive therapies is a limiting factor of availability for the suffering patients. For these reasons, a novel and better therapeutic option is required to be developed which is cost effective, safe and readily available. In recent years, modern day therapeutics are turning towards alternative and oriental medicinal practices for treating chronic diseases including cancer as they are highly effective and have seemingly lesser or no side effects as compared to synthetic drugs. Naturopathy is gaining popularity in the field of drug discovery also because of the fact that it is cost effective and of easy availability. Herbal therapies and zootherapies are given wide attention amongst researchers for their isolated bioactive components. A substantial proportion of these nature derived pharmacologically active principles have anti-oxidant, anti-inflammatory, anti-hyperglycemic, anti-microbial, anti-cancer and anti-nociceptive properties that can play important roles in powerful drug discovery against different diseases as well as cancer [17-22]. One such nature’s treasure is insect venom. Venoms are secreted by a large number of insect groups as a strategy of defense as well as predation mechanism. It is secreted by a specialized organ or tissue called venom gland and introduced into the prey or predator’s body by means of parenteral applicators such as teeth, fangs, nematocytes, setae, spines etc. [23]. Insect venom contains a plethora of bioactive principles that can target different signaling pathways of the cell, which are involved in inducing discomfort, pain, inflammation, headache, vomiting and breathlessness in extreme cases. Most of the animal venoms studied from scorpion, spider, snakes, and wasps are a heterogenous mixture of enzymes of M.W. greater than 10 kDa (mainly Phospholipases and proteases), inorganic salts, polypeptides and small organic molecules [24-26]. Various published and ongoing research works have established that the complex molecular scaffolds present in venom components can modulate the intrinsic signaling pathways that are associated with pain, apoptosis, necrosis, inflammation etc. [27,20]. This property may possess a paramount of importance and possibility in modern day drug discovery, in other words discovery of pharmaceutical liquid gold from proteinaceous venom [19]. Another advantage associated with considering venom as a template for drug designing is that they have undergone a process of evolutionary refinement (natural selection) and evolved to perform optimally and selectively on their target [28]. Here it is tried to cover the later in this review in relation to their mechanism of target modulation.
2. General Mechanism of Venom Action
It has become evident from the various studies performed in past three decades that insect venoms are endowed with intricate mixture of numerous bioactive principles that targets different cell membrane receptors, thereby modulating signaling pathways and ion channels activating nociceptive pathways, grossly put under cytotoxins. Again, some other type of venom principles, classified under neurotoxins, generate action potential by acting on the nervous system and incapacitate the prey or predator organism [29,20]. Both of these venom delivering mechanisms targets different signal receptors of cells and causes pain, inflammation and asphyxiation in some cases. Venoms of hymenopteran insect’s wasps, honey bees contain cytotoxic peptides mastoparan and melittin respectively. These peptides being amphipathic in nature, possess the capacity to disrupt the integrity of the plasmamembrane by interacting with the phospholipids which ultimately leads to pore formation and cell lysis. The cell leaks out the viable cell organelles and approaches to death. Bradykinin is another peptide component of wasp’s venom that acts synergistically with mastoparan (MPN). An enzyme called phospholipase A2 (PLA2) is responsible for further damage of the phospholipid bilayer and exposes the lipids of the inner leaflet (such as phospatidylserine, phosphatidyl ethanolamine). This in turn activates apoptosis by sending “eat me” signals. [30-33]. Neurotoxic venoms of scorpions, centipedes and spiders have pharmacological properties of targeting ion channels directly related to pain inducing paralysis. Their venoms are a deadly cocktail of peptides, proteins and enzymes with diverse bioactives. Insect neurotoxins mainly reacts with voltage gated calcium channels (Cav), sodium channels (Nav), potassium channels (Kv), acid sensitive ion channels etc. Moreover they show other significant physiological activity, viz. anticoagulant activity, PLA2 activity, platelet aggregating, trypsin inhibitory activity etc. [34-38].
3. Important Bioactive Components Isolated from Insect Venoms and their Sources
3.1 Phospholipase A2
PLA2 is a major component isolated from venom of wide range insects such as wasps, bees, scorpion, centipede that performs wide range of catalytic activities [39-41]. The glycerophospholipids of the plasmamembrane are hydrolysed by PLA2 at the ester bond in the sn-2 position releasing fatty acids such as oleic acid, arachidonic acid etc. and lysophospholipids (viz. lysophospatidyl choline, lysophospatidyl inositol, lysophospatidyl ethanolamine etc. [41,42]. All these act as a precursor of a class of lipid derived hormones known as eicosanoids. This is an enzyme assisted process, where enzymes namely cyclooxygenase (COX), cytochrome P450 (CYP450), lipooxygenase (LOX) are involved in this conversion mechanism. Elevated levels of eicosanoids in the circulation is directly related to inflammation, pain, swelling, redness etc. [43-46]. Thus eicosanoids are related to immunomodulatory functions. The pro-inflammatory signalling molecules, such as prostaglandins, leukotrienes, thromboxanes etc., derived from hydrolysis of phospholipids of the cell membrane by the PLA2, depolarises the nerve fibres associated with nociception. These signalling molecules bind to the neuron membrane receptors, e.g., ionotropic receptors, G-protein coupled receptors (GPCR), tyrosin kinase receptors etc., elevating the sensitivity of nerve endings or causing hyperalgesia [47,48]. PLA2 derived from honey bee is categorized under group III of secretory phospholipase A2 (sPLA2) containing a total of eight disulphide bonds [49]. PLA2 isolated from Egyptian honey bee, Apis mellifera lamarckii has reportedly shown anti- coagulation and anti- platelet aggregating activities by prolonging prothrombin time [50]. It showed analgesic activity against sensory neuropathic sign of pain induced by oxaliplatin, a cancer drug used to treat metastatic colorectal cancer [51]. Venom of Vespids and fire ants predominantly contain phospholipase A1 (PLA1) that also functions similar to PLA2 causing hypersensitivity reactions [52].
3.2 Mastoparan
The most predominant peptide of wasp venom is mastoparan (MPN). It is a cationic decapeptide with the amino acid sequence of Ile-Asn-Leu-Lys-Ala-Leu-Ala-Ala-Leu-Ala-Lys-Lys-Ile-Leu-NH2. MPN directly interacts with membrane phospholipids causing destabilization and membrane lysis (pore formation) leading to leakage out of vital cell organelles causing cell death [53]. It is linked with stimulatory secretion of histamine from mast cells [54-56]. Reports showed that MPN can directly interact with the G-protein to activate it and this bound conformation mimics the G-protein coupled receptor (GPCR) of cell membrane [57,58]. Besides histamine, this peptide is also involved with other secretory activities from variety of mammalian cells, such as serotonin, insulin, catecholamines, surfactants from platelets, pancreatic islet cells (b cells), type 2 pulmonary alveolar cells, chromaffin cells respectively [38,59,60,56,61]. It also stimulates Ca2+ influx and increases intracellular Ca2+ concentration [62]. It interacts with the membrane phospholipids of mitochondria resulting into formation of permeability transition pore which in turn leads to swelling and rupturing of outer and inner mitochondrial membranes [62,63]. Involvement of MPN in stimulating G protein that is pertussis toxin sensitive and regulating activities of phospholipase A2 and C in Swiss 3T3 cells, was also established [64].
3.3 Bradykinin / Wasp kinins
The heterogeneous mixture of wasp and ant venoms contain kinin polypeptides of about 8-19 amino acid sequences. A venom constituent similar to bradykinin was first reported in the venom of Vespa vulgaris and categorised under wasp kinin [65-68]. Wasp kinins are neurotoxic in nature and similar to the sequence of bradykinin [69]. The bradykinin like wasps kinins differ in the presence of amino acid residues in the positions 3 or 6. On this basis, wasps kinins are of three main types, bradykinin, Hyp3-bradykinin (hydroxyproline replaces proline) or Thr6-bradykinin (threonine replaces serine) [66,70]. The latter Thr6 is more potent in blocking the pre-synaptic signal transmission in insect central nervous system (CNS) than bradykinin itself [71,72]. Kinins can permanently paralyze the prey by irreversible blockage of CNS. Cascade of reports have established that kinin peptide causes contractions and relaxations of smooth muscle preparations of visceral organs such as fundus, colon, rectum (mild contractions) and duodenum and ileum (strong contractions) in rat [73-75]. Slow and delayed contraction of guineapig ileum and rabbit intestine along with reducing blood pressure in cat and rabbit, were also recorded upon treatment with wasp kinin [76].
3.4 Melittin
The predominant component of bee venom (apitoxin) is a small basic peptide of 26 amino acid residues called melittin. The sequence reads as Gly-Ile-Gly-Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro-Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-Gln-NH2 [77-79]. Being amphipathic in nature, this peptide portrays surfactant and detergent like activities and interacts with cell membrane by wedge and edge effects to create membrane pores [79]. It binds with anionic phospholipids (viz. phospatidyl-serine, phospatidyl-ethanolamine, phosphatidyl-inositol etc.) and disrupts the bilayer structure leading exocytosis and cell death [80]. Melittin carries out lytic action along with secretion of histamine from mast cells and liberates haemoglobin from red blood cells (haemolytic agent) [81-83]. It is also associated with nerve fibre depolarisation, secretion, stimulation and activation of various hormones, enzymes including leuteinizing hormone, phospholipase C and D, adenylate cyclase, protein kinase C, G-protein etc. [84-88]. Another physiological activity of melittin is that it assists in enhancement of phospholipase A2 (PLA2) activity on cell (Shier, 1979).
3.5 Apamin
Apamin of bee venom is a neuropeptide component of about 18 amino acid long sequence (-Cys-Asn-Cys-Lys-Ala-Pro-Glu-Thr-Ala-Leu-Cys-Ala-Arg-Arg-Cys-Gln-Gln-His-NH2). This peptide shows a very specific mode of action, unlike melittin, which effects various physiological activities of the cell. Apamin interacts and blocks (allosteric inhibition) the Ca2+ dependent K+ channel pores and inhibits the actions of small conductance channels (SK channels), widely expressed in the CNS. Thus, it depresses the amplitude of various after hyperpolarisation signals. These signals are important for stimulation of Ca2+ dependent SK channels and this activation is mediated by calmodulin. Excitation of SK channels plays a crucial role in functioning of different cell types, viz. muscle cells, neurons, epithelial cells, T lymphocytes etc and in blockage of hyperpolarising inhibitions, such as cholinergic, adrenergic excitations [89-99,30].
3.6 Hyaluronidase
Hyaluronidase is a venom allergen found in venom of several stinging and biting insects including bees, wasps, ants, fleas, scorpion, spider, hornet etc [100-105]. Its presence in insect venom was first reported in Phoneutria nigriventer and Lycosa raptoral in 1953, but purified and characterized from the venom of Dugesiella hentzi tarantula in the year of 1973 [106,107]. Hyaluronidase is a glycoprotein that primarily acts on hyaluronan, chondroitin sulfate, dermatan sulfate to degrade these into disaccharides and tetrasaccharides [105]. Its hydrolysing activity is enhanced and inhibited by the presence of modulators such as, histamine, anti-histamine, adrenaline, acid phosphatase, heparin, flavonoids, vitamin C etc. [108,109]. This venom glycoprotein is not toxic itself but labelled as “spreading factor” due to its role in hydrolysing the extracellular matrix opening the gap junctions, which in turn leads to enhancement of diffusion of other venom toxins in the blood circulation of their prey, which in turn increases the physiological and pathological effects of envenomation [110-115]. Moreover, it is identified as an venom allergen as it is responsible for inducing IgE mediated fatal anaphylactic shock and hypersensitivity in case of human encounter [103,110].
3.7 Ectatomin
Ectatomin is a class of novel positively charged proteins isolated from the venom of tropical ant species Ectatomma tuberculatum and E. qudridens. The toxicity level of ectatomin surpasses that of bees and solitary wasps [116]. The structural moiety of this class of venom consists of two amphiphilic homologous polypeptide chains, each consisting of 34-37 amino acid sequences with clusters of basic lysine residue. These two chains are connected by disulphide bond at the centre (between Cys22 of A and Cys20 of B chain) [117]. Two subgroups of ectatomins are Et and Eq, subdivided on the basis of position and presence or absence of intrachain disulphide bond. Ectatomin shares homology with interferon g-inducible protein (IFN-g- inducible protein) and tyrosine-related transforming proteins. It acts as a cell penetrating peptide or pore forming peptide by interacting with membrane receptors and activating a cascade of reactions (specifically protein kinase A, C, tyrosine kinase etc.). It ultimately leads to leakage of ions across membrane, lysis and cell death [118]. This cytolysin toxin is also involved in modulation of Ca2+ channel activities, which results in conformational change of Ca2+ channel and elevated calcium currents. This presumably modulates the cascade of b adrenergic signal transduction reactions [117,119,120].
3.8 Mast Cell Degranulating Peptide (MCD)
MCD is another cytolysin toxic peptide component of bee venom consisting of 22 amino acid residues, predominantly containing arginine or lysine (basic in nature). 1H-NMR analysis showed a similar built structure to apamin, of a helical chains with two disulphide bridges [121-123]. As the name indicates, MCD degranulates mast cells, releasing histamines (De Souza and Palma 2009; Nakajima, 1986). Thus, this peptide is associated with activation of histamine mediated cellular responses, viz. inflammation, reddening, pain at the site of encounter by bee stinging [124]. MCD also targets Kv channels leading prolonged action potential of neurons, hyper-excitation of central nervous system (CNS) and seizures [125,126].
3.9 Other Arthropods as Venom Source
3.9.1 Scorpion Toxin: Genus Scorpio includes one of the most potent toxin producing group of arachnids, scorpions. Scorpion venom is a deadly amalgamation of neurotoxic peptides, proteins, amines and nucleotides, which they use against prey or predators to paralyze them in instant [127,128]. This toxin primarily targets the ion channels associated with autonomic nervous system, such as, voltage gated KV, Cav, Nav, Clv channels. It forms a stable interaction with the channel proteins owing to the fact that it has highly stable three dimensional backbone with 3-4 disulphide bonds [129]. This neuropeptide blocks KV and Clv channels and also acts upon voltage sensitive Nav and Cav channels [130-132]. By affecting opening and closing of these ion channels, it prolongs depolarisation of the membranes of nerve, skeletal muscle and cardiac muscle cells, increases action potential and neurotransmitters get released. It slows down inactivation of Nav channels, which in turn results in multiple repetitive stimulus firing in motor nerves of the prey leading to immediate paralysis [133]. First isolated scorpion toxin with highly specific excitatory anti-insect property is AaH IT, which portrays highest affinity towards Nav channels of arthropods [134]. Chlorotoxin is small peptide component of scorpion venom (36 amino acid residues long) that modulates functioning of Clv channels and paralyzes normal cells in insects but not toxic towards normal human cells [135,136].
3.9.2 Spider Toxin: After insects, spiders occupy the second position of largest taxonomic group of phylum Arthropoda. Spider venom is a neurotoxic combination of different components, viz., polyamines, amines, nucleotides, ions, organic acids (primarily citric acid), neuropeptides, enzymes etc. [137,138]. Venoms of some species of spiders including Cupiennius salei and Aphonopelma hentzi contain higher concentration of potassium and lower concentration of sodium ions. This causes increased excitation and depolarisation across cell membranes, in turn leading to rapid paralysis of the victim [139]. A cytolytic agent named cupiennin 1 isolated from the venom of C. salei was reported to act synergistically with neurotoxic component CSTX-1, enhancing its toxicity [140-142]. Cationic peptide components of spider venom are cytolytic and antimicrobial in nature. These peptides bear positively charged amino acid side chains that interact with negatively charged polar heads of the phospholipids of the cell membrane. This ultimately changes the lipid bilayer membrane configuration and leads to pore formation. Venom peptides that are rich in cystine residues act as neurotoxic peptides owing to formation of stable complexes with various receptors and ion-channels of cell membranes [143]. Some ion channel blocker constituents of spider venom are namely, w-agatoxin-1a (targets on inhibition of Cav channels), m-diguetoxin-Dc1a (Nav channels blocker), k-hexatoxin-Hv1c (Kv channels blocker), p-theraphotoxin-Pc1a (blocks acid sensing ion channel) etc. [144,145].
3.9.3 Centepede Toxin: Centepedes belong to class Chilopoda of terrestrial arthropods and of predatory in nature. In this group of arthropods, the first pair of appendages are modified into poison claws or forcipules that act as piercing forceps. Venom of centipede is a neurotoxic pool of different peptides, enzymatic and non-enzymatic components, that once injected can initiate rapid paralysation of the prey [35,146]. These neurotoxic peptides principally acts on voltage gated ion- channels as well as showcases different physiological activities, viz, anticoagulantion, platelet aggregation, inhibition of trypsin activity, PLA2 activity etc. [34]. Summation of all these activities leads to cell lysis and tissue damage. Some isolated neurotoxic peptide constituents from the venom of S. subspinipes mutilans are namely, κ-SLPTX-Ssm1a (modulates Nav, Kv channels), κ-SLPTX-Ssm2a (inhibitor of Kv channels), κ-SLPTX-Ssm3a (selective inhibitor of Kv channel) etc. (Yang et al., 2012). Another two toxic peptide components called ω-SLPTX-Ssm1a and ω-SLPTX-Ssm2a are reported by Yang et al., to act on Cav channels and modulate the rate of calcium influx. Peptide toxin SsmTx-I from S. subspinipes mutilans was reported to act as KV2.1 modulator by inhibiting KV2.1 current [147].
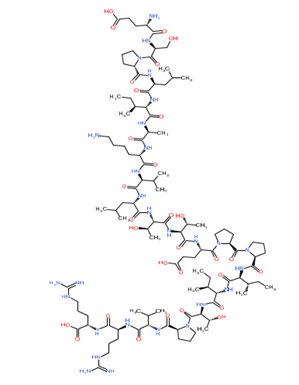
Phospholipase A
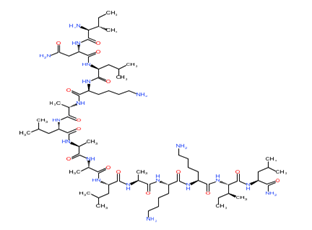
Mastoparan
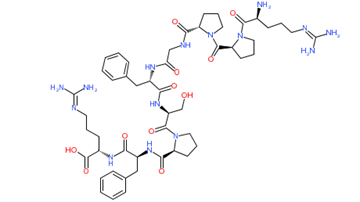
Bradykinin
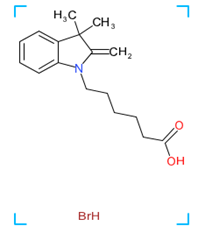
Hyaluronidase
Melittin
Apamin
Mast cell degranulating peptide
Scorpion toxin (cupiennin 1)
Figure 1: Chemical structure of different venom toxins.
4. Regulating Pathways of Different Venom Toxins
4.1 Phospholipase A2
Phospholipase A2 triggers the activation of arachidonic acid signaling pathway which leads to thromboxane A2 (TXA2) synthesis in a reaction catalyzed by thromboxane synthetase (to form TXA2) and cyclooxygenase-1 (COX-1) (to form prostaglandin G2/H2). When TXA2 is released to the bloodstream, it binds to TXA receptors present on the surface of circulating inflammatory cells, adjacent platelets, and atherosclerotic plaque components and this amplifies and perpetuates the atherothrombotic process [148].
4.2 Mastoparan
The disruption of p38 MAPK activity secondary to the disruption of G protein-coupled signaling caused by mastoparan results in decreases in both IL-6 and NF-kB reporter activities in human dermal microvessel endothelial cells and in a murine macrophage cell line [149].
4.3 Bradykinin
Kinins and their cognate receptors can take part in regulation of cell proliferation [150]. Therefore, bradykinin-mediated activation of ERK/MAP kinase pathways is well studied and several different cellular mechanisms are suggested [151]. It is observed that increase in intracellular calcium concentrations in endothelial cells, produced either by GPCR stimulation or artificially, activates tyrosine kinases and ERK/MAP kinase module [152].
4.4 Melittin
Bee venom, melittin has significant inhibitory effects on the EGF-induced invasion and migration of breast cancer cells. Also, melittin reduces the EGF-stimulated F-actin reorganization at the leading edge. Particularly, melittin inhibits the EGF-induced MMP-9 expression through blocking the PI3K/Akt/mTOR and NF-κB pathway. In addition, melittin effectively suppresses the EGF-induced FAK phosphorylation through the inhibition of mTOR/p70S6K/4E-BP1 pathway [153].
4.5 Apamin
Apamin inhibits IFN-γ- and TNF-α- induced inflammatory cytokines and chemokines through the suppression of STAT and NF-κB signaling pathway in human keratinocytes [154].
4.6 Hyaluronidase
In a non-canonical signal pathway, hyaluronidase (HYAL-2) serves as a receptor for TGF-β to signal with downstream tumor suppressors, namely SMAD4 and WWOX to control gene transcription. Cell death occurs when SMAD4 responsive element is overly driven by the HYAL-2–WWOX–SMAD4 signaling complex. In case of rats subjected to traumatic brain injury, over accumulation of a HYAL-2–WWOX complex occurs in the nucleus which causes neuronal death. Hyaluronan induces the signaling of HYAL-2–WWOX–SMAD4 and relocation of the signaling complex to the nucleus. When the signaling complex is overexpressed, WWOX-expressing cells face bubbling cell death [155].
4.7 Ectatomin
Ectatomin can get efficiently inserted into the plasma membrane, where it can form channels. Ectatomin was found to inhibit L-type calcium currents in isolated rat cardiac myocytes [117]. In these cells, ectatomin induces a gradual and irreversible increase in ion leakage across the membrane that can lead to cell death.
4.8 Mast Cell Degranulating Peptide
Mast cell degranulating peptide is the most potent peptide of naturally occurring mast cell secretagogues. It is found to stimulate the GTPase activity of G proteins (G0/Gi) in a concentration dependent manner [156].
4.9 Scorpion Toxin (Chlorotoxin)
Chlorotoxin can directly bind to ERα and change the protein secondary structure of its LBD domain, hence inhibiting the ERα signaling pathway. Vasodilator stimulated phosphoprotein (VASP) is a target gene of ERα signaling pathway. Chlorotoxin can inhibit breast cancer cell proliferation, migration, and invasion via ERα/VASP signaling pathway [157].
4.10 Spider Toxin (ω-agatoxin-1a)
Phorbol-12 myristate-13 acetate - promoted calcium influx can be inhibited by spider toxin such as ω-agatoxin-1a which is a calcium channel blocker specific for Cav2.1 channels [158].
|
Arthropod category |
Genus/Species |
Toxic component |
Chemical nature |
Nature of cellular activity |
Physiological target |
|
Wasps |
Vespula lewisii |
Mastoparan |
Peptide |
Cytolytic |
Membrane phospholipids, cell lysis |
|
Honey bee |
Apis mellifera |
Melittin |
Peptide |
Cytolytic |
Cell membrane, pore formation |
|
Apamin |
Peptide |
Cytotoxic |
Ca2+ dependent K+ channel, SK channels |
||
|
MCD |
Peptide |
Cytotoxic |
Mast cells, releases histamine |
||
|
Vespa, fire ants, bees |
Several species of Vespa, Vespula, Polybia, Polistes, Solenopsis invicta |
Phospholipase A1 |
Protein |
Cytotoxic |
Membrane glycerophospholipids |
|
Wasps, bees, centipedes |
Many species of Apis, Polybia, Agelaia, Bombus, Scolopendra genera |
Phospholipase A2 |
Protein |
Cytotoxic |
Same as PLA1 |
|
Bees, wasps, ants, fleas, scorpions, spiders, hornets |
First isolated from Dugesiella hentzi, many members of Apis, Vespa, Scorpio, Solenopsis |
Hyaluronidase |
Glycoprotein |
“Spreading factor” |
Hyaluronan, chondroitin sulfate, dermatan sulfate |
|
Ants |
Ectatomma tuberculatum |
Ectatomin |
Peptide |
Cytolytic |
Membrane receptors |
|
Paraponera clavata |
Poneratoxin |
Peptide |
Neurotoxic |
Voltage gated sodium channels |
|
|
Pachycondyla goeldii |
Ponericin |
Peptide |
Cytotoxic |
Cell membrane (pore formation) |
|
|
Wasps, bees and ants |
Several species of Vespa, Apis and Pogonomyrmex |
kinin |
Peptide |
Neurotoxic |
Central nervous system (CNS) |
|
Hornets |
Vespa crabro |
Crabrolin |
Peptide |
Cytotoxic |
Mast cell, RBCs |
|
Bumblebees |
Megabombus pennsyluanicus. |
Bombolitin |
Peptide |
Cytotoxic |
Cell membrane penetration |
|
Scorpions |
Leiurus quinquestriatus hebraeus |
Chlorotoxin |
Peptide |
Neurotoxic |
Voltage gated chloride channels |
|
Scorpions |
Pandinus imperator |
Scorpine |
Peptide |
Neurotoxic |
Voltage sensitive ion channels |
|
Blister beetle |
Cantharis vesicatoria |
Cantharidin |
Fatty terpenoid |
Cytotoxic |
Outer layer of skin, desmosomal plaque |
|
Centipede |
Scolopendra subspinipes mutilans |
SsmTx-I |
Peptide |
Neurotoxic |
Voltage gated potassium channel |
Table 1: General physiological action of some important insect venom components.
Figure 2: Biological activity of bioactive components present in insect venoms.
|
Sl. No. |
Venom |
Insect (s) |
Regulation of cell signaling |
Reference |
|
1 |
Phospholipase A2 |
Wasp, bee, scorpion, centipede |
Arachidonic acid pathway ↑ |
[148] |
|
2 |
Mastoparan |
Wasp |
G protein-coupled signaling ↓ |
[149] |
|
3 |
Bradykinin |
Wasp, ant |
ERK/MAP kinase |
[151] |
|
pathway ↑ |
||||
|
4 |
Melittin |
Bee |
PI3K/Akt/mTOR pathway ↓; NF-κB pathway ↓; mTOR/p70S6K/4E-BP1 pathway ↓ |
[153] |
|
5 |
Apamin |
Bee |
NF- κB signaling pathway ↓; STAT signaling pathway ↓ |
[154] |
|
6 |
Hyaluronidase |
Bee, wasp, ant, flea, scorpion, spider, hornet |
HYAL-2–WWOX–SMAD4 signaling ↑ |
[155] |
|
7 |
Ectatomin |
Ants |
Ion leakage across membrane ↑; L-type calcium currents in myocytes ↓ |
[117] |
|
8 |
Mast cell degranulating peptide |
Bee |
G protein-coupled signaling ↑ |
[156] |
|
9 |
Scorpion toxin (Chlorotoxin) |
Scorpion |
ERα signaling pathway ↓ |
[157] |
|
10 |
Spider toxin (ω-agatoxin-1a) |
Spider |
Calcium influx activity ↓ |
[158] |
|
11 |
Centepede toxin (κ-SLPTX-Ssm2a) |
Centepede |
Kv channel activity ↓ |
[35] |
Table 2: Cellular Regulatory pathways of different venom toxins.
(upregulation and ¯ downregulation)
5. Therapeutic Potential of Venom in Chronic Diseases
5.1 In cancer
Insect venom can play a promising role to tackle the growing burden of cancer, as they are packed with target specific bioactive components. Many of the insect venom principles show affinity towards membrane phospholipids expressed on cancer cell and membrane receptors. They exert cytotoxicity and cell lysis. This property can be useful in targeting cancer cells and development of novel target specific drugs. Mastoparan, one key component of wasp venom, mediates cancer cell cytotoxicity and death by binding with anionic phospholipids expressed on the membrane of cancer cells. This interaction leads to pore formation and leakage of vital cell organelles out of the cell. It also affects the mitochondrial membrane permeability transition causing swelling and ruptures outer and partially inner mitochondrial membranes [63]. Mastoparan induces release of LDH from leukemia cells, suggestive of the fact that it is an anti-cancer peptide (ACP) with lytic property [159]. Report has shown that peritumoral mastoparan administration in murine melanoma model delayed tumor growth by activating apoptosis [160]. Similar potential is seen in case of bee venom peptides. Melittin acts as a surfactant, binds with membrane phospholipids and disrupts the bilayer leading to exocytosis. It inhibits calcium binding protein calmodulin and restricts the growth of leukemic cells [161]. It shows anti-tumor potential by blocking COX-2 and VEGFR-2 mediated MAPK signaling pathway [162]. Solenopsin, isolated from fire ant S. invicta shows anti-angiogenic property and inhibits PI3K signaling [163]. Huh et al. reported that bee venom can downregulate vascular endothelial growth factor (VEGFR-2) signalling pathway, in turn inhibits tumour proliferation, angiogenesis and metastasis. A potent anti-cancer agent isolated from Chinese blister beetle (Mylabris phalerata), called cantharidin can arrest cell cycle in G2/M phase and induces apoptosis in T 24 and HT 29 cell lines (Huan et al., 2006). It also suppresses pro-tumor autophagy and induces cell death in triple negative breast cancer cell lines [164]. BmHYA1, a hyaluronidase from Buthus martensi showed anti-proliferative activity by reducing expression of CD44 variant in breast cancer cell line [165]. Various potent anti-cancer agents are also reported in the venom of scorpion Buthus martensii Karsch, such as BmK AGAP, BmKCT, that targets cancer signaling pathways, viz., Bcl-2, NFkB, MAPK pathways and inhibits NaV and chloride current in cancer cell lines (Zhao et al., 2011; Fu et al., 2007; Fan et al., 2010).
5.2 In Rheumatoid Arthritis (RA)
RA is a chronic auto-immune disorder characterised by inflammation, joint pain, redness and ankylosis, which can lead to deformities and permanent disability. Hydrolysis of glycerophospholipids by PLA2 releasing lysophospholipids, precursor of eicosanoids. Eicosanoid level elevation in circulation is related to patho-physiologicial conditions as pain, inflammation etc. Blocking the voltage sensitive ion channels (such as NaV, CaV, acid sensitive ion channels) and receptors (e.g., purinergic receptors) associated with nociception can reduce the pain and inflammatory condition of RA. Analgesic effects of bee venom components (melittin and apamin) are already reported in a number of research works [166-168]. These bio-actives can inhibit enzymatic activity of sPLA2 and reduce inflammation [169,170]. Nipate et al. in 2015, evaluated anti-arthritic, anti-inflammatory property of Indian honey bee (Apis dorsata) venom in FCA and CIA-induced rat arthritic model. Random clinical trials on using bee venom acupuncture in treating RA showed improvement of joint pain and swelling in RA patients [171]. Huwentoxin-I and Huwentoxin-IV toxins isolated from Ornithoctonus huwena, inhibits voltage sensitive sodium channels (NaV1.7) and tetrodotoxin- sensitive channels [172]. These channels are directly related to depolarisation of nerve fibres associated with nociception. μ-scoloptoxin-Ssm6a, a toxic principle isolated from S. subspinipes mutilans, inhibits NaV1.7 channels portraying anti-nociceptive potential against formalin-induced pain in rat model, which is many fold higher than morphine [173]. SsmTx-I is another active component from S. mutilans that showed anti-inflammatory property by blocking voltage gated potassium channels Kv2.1 in murine model system [147].
5.3 In diabetes
Type I diabetes, also called diabetes mellitus is an autoimmune disorder characterized by impairment of insulin action and secretion and hyperglycemia whereas, type II diabetes, known as diabetes insipidus is related to dietary habits and resistance towards insulin action as well as hyperglycemia. Active toxic component of wasp venom, mastoparan has been reported to increase insulin release on glucose augmentation from pancreatic islet cell of human [174]. SsmTx-I, from Scolopendra subspinipes mutilans, can specifically blocks voltage gated potassium channels (KV2.1) found in pancreatic b cells and stimulates insulin secretion [147]. Hassan et al. in 2019 reported that bee venom components also possess the property to suppress plasma glucose level and increases insulin level in albino rat model system. Active toxic component GxTX-1 from the venom of tarantula Plesiophrictus guangxiensis, inhibits delayed rectifier potassium current in b cells and increased glucose stimulated secretion of insulin (Herrington et al., 2006).
5.4 In asthma
Asthma is a chronic disorder characterized by inflammation, narrowing of lungs airways and excessive mucous production. It can be life threatening by making the patient experience difficulty in breathing and shortness of breath. This pathophysiological condition is related to infiltration of CD4 T cells that expresses IL-2 and IL-17 cytokines responsible for inflammatory condition of asthma. This proliferation of type 2 helper T cells (Th2) occurs due to exposure to allergens. Bee venom reportedly decreased the levels of Th2 cytokines and serum IgE levels in ovalbumin induced asthma in mice (Choi et al., 2013). Maurotoxin isolated from Scorpio maurus palmatus has been found to inhibit intermediate conductance channel, KCa channel, in human T lymphocyte cells in concentration dependent manner [175]. T lymphocyte cell activation is modulated by KCa channels [176], hence blocking these channels can reduce expression of inflammatory cytokines.
5.5 Other Chronic and Genetic Disorders
A potent toxin GsMtx-4, isolated from venom of tarantula Grammostola spatulate, has shown inhibitory actions on mechanosensitive ion channels as well as supressed arterial fibrillation, indicating anti-arrhythmic property [177]. Another toxic component PhKv isolated from the venom of Brazilian spider Phoneutria nigriventer, has shown remarkable ability to reduce nociception by blocking AchE activity and inactivating cholinergic transmission in mice model [178]. This property might play important role in treating Alzheimer disease. PhTx4-5-5 toxin from the venom of same spider species showed neuro-protective activity by inhibiting glutamate excitotoxicity responsible for cell death in neurons of mice [179]. Bee venom and its component apamin can significantly increase striatal dopamine level and decreasing MPTP-induced neuron cell loss. Thus it acts as neuro-protective agent against Parkinson disease [180]. It is also excellent in reducing the serum nitrate, TNFa levels and suppressing multiple sclerosis symptoms in experimental encephalomyelitic rat model [181]. Apamin also exhibits anti-atherosclerotic property in lipopolysaccharide- induced atherosclerotic mice model by inhibiting macrophage apoptosis and suppressing expressions of members of Bcl2 family, Cyctochrome C, caspase 3 and PARP [182]. Proulx et al. in 2019 reported that apamin can boost nicotinic excitation improving cognitive function and attention acquisition in transgenic mice model system, which is indicative of a novel anti-Alzheimer agent. FrPbAII and Parawixin 10 are two toxins isolated from venom of Brazilian spider Parawixia bistriata have shown remarkable neuroprotective abilities in treating retinal glaucoma in rat [183,184].
|
Venom component |
Experimental model |
Physiological role |
Therapeutic potential |
References |
|
Melittin |
Hodgkin Lymphoma (HL) cell lines KM-H2 and L-428 |
Plays synergistic role with cisplatin |
Anti-neoplastic |
[185] |
|
Melittin |
Human hepatocellular carcinoma cell lines: SMMC-7721 and BEL-7402 |
Activates CaMKII-TAK1-JNK/p38 and inhibits IkBa kinase- NFkB |
Anti- timour |
[186] |
|
Melittin |
Caki-1, Caski, SK-BR-3 cell lines |
Inhibits matrix metalloproteinase-9 (MMP-9) gene expression by blocking activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB) |
Anti- metastatic |
[187] |
|
Melittin |
human breast carcinoma MCF-7 cell line |
Downregulation of cluster of differentiation (CD)147 and (MMP-9) expressions |
Anti-invasive and anti-metastatic |
Wang et al., 2016 |
|
Melittin |
Human peripheral blood leukocytes |
Inhibits neutrophil O2- production. |
Anti-inflammatory |
[188] |
|
Melittin-like peptide 101 |
In vitro: LNCaP-LN3, DU-145, C3 cell lines |
Peptide 101-immunoconjugates showed more affinity towards cell binding and cell killing, delaying tumor growth |
Cytotoxic, anti- tumor |
[189] |
|
*** |
||||
|
Melittin |
Human HCC cells (BEL-7402) |
Apoptosis and growth arrest, up-regulates Fas expression |
Anti-proliferative |
[190] |
|
Melittin |
human acute T lymphocyte leukemia cell line 6T-CEM |
Apoptosis, cell death |
Cytotoxic, anti-proliferative |
[191] |
|
Ad-rAFP-Mel (Recombinant adenovirus carrying melittin gene) |
In vitro: BEL-7402 cell line In vivo: BALB/c-nu/nu athymic mice (male) |
Reduced rate of tumorigenicity and detection of significant anti-neoplastic effect |
Anti-neoplastic |
[192] |
|
Melittin |
Murine leukemic lymphocyte cell lines (L1210 and L5178Y) and human promyelocytic leukemic granulocyte cell line (HL-60) |
Calmodulin inhibition, inhibition of cell growth and clonogenicity |
Anti-proliferative |
[193] |
|
Melittin |
Ras transformed cells |
Hyper-activation of PLA2 and enhanced Ca2+ influx |
Anti-neoplastic |
[194] |
|
Bee venom (whole) |
Patients with sepsis from Intensive Care Unit |
Decreases generation of inducible Nitic Oxide (NO) synthase and TNFa |
Anti-arthritic |
[195] |
|
Bee venom (Water soluble sub fractionated part) |
J774A.1 (mouse macrophage cell line), A549 ( human airway epithelial cell line), U937 (human myelomonocytic cell line) |
Inhibits COX2 activity, pro-inflammatory cytokines: TNF-a and IL-1b |
Anti-arthritic |
[196] |
|
Bee venom (whole) |
Male Lewis rats, Charles River CD strain male rats |
Suppression of adjuvant arthritis and carrageenan induced paw edema in time and dose dependent manner |
Anti-arthritic |
[197] |
|
Bee venom (Apis mellifera) |
Albino rats (male) |
Decreases plasma glucose level and increases plasma insulin level |
Ant-diabeteic |
[198] |
|
Adolapin |
Rats |
Anti-inflammatory activity in carrageenan, prostaglandin (PG) and adjuvant-induced paw edema by cyclo-oxygenase, PG synthesis inhibitory activities |
Anti-arthritic |
[199] |
|
Bee venom |
Lewis rats (male and female) |
Depression of cytochrome p450 level and Ethylmorphine N-demethylase activity |
Anti-arthritic |
[200] |
|
Bee venom (whole) (Apis mellifera) |
Sprague-Dawley rats (male) |
Decrease in erosions of articular cartilage and infiltration of inflammatory cells in adjuvant-induced hind paw arthritis |
Anti-arthritic |
[201] |
|
Apamin |
AML12 cell line |
Suppresses epithelial mesenchymal transition induced by TGF-b1 and inhibits smad dependent/ independent signalling pathways |
Anti- hepato fibrotic |
[202] |
|
Apamin |
HaCaT (human keratinocyte cell line) |
Inhibits NF-kB and JAK/STAT signaling pathways; suppresses inflammatory chemokines and cytokines |
Anti- inflammatory |
[203] |
|
Apamin |
Transgenic TgCRND8 mice |
Improves cognitive function and attention acquisition |
Anti-Alzheimer agent |
[204] |
|
Apamin |
In vitro: THP-1 (human monocyte cell line) In vivo: C57BL/6 mice (male) |
Inhibits apoptosis of macrophages by suppressing levels of Bcl-2 family, cytochrome-c, caspase-3 and PARP |
Anti-atherosclerotic |
[182] |
|
Apamin |
In vivo: HSC-T6 ( rat hepatic stellate cell line) In vitro: C57BL/6 mice (male) |
Attenuates IL-6, IFN-γ, TNF-α, IL-1β expressions and inhibits HSC activation by Smad signaling pathway |
Anti-cholestatic |
[203] |
|
Apamin |
C57/Bl6 mice (male) |
Decreases MPTP-induced dopamine neuron cell loss and increases striatal dopamine levels |
Anti-parkinsonian agent |
[180] |
|
Solenopsin (Solenopsis invicta) |
Transgenic zebrafish Fli-EGFP (embryos) |
Inhibits PI3K activation and delayed angiogenesis |
Anti-angiogenic |
[163] |
|
Cantharidin (Lytta vesicatoria) |
CCRF-CEM (leukemia cell line) and its sub-lines: CEM/ADR5000, CEM/VLB100, CEM/E1000 |
Induces apoptosis of multidrug resistant cells by p53 dependent mechanism |
Anti-cancer |
[205] |
|
Cantharidin (Mylabris phalerata) |
T 24 (human bladder carcinoma) and HT 29 (human colon carcinoma) cell lines |
Arrests cell cycle at G2/M phase and induces apoptosis |
Cytotoxic, anti-cancer |
[206] |
|
Cantharidin |
In vitro: MDA-MB-231, MDA-MB-468 (human breast cancer cell lines) In vivo: BALB/c nude mice |
Reduces cell viability in dose dependent manner, induces apoptosis, suppresses pro-tumor autophagy |
Apoptotic |
[207] |
|
Scorpion venom (Heterometrus bengalensis Koch) |
U937 and K562 (human leukemia cell lines) |
Arrests cell cycle, induces apoptosis by membrane blebbing and DNA damage |
Anti-proliferative |
[208] |
|
Chlorotoxin |
Various human and animal cell lines |
Tumour-specific binding with glioma cells, inhibits cell invasion |
Anti-metastatic |
[209] |
|
Maurotoxin (Scorpio maurus palmatus) |
B82 (mouse fibroblast cells) and Ovary cells of Chinese hamster |
Inhibition of intermediate conductance subclass of KCa channels |
Anti-asthmatic |
[175] |
|
Hyaluronidase BmHYA1 (Buthus martensi) |
MDA-MB-231 (Breast cancer cell line) |
Reduces CD44 variant expression |
Anti-tumor |
[165] |
|
Hyaluronidase (Tityus serrulatus) |
C57BL6/6 mice |
Reduces bleomycin induced pulmonary fibrosis by decreasing collagen deposition and TGFb expression |
Anti-fibrotic |
[210] |
|
Mastoparan |
In vito: Jurkat, THP-1 (human leukaemia cell line), and HOPC (murine myeloma cells line) |
Induces cell death in concentration dependent manner, reduces tumour growth and acts synergistically with chemotherapeutic drug (gemcitabine) |
Anti-cancer |
[159] |
|
In vivo: T41 mammary carcinoma induced immune competent mice |
||||
|
All-D Mastoparan M |
Colo 225, KB, Hep-2, H226Br and HeLa cell lines |
Inhibits tumor growth by direct lysis of cancer cells |
Cytolytic, anti-tumor |
[211] |
|
Mitoparan and analogues |
U373MG and ECV304 cell lines |
Causes DNA fragmentation, modulates mitochondrial membrane permeability, initiates apoptosis |
Apoptotic |
[212] |
|
Mastoparan |
In vitro: B16F10, B16F10-Nex2, A2058, SiHa, Jurkat, MCF-7, MDA-MB-231, U87, SK-BR-3, Melan-a cell lines In vivo: C57BL/6 mice (male) |
Increases ROS level and decreases pro-caspases-3, 9 and 12, leading intrinsic pathway of cell death |
Anti-tumor, apoptotic |
[160] |
|
Mastoparan |
Sprague-Dawley rats (male) Human pancreatic islet cells |
Many fold increase in mastopran-induced insulin release over glucose augmentation |
Anti-hyper glycemic, anti-diabetic |
[174] |
|
SsmTx-I (Scolopendra subspinipes mutilans L. Koch) |
Sprague–Dawley rats |
Blocks voltage sensitive potassium channel (Kv 2.1) |
Anti-asthmatic, anti-diabetic, anti- inflammatory |
[147] Herrington et al., 2006 |
|
Ssm6a (S. subspinipes mutilans ) |
In vitro: HEK293T cell line In vivo: mice model |
Blocks voltage gated sodim channel (NaV1.7) and pain reliving effectiveness is manyfold higher than morphine |
Antinociceptive, analgesic |
[173] |
|
Scolopendrasin VII (S. subspinipes mutilans ) |
U937 and Jurkat cell lines |
Reduces viability of cancer cells, induces necrosis by interacting with membrane phosphatidylserine |
Anti- cancer |
[213] |
|
Centipede venom (Scolopendra viridicornis) |
In vitro: Hep 3B, HBL-100, IMR-32, HEL 92.1.7, ACHN In vivo: Swiss albino mice |
Substantially decreases tumor growth |
Anti-tumor |
[214] |
|
S. subspinipes mutilans extract |
A375 cell line |
Arrests cell cycle and promotes cell death, decreases Bcl-2, increases Bak, Bax and Bad expressions |
Apoptotic |
[215] |
|
Psalmotoxin 1 (Psalmopoeus cambridgei) |
Rats |
Blocks ASIC1a (acid sensing ion channel), opioid activity similar to morphine |
Antinociceptive |
[216] |
|
β-TRTX-Gr1b (Grammostola spatulata) |
Rats |
Interacts with Cav channels, relieves pain |
Analgesic |
[217] |
|
GsMtx-4 (Grammostola spatulata) |
Rabbit |
Inhibits mechanosensitive channels, suppresses atrial fibrillation |
Antiarrhythmic |
[177] |
***In vivo: LN3 or DU-145 human CaP cell xenografts established in Athymic BALB/c nu/nu (nude) male mice
Table 3: Therapeutic potential of whole venom or venom bioactive components in chronic diseases.
6. Venom- a Future Prospective of Drug Discovery
Despite being a source of paramount of bioactive principles with diverse physiological properties and actions, only a few drugs have been developed from insect venoms that are under clinical and pre-clinical trials. Most of the toxin based approved drugs are derived from the venoms of snake, frog, cone snail and puffer fish (viz. Haemocaogulaseâ from Bothrops atox moojeni, ABT-594 from Epipedobates tricolor) [23]. There are ample of active insect-venom components yet to be explored and documented, that may play crucial role in discovery of potential modern drugs for the treatment of chronic diseases, including cancer. Furthermore, emphasis is given only on more stable venom peptides while selecting for drug designing, such as, presence of Disulphide Bridge in the core peptide. This provides the venom peptides or proteins, the ability to withstand proteolytic digestion and penetration through physiological shuttles or barriers, e.g., blood-brain barrier [218]. Attention is also required on the mode of delivery and bio-availability of the designed drugs. Oral administration of toxin derived drugs designed from large molecular weight proteins is more likely to be a poor option due to the chance of getting digested by proteolytic enzymes. An alternative to improve stability of venom derived drugs is replacement of disulphide bond configuration with diselenide bonds by incorporating Selenocysteine residues [219,220]. Being isosteric in nature with disulphide bonds and less reactive towards biological reductions, diselenide bonds architecture is of highly stable nature [221,222]. Another issue associated with venom derived drug discovery is that their half-life is poor in human gastric juice and serum. This creates a limitation regarding high drug clearance by liver and kidney [223,224]. It can be overcome by using carrier proteins or conjugation with non-immunogenic polyethylene glycol (PEG) [225,226]. Some of insect venom derived drugs under clinical or pre-clinical trials are listed in table 3.
|
Venom protein or analogue |
Source |
Mode of drug delivery |
Trial stage |
Refrences |
|
Chlorotoxin (Conjugated with Cy5.5) |
Scorpion (Leiurus quinquestriatus) |
Intravenous (parenteral) |
Clinical phase I |
[227,228] |
|
131I-TM-601 (radiolabelled chlorotoxin) |
Scorpion (Leiurus quinquestriatus) |
Injection on tumor site (intracavitary) |
Clinical phase II |
[228,229] |
|
Tozuleristide (BLZ-100) |
Scorpion (Leiurus quinquestriatus) |
Intravenous |
Clinical phase I |
[230] |
|
Bee venom (whole) Alyostal STâ |
Apis mellifera |
Sub-cutaneous |
Clinical phase II |
[231] |
|
ARC-520 (melittin) |
Honey bee |
Intravenous |
Terminated at phase II |
[232] |
|
BZ371 |
Armed spider P. nigriventer |
Topical application |
Ist clinical trial concluded |
[233-240] |
Table 4: Drugs derived from insect venom under clinical trials.
7. Conclusion
Exploring venomics is like exploring the treasure house of novel therapeutics. Despite large number of research works completed on insect venom and their therapeutical potentials, few works have been published on practical application as bio-available drugs. Thorough optimization on some issues, such as, their mode of action, route of delivery, side effects and safety, can lead to find out plausible compositions of highly selective target- specific drugs. This area needs more focus and attention to meet success in combating chronic diseases as well as cancer.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
Authors are highly grateful to the Head, Cell and Molecular Biology Division, Department of Zoology, Cotton University for providing the lab facilities to carry out the work.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ding J, Chua PJ, Bay BH, et al. Scorpion venoms as a potential source of novel cancer therapeutic compounds. Experimental biology and medicine 239 (2014): 387-393.
- Boulidam S. Edible insects in a Lao market economy. Edible forest insects (2010): 131-141.
- Mitsuhashi J. Insects as traditional foods in Japan. Ecology of Food and Nutrition 36 (1997): 187-199.
- Mercer CWL. Sago grub production in Labu swamp near Lae, Papua New Guinea. Klinkii 5 (1994): 30-34.
- Jeong H, Kim JM, Kim B, et al. Nutritional value of the larvae of the alien invasive wasp Vespa velutina nigrithorax and amino acid composition of the larval saliva. Foods 9 (2020): 885.
- Zhou XH, Yang D, Zhang JH, et al. Purification and N-terminal partial sequence of anti-epilepsy peptide from venom of the scorpion Buthus martensiiBiochemical Journal, 257 (1989): 509-517.
- Shao J, Kang N, Liu Y, et al. Purification and characterization of an analgesic peptide from Buthus martensii Biomedical Chromatography 21(2007): 1266-1271.
- Huis AV, Itterbeeck JV, Klunder H, et al. Insects: Future prospects for food and feed security. FAO Forestry paper 171 (2013).
- Bequaert JC. Insects as food: how they have augmented the food supply of mankind in early and recent times. American Museum of Natural History (1921).
- Bergier E. Peuples entomophages et insectes comestibles: étude sur les moeurs de l'homme et de l'insecte. N. Boubée (1986).
- Chavanduka DM. Insects as a source of protein to the Africain. The Rhodesia Science News 9 (1976): 217-220.
- Roulon-Doko P. Chasse, cueillette et cultures chez les Gbaya de Centrafrique l'Harmattan. (1998): 540.
- Paoletti MG, Buscardo E, Vanderjagt DJ, et al. Nutrient content of termites (Syntermes soldiers) consumed by Makiritare Amerindians of the Alto Orinoco of Venezuela. Ecology of Food and Nutrition 42 (2003): 177-191.
- Aggarwal BB, Sethi G, Baladandayuthapani V, et al. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. Journal of cellular biochemistry 102 (2007): 580-592.
- Shanmugam MK, Kannaiyan R, Sethi G. Targeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancer. Nutrition and cancer 63 (2011): 161-173.
- Kirtonia A, Gala K, Fernandes SG, et al. Repurposing of drugs: an attractive pharmacological strategy for cancer therapeutics. In Seminars in cancer biology. Academic Press 68 (2021): 258-278.
- Monisha J, Padmavathi G, Roy NK, et al. NF-κB blockers gifted by Mother Nature: Prospectives in cancer cell chemosensitization. Current pharmaceutical design 22 (2016): 4173-4200.
- Merarchi M, Sethi G, Shanmugam M, et al. Role of natural products in modulating histone deacetylases in cancer. Molecules 24 (2019): 1047.
- Trim SA, Trim CM. Venom: The sharp end of pain therapeutics. British journal of pain 7 (2013): 179-188.
- Monge-Fuentes V, Arenas C, Galante P, et al. Arthropod toxins and their antinociceptive properties: From venoms to painkillers. Pharmacology & therapeutics 188 (2018): 176-185.
- Escoubas P, King GF. Venomics as a drug discovery platform Expert Rev. (2009).
- Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nature reviews drug discovery 2 (2003): 790-802.
- Fox JW, Serrano SM. Approaching the golden age of natural product pharmaceuticals from venom libraries: an overview of toxins and toxin-derivatives currently involved in therapeutic or diagnostic applications. Current pharmaceutical design 13 (2007): 2927-2934.
- King GF. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert opinion on biological therapy 11 (2011): 1469-1484.
- Konno K, Kazuma K, Nihei KI. Peptide toxins in solitary wasp venoms. Toxins 8 (2016):114.
- Casewell NR, Wüster W, Vonk FJ, et al. Complex cocktails: the evolutionary novelty of venoms. Trends in ecology & evolution 28 (2013): 219-229.
- Pratheeshkumar P, Sreekala C, Zhang Z, et al. Cancer prevention with promising natural products: mechanisms of action and molecular targets. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 12 (2012): 1159-1184.
- Ménez A, Stocklin R, Mebs D. 'Venomics' or: The venomous systems genome project. Toxicon (Oxford) 47 (2006): 255-259.
- Escoubas P, Quinton L, Nicholson GM. Venomics: unravelling the complexity of animal venoms with mass spectrometry. Journal of mass spectrometry 43 (2008): 279-295.
- Moreno M, Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins 7 (2015): 1126-1150.
- Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Bioscience reports 27 (2007): 189-223.
- Damianoglou A, Rodger A, Pridmore C, et al. The synergistic action of melittin and phospholipase A2 with lipid membranes: development of linear dichroism for membrane-insertion kinetics. Protein and peptide letters 17 (2010): 1351-1362.
- Rocha T, de Souza BM, Palma MS, et al. Myotoxic effects of mastoparan from Polybia paulista (Hymenoptera, Epiponini) wasp venom in mice skeletal muscle. Toxicon 50 (2007): 589-599.
- Hakim MA, Yang S, Lai R. Centipede venoms and their components: resources for potential therapeutic applications. Toxins 7 (2015): 4832-4851.
- Yang S, Liu Z, Xiao Y et al. Chemical punch packed in venoms makes centipedes excellent predators. Molecular & Cellular Proteomics 11 (2012): 640-650.
- Liu ZC, Zhang R, Zhao F, et al. Venomic and transcriptomic analysis of centipede Scolopendra subspinipes dehaani. Journal of proteome research 11 (2012): 6197-6212.
- Kong Y, Shao Y, Chen H, et al. A novel factor Xa-inhibiting peptide from centipede’s venom. International journal of peptide research and therapeutics 19 (2013): 303-311.
- Ozaki Y, Matsumoto Y, Yatomi Y, et al. Mastoparan, a wasp venom, activates platelets via pertussis toxin-sensitive GTP-binding proteins. Biochemical and biophysical research communications 170 (1990): 779-785.
- McIntosh JM, Ghomashchi F, Gelb MH, et al. Conodipine-M, a Novel Phospholipase A2 Isolated from the Venom of the Marine Snail Conus magus. Journal of biological chemistry 270 (1995): 3518-3526.
- Fry BG, Roelants K, Champagne DE, et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annual review of genomics and human genetics 10 (2009): 483-511.
- Undheim EA, Fry BG, King GF. Centipede venom: recent discoveries and current state of knowledge. Toxins 7 (2015): 679-704.
- Dabral D, van den Bogaart G. The Roles of Phospholipase A2 in Phagocytes. Frontiers in Cell and Developmental Biology 9 (2021):1423.
- Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nature Reviews Immunology 15 (2015): 511-523.
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual review of biochemistry 69 (2000): 145-182.
- Kühn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Progress in lipid research 45 (2006): 334-356.
- Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nature Reviews Immunology 2 (2002): 787-795.
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nature reviews Drug discovery 13 (2014): 533-548.
- Zambelli VO, Gross ER, Chen CH, et al. Aldehyde dehydrogenase-2 regulates nociception in rodent models of acute inflammatory pain. Science translational medicine 6 (2014): 251ra118-251ra118.
- Zambelli VO, Picolo G, Fernandes CA, et al. Secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins 9 (2017): 406.
- Darwish DA, Masoud HM, Abdel-Monsef MM, et al. Phospholipase A2 enzyme from the venom of Egyptian honey bee Apis mellifera lamarckii with anti-platelet aggregation and anti-coagulation activities. Journal of Genetic Engineering and Biotechnology 19 (2021): 1-8.
- Li D, Lee Y, Kim W, et al. Analgesic effects of bee venom derived phospholipase A2 in a mouse model of oxaliplatin-induced neuropathic pain. Toxins 7 (2015): 2422-2434.
- Perez-Riverol A, Lasa AM, dos Santos-Pinto JRA, et al. Insect venom phospholipases A1 and A2: Roles in the envenoming process and allergy. Insect biochemistry and molecular biology 105 (2019): 10-24.
- Katsu T, Kuroko M, Morikawa T, et al. Interaction of wasp venom mastoparan with biomembranes. Biochimica et Biophysica Acta (BBA)-Biomembranes 1027 (1990): 185-190.
- Hirai Y, Yasuhara T, Yoshida H, et al. A new mast cell degranulating peptide" mastoparan" in the venom of Vespula lewisii. Chemical and Pharmaceutical Bulletin 27 (1979): 1942-1944.
- Kurihara H, Kitajima K, Senda T, et al. Multigranular exocytosis induced by phospholipase A 2-activators, melittin and mastoparan, in rat anterior pituitary cells. Cell and tissue research 243 (1986): 311-316.
- Kuroda Y, Yoshioka M, Kumakura K, et al. Effects of Peptides on the Release of Catecholamines and Adenine Nucleotides from Cultured Adrenal Chromaffin Cells Mastoparan-Induced Release. Proceedings of the Japan Academy, Series B 56 (1980): 660-664.
- Higashijima T, Uzu S, Nakajima T, et al. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). Journal of Biological Chemistry 263 (1988): 6491-6494.
- Sukumar M, Higashijima T. G protein-bound conformation of mastoparan-X, a receptor-mimetic peptide. Journal of Biological Chemistry, 267 (1992): 21421-21424.
- Yokokawa N, Komatsu M, Takeda T, et al. Mastoparan, a wasp venom, stimulates insulin release by pancreatic islets through pertussis toxin sensitive GTP-binding protein. Biochemical and biophysical research communications 158 (1989): 712-716.
- Joyce-Brady M, Rubins JB, Panchenko MP, et al. Mechanisms of mastoparan-stimulated surfactant secretion from isolated pulmonary alveolar type 2 cells. Journal of Biological Chemistry 266 (1991): 6859-6865.
- Vitale N, Mukai H, Rouot B, et al. Exocytosis in chromaffin cells. Possible involvement of the heterotrimeric GTP-binding protein Gi. Journal of Biological Chemistry 268 (1993): 14715-14723.
- Perianin AXEL, Snyderman RALPH. Mastoparan, a wasp venom peptide, identifies two discrete mechanisms for elevating cytosolic calcium and inositol trisphosphates in human polymorphonuclear leukocytes. The Journal of Immunology 143 (1989): 1669-1673.
- Yamamoto T, Ito M, Kageyama K, et al. Mastoparan peptide causes mitochondrial permeability transition not by interacting with specific membrane proteins but by interacting with the phospholipid phase. The FEBS journal 281 (2014): 3933-3944.
- Gil J, Higgins T, Rozengurt E. Mastoparan, a novel mitogen for Swiss 3T3 cells, stimulates pertussis toxin-sensitive arachidonic acid release without inositol phosphate accumulation. The Journal of cell biology 113 (1991): 943-950.
- Schachter M, Thain EM. Chemical and pharmacological properties of the potent, slow contracting substance (kinin) in wasp venom. British journal of pharmacology and chemotherapy 9 (1954): 352.
- Kishimura H, Yasuhara T, Yoshida H, et al. & Nakajima T. Vespakinin-M, a novel bradykinin analogue containing hydroxyproline, in the venom of Vespa mandarinia Smith. Chemical and Pharmaceutical Bulletin 24 (1976): 2896-2897.
- Griesbacher T, Legat FJ. Effects of FR173657, a non-peptide B2 antagonist, on kinin-induced hypotension, visceral and peripheral oedema formation and bronchoconstriction. British journal of pharmacology 120 (1997): 933-939.
- Nakajima T. Pharmacological biochemistry of vespid venoms. Venoms of hymenoptera (1986).
- Piek T. Neurotoxic kinins from wasp and ant venoms. Toxicon 29 (1991): 139-149.
- Yesuhara T, Yoshida H,Nakajima T. Chemical Investigation of the Hornet (Vespa xanthoptera Cameron)) Venom. The structure of a new Bradykinin analogue" Vespakinin-X". Chemical and Pharmaceutical Bulletin 25 (1977): 936-941.
- Hue B, Piek T. Effects of kinins and related peptides on synaptic transmission in the insect CNS. Neurotox 88 (1988): 27-33.
- Hue B, Piek, T. Irreversible presynaptic activation-induced block of transmission in the insect CNS by hemicholinium-3 and threonine-6-bradykinin. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 93 (1989): 87-89.
- Piek T, Buitenhuis A, Simonthomas RT, et al. Smooth muscle contracting compounds in the venom of Megascolia flavifrons (Hym: Scoliidae) with notes on the stinging behaviour. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 75 (1983): 145-152.
- Piek T, Hue B, Mantel P, et al. Pharmacological characterization and chemical fractionation of the venom of the ponerine ant, Paraponera clavata (F.). Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 99 (1901): 481-486.
- Piek T, Duval A, Hue B, et al. Poneratoxin, a novel peptide neurotoxin from the venom of the ant, Paraponera clavata. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 99 (1991): 487-495.
- Schachter M, Thain EM. Chemical and pharmacological properties of the potent, slow contracting substance (kinin) in wasp venom. British journal of pharmacology and chemotherapy 9 (1954): 352.
- Terwilliger TC, Eisenberg D. The structure of melittin. I. Structure determination and partial refinement. Journal of Biological Chemistry 257 (1982): 6010-6015.
- Terwilliger TC, Eisenberg D. The structure of melittin. II. Interpretation of the structure. Journal of Biological Chemistry 257 (1982): 6016-6022.
- Terwilliger TC, Weissman L, Eisenberg D. The structure of melittin in the form I crystals and its implication for melittin's lytic and surface activities. Biophysical journal 37 (1982): 353-361.
- Dawson CR, Drake AF, Helliwell J, et al. The interaction of bee melittin with lipid bilayer membranes. Biochimica et Biophysica Acta (BBA)-Biomembranes 510 (1978): 75-86.
- Habermann Bee and wasp venoms. The biochemistry and pharmacology of their peptides and enzymes are reviewed. Science 177 (1972): 314-322.
- Tosteson MT, Holmes SJ, Razin M, et al. Melittin lysis of red cells. The Journal of membrane biology 87 (1985): 35-44.
- DeGrado WF, Musso GF, Lieber M, et al. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophysical journal 37 (1982): 329-338.
- Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. The Journal of membrane biology 156 (1997): 197-211.
- Kiesel L, Rabe T, Hauser G, et al. Stimulation of luteinizing hormone release by melittin and phospholipase A2 in rat pituitary cells. Molecular and cellular endocrinology 51 (1987): 1-6.
- Haase I, Czarnetzki BM, Rosenbach T. Thrombin and melittin activate phospholipase C in human HaCaT keratinocytes. Experimental dermatology 5 (1996): 84-88.
- Knowles BH, Farndale RW. Activation of insect cell adenylate cyclase by Bacillus thuringiensis δ-endotoxins and melittin. Toxicity is independent of cyclic AMP. Biochemical journal 253 (1988): 235-241.
- Mau SE, Vilhardt H. Cross talk between substance P and melittin-activated cellular signaling pathways in rat lactotroph-enriched cell cultures. Journal of neurochemistry 69 (1997): 762-772.
- Habermann E. Apamin. Pharmacology & therapeutics 25 (1984): 255-270.
- Strong PN. Potassium channel toxins. Pharmacology & therapeutics 46 (1990): 137-162.
- Strong PN, Brewster BS. Apamin: A Probe for Small-Conductance, Calcium-Activated Potassium Channels. In Methods in Neurosciences.Academic Press 8 (1992): 15-24.
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends in neurosciences 19 (1996): 150-154.
- Pease JH, Wemmer DE. Solution structure of apamin determined by nuclear magnetic resonance and distance geometry. Biochemistry 27 (1988): 8491-8498.
- Lazdunski M. Apamin, a neurotoxin specific for one class of Ca2+-dependent K+ channels. Cell calcium 4 (1983): 421-428.
- Lazdunski M, Fosset M, Hugues M, et al. The Apamin-Sensitive Ca 2+-Dependent K+ Channel. Molecular Properties, Differentiation, and Endogeneous Ligands in Mammalian Brain. Neurobiochemistry (1985): 164-171.
- Lamy C, Goodchild SJ, Weatherall KL, et al. Allosteric block of KCa2 channels by apamin. Journal of Biological Chemistry 285 (2010): 27067-27077.
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annual review of physiology 74 (2012): 245-269.
- Begenisich T, Nakamoto T, Ovitt CE, et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. Journal of Biological Chemistry 279 (2004): 47681-47687.
- Schumacher MA, Rivard AF, Bächinger HP, et al. Structure of the gating domain of a Ca 2+-activated K+ channel complexed with Ca 2+/calmodulin. Nature 410 (2001): 1120-1124.
- Jacomini DLJ, Pereira FDC, dos Santos Pinto JRA, et al. Hyaluronidase from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae): Cloning, structural modeling, purification, and immunological analysis. Toxicon 64 (2013): 70-80.
- Pessini AC, Takao TT, Cavalheiro EC, et al. A hyaluronidase from Tityus serrulatus scorpion venom: isolation, characterization and inhibition by flavonoids. Toxicon 39 (2001): 1495-1504.
- Kemeny DM, Dalton N, Lawrence AJ, et al. The purification and characterisation of hyaluronidase from the venom of the honey bee, Apis mellifera. European journal of biochemistry 139 (1984): 217-223.
- Lu G, Kochoumian L. Sequence Identity and Antigenic Cross-reactivity of White Face Hornet Venom Allergen, Also a Hyaluronidase, with Other Proteins. Journal of Biological Chemistry 270 (1995): 4457-4465.
- Nagaraju S, Devaraja S, Kemparaju K. Purification and properties of hyaluronidase from Hippasa partita (funnel web spider) venom gland extract. Toxicon 50 (2007): 383-393.
- Bordon KC, Wiezel GA, Amorim FG, et al. Arthropod venom Hyaluronidases: biochemical properties and potential applications in medicine and biotechnology. Journal of Venomous Animals and Toxins including Tropical Diseases 21 (2015): 4-4.
- Kaiser E. Trypsin and hyaluronidase inhibitor of human serum; the inhibition of the proteolytic and hyaluronic acid cleavage enzymes of snake and spider venoms by human serum. Biochemische Zeitschrift 324 (1953): 344-350.
- Schanbacher FL, Lee CK, Wilson IB, et al. Purification and characterization of tarantula, Dugesiella hentzi (girard) venom Hyaluronidase. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 44 (1973): 389-396.
- Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer letters 131 (1998): 3-11.
- Li MW, Yudin AI, VandeVoort CA, et al. Inhibition of monkey sperm hyaluronidase activity and heterologous cumulus penetration by flavonoids. Biology of reproduction 56 (1997): 1383-1389.
- Kolarich D, Léonard R, Hemmer W, et al. The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. The FEBS journal 272 (2005): 5182-5190.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life sciences 80 (2007): 1921-1943.
- Morey SS, Kiran KM, Gadag JR. Purification and properties of hyaluronidase from Palamneus gravimanus (Indian black scorpion) venom. Toxicon 47 (2006): 188-195.
- Poh CH, Yuen R, Chung MC, et al. Purification and partial characterization of hyaluronidase from stonefish (Synanceja horrida) venom. Comparative biochemistry and physiology. B, Comparative biochemistry 101 (1992): 159-163.
- Duran-Reynals F. Studies on a certain spreading factor existing in bacteria and its significance for bacterial invasiveness. The Journal of experimental medicine 58 (1933): 161.
- Tu AT, Hendon RR. Characterization of lizard venom hyaluronidase and evidence for its action as a spreading factor. Comparative biochemistry and physiology. B, Comparative biochemistry 76 (1983): 377-383.
- Pluzhnikov KA, Shevchenko LV, Grishin EV. Ant polypeptide toxins. Methods and Tools in Biosciences and Medicine. Animal Toxins: Facts and Protocols, ed. by H. Rochat and M.-F. Martin-Eauclaire © 2000 Birkhauser Verlag Basel/Switzerland. (2000): 90-98.
- Pluzhnikov K, Nosyreva E, Shevchenko L, et al. Analysis of ectatomin action on cell membranes. European journal of biochemistry 262 (1999): 501-506.
- Pluzhnikov KA, Nolde DE, Tertishnikova SM, et al. Structural and functional-studies of toxic principle of ectatomma-tuberculatum ant venom. Bioorganicheskaya Khimiya 20 (1994): 857-871.
- Yoshida A, Takahashi M, Nishimura S, et al. Cyclic AMP-dependent phosphorylation and regulation of the cardiac dihydropyridine-sensitive Ca channel. FEBS letters 309 (1992): 343-349.
- Dolphin AC. Facilitation of Ca2+ current in excitable cells. Trends in neurosciences 19 (1996): 35-43.
- Kumar NV, Wemmer DE, Kallenbach NR. Structure of P401 (mast cell degranulating peptide) in solution. Biophysical chemistry 31 (1988): 113-119.
- Dohtsu K, Okumura K, Hagiwara KI, et al. Isolation and sequence analysis of peptides from the venom of Protonectarina sylveirae (hymenoptera-vespidae). Natural toxins, 1 (1993): 271-276.
- Argiolas A, Pisano JJ. Bombolitins, a new class of mast cell degranulating peptides from the venom of the bumblebee Megabombus pennsylvanicus. Journal of Biological Chemistry 260 (1985): 1437-1444.
- De Souza BM, Palma MS. Peptides from Hymenoptera Venoms: Biochemistry, pharmacology and potential applications in health and biotechnology. Animal Toxins: The State of Art. Perspectives on Health and Biotechnology (2009): 273-297.
- Simmons MA. Mast Cell Degranulating Peptide. xPharm: The Comprehensive Pharmacology Reference (2007): 1-3.
- Mourre C, Lazdunski M, Jarrard LE. Behaviors and neurodegeneration induced by two blockers of K+ channels, the mast cell degranulating peptide and Dendrotoxin I. Brain research 762 (1997): 223-227.
- Miranda F, Kupeyan C, Rochat H, et al. Purification of animal neurotoxins: isolation and characterization of eleven neurotoxins from the venoms of the scorpions Androctonus australis Hector, Buthus occitanus tunetanus and Leiurus quinquestriatus quinquestriatus. European journal of biochemistry 16 (1970): 514-523.
- Martin-Eauclaire MF, Rochat H. Purification and characterization of scorpion toxins acting on voltage-sensitive Na+ channels. In Animal Toxins, Birkhäuser, Basel (2000): 152-168.
- Hmed BN, Serria HT, Mounir ZK. Scorpion peptides: potential use for new drug development. Journal of toxicology (2013).
- Smith JJ, Jones A, Alewood PF. Mass landscapes of seven scorpion species: The first analyses of Australian species with 1, 5-DAN matrix. Journal of venom research 3 (2012): 7.
- Petricevich VL. Scorpion venom and the inflammatory response. Mediators of inflammation (2010).
- Possani LD, Merino E, Corona M, et al. Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 82 (2000): 861-868.
- Zlotkin E, Miranda F, Lissitzky S. Proteins in scorpion venoms toxic to mammals and insects. Toxicon 10 (1972): 207-209.
- De Dianous S, Hoarau F, Rochat H. Re-examination of the specificity of the scorpion Androctonus australis Hector insect toxin towards arthropods. Toxicon 25 (1987): 411-417.
- DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. American journal of physiology-cell physiology 264 (1993): C361-C369.
- Dardevet L, Rani D, Aziz TAE, et al. Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 7 (2015):1079-1101.
- Kuhn-Nentwig L, Stöcklin R, Nentwig W. Venom composition and strategies in spiders: is everything possible? Advances in insect physiology 40 (2011): 1-86.
- Langenegger N, Nentwig W, Kuhn-Nentwig L. Spider venom: components, modes of action, and novel strategies in transcriptomic and proteomic analyses. Toxins 11 (2019): 611.
- Wullschleger B, Nentwig W, Kuhn-Nentwig L. Spider venom: enhancement of venom efficacy mediated by different synergistic strategies in Cupiennius salei.Journal of experimental biology 208 (2005): 2115-2121.
- Wullschleger B, Kuhn-Nentwig L, Tromp J, et al. CSTX-13, a highly synergistically acting two-chain neurotoxic enhancer in the venom of the spider Cupiennius salei (Ctenidae). Proceedings of the National Academy of Sciences 101 (2004): 11251-11256.
- Kuhn-Nentwig L, Muller J, Schaller J, et al. Cupiennin 1, a new family of highly basic antimicrobial peptides in the venom of the spider Cupiennius salei (Ctenidae). Journal of Biological Chemistry 277 (2002): 11208-11216.
- Kuhn-Nentwig L, Schaller J, Nentwig W. Biochemistry, toxicology and ecology of the venom of the spider Cupiennius salei (Ctenidae). Toxicon 43 (2004): 543-553.
- Kuhn-Nentwig L, Stöcklin R, Nentwig W. Venom composition and strategies in spiders: is everything possible? Advances in insect physiology 40 (2011): 1-86.
- Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430 (2004): 232-235.
- Mazzuca M, Heurteaux C, Alloui A, et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nature neuroscience 10 (2007): 943-945.
- González-Morales L, Diego-García E, Segovia L, et al. Venom from the centipede Scolopendra viridis Say: Purification, gene cloning and phylogenetic analysis of a phospholipase A2. Toxicon 54 (2009): 8-15.
- Chen M, Li J, Zhang F, et al. Isolation and characterization of SsmTx-I, a Specific Kv2. 1 blocker from the venom of the centipede Scolopendra Subspinipes Mutilans Koch. Journal of Peptide Science 20 (2014): 159-164.
- Badimon L. Pathogenesis of ST-Elevation Myocardial Infarction. In Coronary Microvascular Obstruction in Acute Myocardial Infarction. Academic Press (2018): 1-13.
- Lentschat A, Karahashi H, Michelsen KS, et al. Mastoparan, a G protein agonist peptide, differentially modulates TLR4-and TLR2-mediated signaling in human endothelial cells and murine macrophages. The Journal of Immunology 174 (2005): 4252-4261.
- Sethi T, Rozengurt E. Multiple neuropeptides stimulate clonal growth of small cell lung cancer: effects of bradykinin, vasopressin, cholecystokinin, galanin, and neurotensin. Cancer research 51(1991): 3621-3623.
- Blaukat A. Structure and signalling pathways of kinin receptors. Andrologia 35 (2003): 17-23.
- Fleming I, Fisslthaler B, Busse R. Calcium signaling in endothelial cells involves activation of tyrosine kinases and leads to activation of mitogen-activated protein kinases. Circulation research 76 (1995): 522-529.
- Jeong YJ, Choi Y, Shin JM, et al. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 68 (2014): 218-225.
- Kim WH, An HJ, Kim JY, et al. Apamin inhibits TNF-α- and IFN-γ-induced inflammatory cytokines and chemokines via suppressions of NF-κB signaling pathway and STAT in human keratinocytes. Pharmacological reports: PR 69 (2017): 1030-1035.
- Hsu LJ, Chiang MF, Sze CI, et al. HYAL-2–WWOX–SMAD4 signaling in cell death and anticancer response. Frontiers in cell and developmental biology 4 (2016): 141.
- Watt AP. Mast cells and peptide induced histamine release. Inflammopharmacology 9 (2001): 421-434.
- Wang Y, Li K, Han S, et al. Chlorotoxin targets ERα/VASP signaling pathway to combat breast cancer. Cancer medicine 8 (2019): 1679-1693.
- Andrews DA, Yang L, Low PS. Phorbol ester stimulates a protein kinase C-mediated agatoxin-TK-sensitive calcium permeability pathway in human red blood cells. Blood 100 (2002): 3392-3399.
- Hilchie AL, Sharon AJ, Haney EF, et al. Mastoparan is a membranolytic anti-cancer peptide that works synergistically with gemcitabine in a mouse model of mammary carcinoma. Biochimica Et Biophysica Acta (BBA)-Biomembranes 1858 (2016): 3195-3204.
- de Azevedo RA, Figueiredo CR, Ferreira AK, et al. Mastoparan induces apoptosis in B16F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Peptides 68 (2015): 113-119.
- Hait WN, Grais L, Benz C, et al. Inhibition of growth of leukemic cells by inhibitors of calmodulin: phenothiazines and melittin. Cancer chemotherapy and pharmacology 14 (1985): 202-205.
- Huh JE, Kang JW, Nam D, et al. Melittin suppresses VEGF-A-induced tumor growth by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway. Journal of natural products 75 (2012): 1922-1929.
- Arbiser JL, Kau T, Konar M, et al. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood 109 (2007): 560-565.
- Li HC, Xia ZH, Chen YF, et al. Cantharidin inhibits the growth of triple-negative breast cancer cells by suppressing autophagy and inducing apoptosis in vitro and in vivo. Cellular Physiology and Biochemistry 43 (2017): 1829-1840.
- Feng L, Gao R, Gopalakrishnakone P. Isolation and characterization of a hyaluronidase from the venom of Chinese red scorpion Buthus martensi. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 148 (2008): 250-257.
- Son DJ, Lee JW, Lee YH, et al. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacology & therapeutics 115 (2007): 246-270.
- Moon DO, Park SY, Lee KJ, et al. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. International immunopharmacology 7 (2007): 1092-1101.
- Nah SS, Ha E, Mun SH, et al. Effects of melittin on the production of matrix metalloproteinase-1 and-3 in rheumatoid arthritis fibroblast-like synoviocytes. Journal of pharmacological sciences 106 (2008): 162-166.
- Saini SS, Peterson JW, Chopra AK. Melittin binds to secretory phospholipase A2and inhibits its enzymatic activity. Biochemical and Biophysical Research Communications 238 (1997): 436-442.
- Nipate SS, Hurali PB, Ghaisas MM. Evaluation of anti-inflammatory, anti-nociceptive, and anti-arthritic activities of Indian Apis dorsata bee venom in experimental animals: biochemical, histological, and radiological assessment. Immunopharmacology and immunotoxicology 37 (2015): 171-184.
- Lee JA, Son MJ, Choi J, et al. Bee venom acupuncture for rheumatoid arthritis: a systematic review of randomised clinical trials. BMJ open 4 (2014): e006140.
- Xiao Y, Bingham J P, Zhu W, et al. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain ii voltage sensor in the closed configuration. Journal of Biological Chemistry 283 (2008): 27300-27313.
- Yang S, Xiao Y, Kang D, et al. Discovery of a selective NaV1. 7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proceedings of the National Academy of Sciences 110 (2013): 17534-17539.
- Straub SG, James RF, Dunne MJ, et al. Glucose augmentation of mastoparan-stimulated insulin secretion in rat and human pancreatic islets. Diabetes 47 (1998): 1053-1057.
- Castle NA, London DO, Creech C, et al. Maurotoxin: a potent inhibitor of intermediate conductance Ca2+-activated potassium channels. Molecular pharmacology 63 (2003): 409-418.
- Rader RK, Kahn LE, Anderson GD, et al. T cell activation is regulated by voltage-dependent and calcium-activated potassium channels. The Journal of Immunology 156 (1996): 1425-1430.
- Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature 409 (2001): 35-36.
- Rigo FK, Rossato MF, Trevisan G, et al. PhKv a toxin isolated from the spider venom induces antinociception by inhibition of cholinesterase activating cholinergic system. Scandinavian journal of pain 17 (2017): 203-210.
- Silva FR, Batista EM, Gomez MV, et al. The Phoneutria nigriventer spider toxin, PnTx4-5-5, promotes neuronal survival by blocking NMDA receptors. Toxicon 112 (2016): 16-21.
- Alvarez-Fischer D, Noelker C, Vulinovi? F, et al. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. Plos one 8 (2013): e61700.
- Karimi A, Ahmadi F, Parivar K, et al. Effect of honey bee venom on lewis rats with experimental allergic encephalomyelitis, a model for multiple sclerosis. Iranian journal of pharmaceutical research: IJPR 11 (2012): 671.
- Kim SJ, Park JH, Kim KH, et al. Apamin inhibits THP-1-derived macrophage apoptosis via mitochondria-related apoptotic pathway. Experimental and molecular pathology 93 (2012): 129-134.
- Beleboni RO, Guizzo R, Fontana ACK, et al. Neurochemical characterization of a neuroprotective compound from Parawixia bistriata spider venom that inhibits synaptosomal uptake of GABA and glycine. Molecular pharmacology 69 (2006): 1998-2006.
- Fachim HA, Mortari MR, Gobbo-Netto L, et al. Neuroprotective activity of parawixin 10, a compound isolated from Parawixia bistriata spider venom (Araneidae: Araneae) in rats undergoing intrahippocampal NMDA microinjection. Pharmacognosy magazine 11 (2015): 579.
- Kreinest T, Volkmer I, Staege MS. Melittin Increases Cisplatin Sensitivity and Kills KM-H2 and L-428 Hodgkin Lymphoma Cells. International Journal of Molecular Sciences 22 (2021): 343.
- Wang C, Chen T, Zhang N, et al. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IκBα kinase-NFκB. Journal of Biological Chemistry 284 (2009): 3804-3813.
- Park JH, Jeong YJ, Park KK, et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-κB and AP-1-dependent MMP-9 expression. Molecules and cells 29 (2010): 209-215.
- Somerfield SD, Stach JL, Mraz C, et al. Bee venom melittin blocks neutrophil O 2− production. Inflammation 10 (1986): 175-182.
- Russell PJ, Hewish D, Carter T, et al. Cytotoxic properties of immunoconjugates containing melittin-like peptide 101 against prostate cancer: in vitro and in vivoCancer Immunology, Immunotherapy 53 (2004): 411-421.
- Li B, Gu W, Zhang C, et al. arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Oncology Research and Treatment 29 (2006): 367-371.
- Huang XQ, Ling CQ, Chen Z, et al. Apoptosis of human acute T lymphocyte leukemia cell line 6T-CEM induced by melittin. J Anhui TCM College 20 (2001): 39-41.
- Ling CQ, Li B, Zhang C, et al. Inhibitory effect of recombinant adenovirus carrying melittin gene on hepatocellular carcinoma. Annals of oncology 16 (2005): 109-115.
- Hait WN, Grais L, Benz C, et al. Inhibition of growth of leukemic cells by inhibitors of calmodulin: phenothiazines and melittin. Cancer chemotherapy and pharmacology 14 (1985): 202-205.
- Sharma SV. Melittin-induced hyperactivation of phospholipase A2 activity and calcium influx in ras-transformed cells. Oncogene 8 (1993): 939-947.
- Green JA, Smith GM, Buchta R, et al. Circulating phospholipase A 2 activity associated with sepsis and septic shock is indistinguishable from that associated with rheumatoid arthritis. Inflammation 15 (1991): 355-367.
- Nam KW, Je KH, Lee JH, et al. Inhibition of COX-2 activity and proinflammatory cytokines (TNF-α and IL-1β) production by water-soluble sub-fractionated parts from bee (Apis mellifera) venom. Archives of Pharmacal Research 26 (2003): 383-388.
- Chang YH, Bliven ML. Anti-arthritic effect of bee venom. Agents and actions 9 (1979): 205-211.
- Hassan AK, El-kotby DA, Tawfik MM, et al. Antidiabetic effect of the Egyptian honey bee (Apis mellifera) venom in alloxan-induced diabetic rats. The Journal of Basic and Applied Zoology 80 (2019): 1-9.
- Shkenderov S, Koburova K. Adolapin-a newly isolated analgetic and anti-inflammatory polypeptide from bee venom. Toxicon 20 (1982): 317-321.
- Eiseman JL, Von Bredow J, Alvares AP. Effect of honeybee (Apis mellifera) venom on the course of adjuvant-induced arthritis and depression of drug metabolism in the rat. Biochemical pharmacology 31 (1982): 1139-1146.
- Kang SS, Pak SC, Choi SH. The effect of whole bee venom on arthritis. The American journal of Chinese medicine 30 (2002): 73-80.
- Lee WR, Kim KH, An HJ, et al. Apamin inhibits hepatic fibrosis through suppression of transforming growth factor β1-induced hepatocyte epithelial–mesenchymal transition. Biochemical and biophysical research communications 450 (2014): 195-201.
- Kim WH, An HJ, Kim JY, et al. Apamin inhibits TNF-α-and IFN-γ-induced inflammatory cytokines and chemokines via suppressions of NF-κB signaling pathway and STAT in human keratinocytes. Pharmacological Reports 69 (2017): 1030-1035.
- Proulx É, Power SK, Oliver DK, et al. Apamin improves prefrontal nicotinic impairment in mouse model of Alzheimer’s disease. Cerebral Cortex 30 (2020): 563-574.
- Rauh R, Kahl S, Boechzelt H, et al. Molecular biology of cantharidin in cancer cells. Chinese Medicine 2 (2007): 1-9.
- Huan SKH, Lee HH, Liu DZ, et al. Cantharidin-induced cytotoxicity and cyclooxygenase 2 expression in human bladder carcinoma cell line. Toxicology 223 (2006): 136-143.
- Li HC, Xia ZH, Chen YF, et al. Cantharidin inhibits the growth of triple-negative breast cancer cells by suppressing autophagy and inducing apoptosis in vitro and in vivo. Cellular Physiology and Biochemistry 43 (2017): 1829-1840.
- Gupta SD, Debnath A, Saha A, et al. Indian black scorpion (Heterometrus bengalensis Koch) venom induced antiproliferative and apoptogenic activity against human leukemic cell lines U937 and K562. Leukemia research 31 (2007): 817-825.
- Lyons SA, O'Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 39 (2002): 162-173.
- Bitencourt CS, Pereira PA, Ramos SG, et al. Hyaluronidase recruits mesenchymal-like cells to the lung and ameliorates fibrosis. Fibrogenesis & tissue repair 4 (2011): 1-14.
- Wu TM, Li ML. The cytolytic action of all-D mastoparan M on tumor cell lines. International journal of tissue reactions 21 (1999): 35-42.
- Jones S, Martel C, Belzacq-Casagrande AS, et al. Mitoparan and target-selective chimeric analogues: membrane translocation and intracellular redistribution induces mitochondrial apoptosis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1783 (2008): 849-863.
- Lee JH, Kim IW, Kim SH, et al. Anticancer activity of the antimicrobial peptide scolopendrasin VII derived from the centipede, Scolopendra subspinipes mutilans. Journal of microbiology and biotechnology 25 (2015): 1275-1280.
- Bhagirath T, Chingtham B, Mohen Y. Venom of a hill centipede Scolopendra viridicornis inhibits growth of human breast tumor in mice. Indian journal of pharmacology 38 (2006): 291.
- Ma W, Liu R, Qi J, et al. Extracts of centipede Scolopendra subspinipes mutilans induce cell cycle arrest and apoptosis in A375 human melanoma cells. Oncology letters 8 (2014): 414-420.
- Mazzuca M, Heurteaux C, Alloui A, et al. A tarantula peptide against pain via ASIC1a channels and opioid mechanisms. Nature neuroscience 10 (2007): 943-945.
- Lampe RA. Analgesic peptides from the venom of Grammostola spatulata. U.S. Patent 5,877,026, 2 March 1999.
- Tedford HW, Sollod BL, Maggio F, et al. Australian funnel-web spiders: master insecticide chemists. Toxicon 43 (2004): 601-618.
- Muttenthaler M, Alewood PF. Selenopeptide chemistry. Journal of peptide science: an official publication of the European Peptide Society 14 (2008): 1223-1239.
- Mobli M, de Araújo AD, Lambert LK, et al. Direct visualization of disulfide bonds through diselenide proxies using 77Se NMR spectroscopy. Angewandte Chemie International Edition 48 (2009): 9312-9314.
- Armishaw CJ, Daly NL, Nevin ST, et al. alpha-Selenoconotoxins, a new class of potent alpha7 neuronal nicotinic receptor antagonists. The Journal of Biological Chemistry 281 (2006): 36-43.
- Moroder L. Isosteric replacement of sulfur with other chalcogens in peptides and proteins. Journal of peptide science: an official publication of the European Peptide Society 11 (2005): 187-214.
- Clark RJ, Jensen J, Nevin ST, et al. The engineering of an orally active conotoxin for the treatment of neuropathic pain. Angewandte Chemie International Edition 49 (2010): 6545-6548.
- Norton RS. µ-Conotoxins as leads in the development of new analgesics. Molecules 15 (2010): 2825-2844.
- Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino acids 30 (2006): 351-367.
- Sato AK, Viswanathan M, Kent RB, et al. Therapeutic peptides: technological advances driving peptides into development. Current opinion in biotechnology 17 (2006): 638-642.
- Veiseh M, Gabikian P, Bahrami SB, et al. Tumor paint: a chlorotoxin: Cy5. 5 bioconjugate for intraoperative visualization of cancer foci. Cancer research 67 (2007): 6882-6888.
- Kesavan K, Ratliff J, Johnson EW, et al. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. Journal of Biological Chemistry 285 (2010): 4366-4374.
- Hockaday DC, Shen S, Fiveash J, et al. Imaging glioma extent with 131I-TM-601. J. Nucl. Med. 46 (2005): 580-586.
- Butte PV, Mamelak A, Parrish-Novak J, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg. Focus 36 (2014): E1.
- Hartmann A, Mullner J, Meier N, et al. Bee venom for the treatment of Parkinson disease - A randomized controlled clinical trial. PloS One 11 (2016): e0158235.
- Yuen M, Schiefke I, Yoon J, et al. Ahn, S. H., Heo, J., Kim, J. H. et al. RNA Interference Therapy with ARC-520 Results in Prolonged HBsAg Response in Patients with Chronic Hepatitis B Infection. Hepatology (2019).
- Biozeus Biopharmaceutical SA. First clinical trial sponsored by Biozeus concluded! (2018).
- Dabral D, Coorssen JR. Phospholipase A2: Potential roles in native membrane fusion. The international journal of biochemistry & cell biology 85 (2017): 1-5.
- Pfeiffer DR, Gudz TI, Novgorodov, SA, et al. The Peptide Mastoparan is a potent facilitator of the mitochondrial permeability transition. Journal of Biological Chemistry 270 (1995): 4923-4932.
- Dudkin SM, Mikchaylova, LI, Severin Jr ES. Mechanisms of cyclic AMP phosphodiesterase regulation. Advances in enzyme regulation 21 (1983): 333-352.
- Shier WT. Activation of high levels of endogenous phospholipase A2 in cultured cells. Proceedings of the National Academy of Sciences 76 (1979): 195-199.
- Buku A. Mast cell degranulating (MCD) peptide: a prototypic peptide in allergy and inflammation. Peptides 20 (1999): 415-420.
- Nakajima T In: Piek, T. editor. Venoms of the Hymenoptera: pharmacology and biochemistry of vespide venoms. London, United Kingdom: Academic Press (1986): 309-327.
- Palma MS. Hymenoptera insect peptides. Handbook of biologically active peptides (2013): 416-422.
