Inhibition of Radioactive Depletion of Hemocytes and Antitumor Effects of Flavonoid
Article Information
Yeun-Hwa Gu1*, Takenori Yamashita2, Tota Inoue3, Ki-Mun Kang4
1Department of Radiological Science, Faculty of Health Science, Junshin Gakuen University, Fukuoka, Japan
2Department of Radiological Science, Faculty of Health Science, Suzuka University of Medical Science, Mie, Japan
3Mie breathing swallowing rehabilitation clinic, Mie, Japan
4Department of Radiation Oncology, Gyeongsang National University School of Medicine and Gyeongsang National University Changwon Hospital, Changwon, South Korea
*Corresponding Author: Dr. Yeun-Hwa Gu, Department of Radiological Science, Faculty of Health Science, Junshin Gakuen University, 1-1-1 chikushigaoka, Minami-ku, Fukuoka, Japan
Received: 16 December 2020; Accepted: 08 January 2021; Published: 14 January 2021
Citation:
Yeun-Hwa Gu, Takenori Yamashita, Tota Inoue, Ki-Mun Kang. Inhibition of Radioactive Depletion of Hemocytes and Antitumor Effects of Flavonoid. Archives of Clinical and Medical Case Reports 5 (2021): 118-128.
View / Download Pdf Share at FacebookAbstract
Natural products are able to inhibit radiation effects and exert an antitumor effect with fewer adverse reactions; however, their antitumor effects are less than those of widely-used synthetic drugs. Flavonoid is a natural material that has attracting attention, and we extracted this material with alcohol and investigated the effect of continuous flavonoid administration on radioactivity-induced reduction of hemocytes, in addition to the antioxidant and antitum or effects of flavonoid. Following a 1-week adjustment period, flavonoid was administered intraperitoneally to male ICR mice at a dose of 100 mg/kg every other day for 2 weeks.
Following administration, 2 Gy whole-body irradiation was performed and the counts of leukocytes, lymphocytes, and granulocytes and monocytes in the peripheral blood were determined 1, 3, 7, 15 and 30 days after irradiation. These cells were considered since they are closely associated with immunity to radioactivity. In a second experiment, flavonoid was similarly administered to the mice for 2 weeks after a 1-week adjustment period, and 2 Gy whole-body irradiation was performed. The antioxidant effects in hemocytes were then investigated using 2,2'-azobis (2-amidinopropane) dihydrochloride (AAPH), a radical generator.
In a third experiment, 1×106 Sarcoma-180 cells were inoculated into the right thigh of mice, which were divided into four groups: control, flavonoid-treated, 6 Gy irradiated and Flavonoid-treated + 6 Gy irradiated groups, and changes in tumor size were measured for 20 days. Statistical analysis was conducted using ANOVA for multiple groups. In the three experiments, administration of Flavonoid inhibited the reduction of hemocytes caused by whole-body irradiation, showed antioxidant effects against radioactivity, and inhibited tumor growth, respectively. In conclusion, our data suggest that the anti
Keywords
Radiation protection; Anti-tumor effects; Lymphocyte; Anti-oxident effect
Radiation protection articles; Anti-tumor effects articles; Lymphocyte articles; Anti-oxident effect articles
Radiation protection articles Radiation protection Research articles Radiation protection review articles Radiation protection PubMed articles Radiation protection PubMed Central articles Radiation protection 2023 articles Radiation protection 2024 articles Radiation protection Scopus articles Radiation protection impact factor journals Radiation protection Scopus journals Radiation protection PubMed journals Radiation protection medical journals Radiation protection free journals Radiation protection best journals Radiation protection top journals Radiation protection free medical journals Radiation protection famous journals Radiation protection Google Scholar indexed journals Radiation articles Radiation Research articles Radiation review articles Radiation PubMed articles Radiation PubMed Central articles Radiation 2023 articles Radiation 2024 articles Radiation Scopus articles Radiation impact factor journals Radiation Scopus journals Radiation PubMed journals Radiation medical journals Radiation free journals Radiation best journals Radiation top journals Radiation free medical journals Radiation famous journals Radiation Google Scholar indexed journals Anti-tumor effects articles Anti-tumor effects Research articles Anti-tumor effects review articles Anti-tumor effects PubMed articles Anti-tumor effects PubMed Central articles Anti-tumor effects 2023 articles Anti-tumor effects 2024 articles Anti-tumor effects Scopus articles Anti-tumor effects impact factor journals Anti-tumor effects Scopus journals Anti-tumor effects PubMed journals Anti-tumor effects medical journals Anti-tumor effects free journals Anti-tumor effects best journals Anti-tumor effects top journals Anti-tumor effects free medical journals Anti-tumor effects famous journals Anti-tumor effects Google Scholar indexed journals tumor effects articles tumor effects Research articles tumor effects review articles tumor effects PubMed articles tumor effects PubMed Central articles tumor effects 2023 articles tumor effects 2024 articles tumor effects Scopus articles tumor effects impact factor journals tumor effects Scopus journals tumor effects PubMed journals tumor effects medical journals tumor effects free journals tumor effects best journals tumor effects top journals tumor effects free medical journals tumor effects famous journals tumor effects Google Scholar indexed journals Lymphocyte articles Lymphocyte Research articles Lymphocyte review articles Lymphocyte PubMed articles Lymphocyte PubMed Central articles Lymphocyte 2023 articles Lymphocyte 2024 articles Lymphocyte Scopus articles Lymphocyte impact factor journals Lymphocyte Scopus journals Lymphocyte PubMed journals Lymphocyte medical journals Lymphocyte free journals Lymphocyte best journals Lymphocyte top journals Lymphocyte free medical journals Lymphocyte famous journals Lymphocyte Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Anti-oxident effect articles Anti-oxident effect Research articles Anti-oxident effect review articles Anti-oxident effect PubMed articles Anti-oxident effect PubMed Central articles Anti-oxident effect 2023 articles Anti-oxident effect 2024 articles Anti-oxident effect Scopus articles Anti-oxident effect impact factor journals Anti-oxident effect Scopus journals Anti-oxident effect PubMed journals Anti-oxident effect medical journals Anti-oxident effect free journals Anti-oxident effect best journals Anti-oxident effect top journals Anti-oxident effect free medical journals Anti-oxident effect famous journals Anti-oxident effect Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals immunotherapy articles immunotherapy Research articles immunotherapy review articles immunotherapy PubMed articles immunotherapy PubMed Central articles immunotherapy 2023 articles immunotherapy 2024 articles immunotherapy Scopus articles immunotherapy impact factor journals immunotherapy Scopus journals immunotherapy PubMed journals immunotherapy medical journals immunotherapy free journals immunotherapy best journals immunotherapy top journals immunotherapy free medical journals immunotherapy famous journals immunotherapy Google Scholar indexed journals tomography articles tomography Research articles tomography review articles tomography PubMed articles tomography PubMed Central articles tomography 2023 articles tomography 2024 articles tomography Scopus articles tomography impact factor journals tomography Scopus journals tomography PubMed journals tomography medical journals tomography free journals tomography best journals tomography top journals tomography free medical journals tomography famous journals tomography Google Scholar indexed journals
Article Details
1. Introduction
Flavonoid is a natural material collected by bees from the skin, sap, and bud and so on of the tree. It is known that flavonoid has contained various elements, polyphenoles, flavonoids, phenolic acid and their esters, caffeic acid and their esters and phenolic aldehydes and ketones, respectively [1]. It has been used in folk medicine. In present studies, flavonoid has been shown various pharmacological effects including anti-inflammatory [2-5], antimicrobial [6], antioxidant [7, 8], immunostimulatory, antihyperglycemic and antitumor activities [9]. Some the influences on the radiation that uses the flavonoid [10] and the researches of the antitumor effect are performed. This time, we report that effects of blood cell and antioxidant for whole-body irradiation of mice and influence of Sarcoma-180 antitumor in vivo using flavonoid.
2. Material and Methods
2.1 Flavonoid
Lumps of crude flavonoid were powdered and mixed with 2l of alcohol for 2 hours. Then the suspended material was separated by centrifugation at 4.5 ´ 104 rpm for 10 min. The resulting pellet was extracted with 2l of alcohol and the material again centrifuged. The combined supernatants were passed through filter paper and freeze-dried to produce flavonoid. Favonoid was suspended in saline and administered to mice at a does of 100 mg/kg every other day. Flavonoid administration continued until the end of the experiments.
2.2 Animals
Five-week-old mail ICR mice with a mean weight of 18-20 g were purchased from Japan SLC Inc. and kept under standard conditions (room temperature 22 ± 3ºC, humidity 60%) with free access to food (CA-1, Japan Clare, Inc.) and drinking alcohol. The mice were acclimated to the breeding and experimental environment for 1 week prior to the experiments (Figure 1).
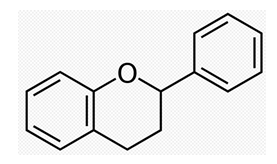
Figure 1: Structural formula of flavonoid basic skeleton flavan.
2.3 Irradiation
X-ray irradiation was administered to each mouse using an X-ray generator designed for animal use (Phillips, Inc.). The table was rotated at a constant speed so that these mice were irradiated evenly. The conditions for irradiation were: source voltage, 200 kV; rate of radiation, 0.35 Gy/min; and supplemental filter, 0.1 mm Cu + 1 mm Al.
2.4 Measurement of peripheral blood cell count
Mice were divided in 4 groups, control, flavonoid, irradiation alone and combined with flavonoid and irradiation. After acclimation, control and irradiation alone group of mice were i.p. injected with saline, flavonoid and combined with flavonoid and irradiation group of mice were injected with flavonoid 2 weeks of every other day. Irradiation groups had 2 Gy whole body irradiation and 10 μl of peripheral blood was collected with capillary tube from tail vein then counted with an automated blood cell counter (Celltac-α MEK-6318, Nippon Koden Inc.). The numbers of peripheral leukocytes, lymphocytes, granulocytes and monocytes, which all have relatively high sensitivity to radiation and primary cells of the immune system, were counted. Measurements were done at 1 day before irradiation and at 1 day, 3 days, 7 days, 15 days and 30 days after irradiation.
2.5 Chemiluminescence measurement
After acclimation, mice were divided in 4 groups same as the experiment of blood cell count. FLAVONOID was injected to mice for 2 weeks. After 2 Gy whole-body irradiation, whole blood was collected from mouse hearts by puncture with 23-G needle under anesthesia, mixed with heparin, and either centrifuged (10 min at 1200 rpm 2ºC) to separate blood plasma. Blood plasma was suspended 1:100 in PBS to 200 μl then added 200 μl of AAPH (2,2’-azobis[2-amidinopropane]). After warm 37ºC for 120 seconds, added 200 μl of luminal, chemiluminescence intensity was recorded using a luminescence reader (ALOKA BLR-201).
2.6 Measurement of flavonoid injection or combined with X-irradiation on tumor growth
After acclimation, mice were divided in 4 groups same as the experiment of blood cell count. Flavonoid was injected to mice for 2 weeks then 1 ´ 106 Sarcoma-180 cells were injected into the right femoral region. When tumor grew to 10 mm in diameter, irradiation groups were treated with 3 times of 2 Gy irradiation every other day and all groups of tumor diameters were measured with a caliper for 20 days. Tumor volumes were calculated using the formula: V=ab2/2, where “a” was the shortest tumor diameter and “b” was the longest tumor diameter measured.
2.7 Statistical analysis
Experimental values are given as mean ± standard error of the mean (SE). All experiments of statistical analysis were performed using a parametric ANOVA test among the groups to determine significant difference.
3. Results
The leukocyte counts in the flavonoid-treated and control groups were 196.26 ´ 102 ± 24.36 ´ 102 cell/μl and 126.18 ´ 102 ± 14.18 ´ 102 cell/μl , respectively (Figure 2-5), showing a significant increased in leukocytes with flavonoid administration (p<0.01). Figure 2- Figure 5 shows the changes in the number of hemocytes, (leukocytes, lymphocytes, monocytes and granulocytes) caused by irradiation. Following 2 Gy whole-body irradiation, the number of hemocytes rapidly decreased and reached a minimum 3 days after irradiation, before recovering gradually. The number of hemocytes in the flavonoid-treated + irradiated group was generally higher than that in the irradiated group, with a significant difference between these groups on the day before irradiation, and after the minimum leukocyte count (day after irradiation: p<0.05; 7, 15 and 30 days after irradiation: p<0.01).
Lymphocytes in the flavonoid and control groups were 112.31 ´ 102 ± 16.78 ´ 102 cell/μl and 83.21 ´ 102 ± 12.14 ´ 102 cell/μl, respectively (Figure 3), showing that flavonoid administration increased the number of lymphocytes significantly (p<0.01). Immediately after irradiation, the number of hemocytes rapidly decreased and all values in the irradiated group were statistically significantly different (3 days after irradiation: p<0.05; 1, 7, 15 and 30 days after irradiation: p<0.01). The number of lymphocytes in the irradiated and flavonoid-treated + irradiated groups 3 days after irradiation (minimum values) decreased from those on the day before irradiation by 9.1% and 22.3%, respectively.
The monocyte counts of the flavonoid-treated and control groups were 44.8 ´ 102 ± 7.3 ´ 102 cell/μl and 26.48 ´ 102 ± 4.39 ´ 102 cell/μl, respectively (Figure 4). Monocytes did not decrease immediately after irradiation, but rather on the day following irradiation, reached a minimum 3 days after irradiation, and then gradually recovered. Flavonoid administration increased the number of monocytes significantly (p<0.05), showing statistically significant differences on the day after irradiation and 15 and 30 days after irradiation (day after irradiation: p<0.05; 15 and 30 days after irradiation: p<0.01). The number of monocytes in the irradiated and flavonoid-treated + irradiated groups 3 days after irradiation (minimum values) decreased from those the day before irradiation by 18.7% and 26.7%, respectively.
The granulocytes also showed a tendency to increase with flavonoid administration (Figure 5). The number of granulocytes in the irradiated group reached a minimum 3 days after irradiation and recovered gradually thereafter; however, that of the flavonoid-treated + irradiated group reached a minimum the day after irradiation and recovered slightly earlier. No statistically significant difference in the number of granulocytes was found between the groups at any time point (Figure 2-5).
Figure 6 shows the antioxidant activity for the control, flavonoid-treated, 2 Gy irradiated and flavonoid-treated + 2 Gy irradiated groups, determined using the AAPH method. In the non-irradiated groups, statistically significant differences were found between the control and flavonoid-treated groups (p<0.05). In irradiated groups, no statistically significant differences were found between the groups, but the flavonoid-treated + irradiated group showed a higher antioxidant activity, compared with the irradiated group (Figure 6). Figure 7 shows the rate of tumor growth in each group for 20 days. Administration of flavonoid delayed tumor growth. Irradiation also inhibited tumor growth, and administration of flavonoid in addition to irradiation further delayed the tumor growth.
However, no statistically significant differences were found among the groups at all determination points. The tumor growth rates (mean ± SE) 20 days after tumor implantation were 16.2 ± 3.4, 11.2 ± 2.6, 8.5 ± 1.6 and 6.7 ± 0.8 for the control, flavonoid-treated, irradiated and flavonoid-treated + Gy irradiated groups, respectively (Figure 7).
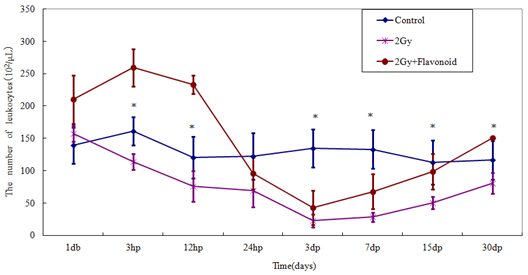
Figure 2: The change in the number of cells in the blood taken from the tail vein of whole body irradiated mice. Each lineargram represents the mean value ± SE in leukocytes from 10 mice (M). Results represent means ± S.E.
*Statistically significant (P<0.05) from the 2Gy group.
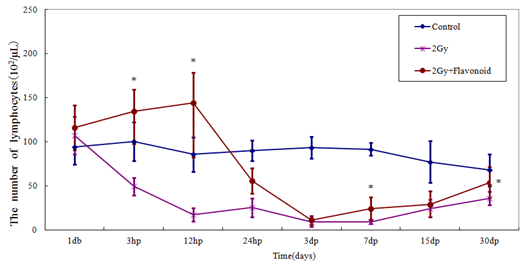
Figure 3: The change in the number of cells in the blood taken from the tail vein of whole body irradiated mice. Each lineargram represents the mean value ± SE in lympocytes from 10 mice (M). Results represent means ± S.E.
*Statistically significant (P<0.05) from the 2Gy group.
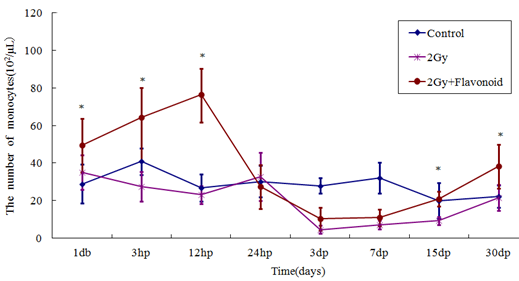
Figure 4: The change in the number of cells in the blood taken from the tail vein of whole body irradiated mice. Each lineargram represents the mean value ± SE in monocytes from 10 mice (M). Results represent means ± S.E. *Statistically significant (P<0.05) from the 2Gy group.
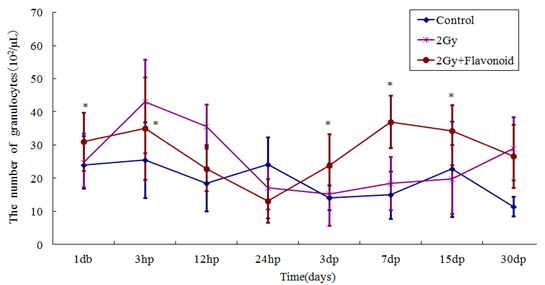
Figure 5: The change in the number of cells in the blood taken from the tail vein of whole body irradiated mice. Each lineargram represents the mean value ± SE in granulocytes from 10 mice (M). Results represent means ± S.E. *Statistically significant (P<0.05)
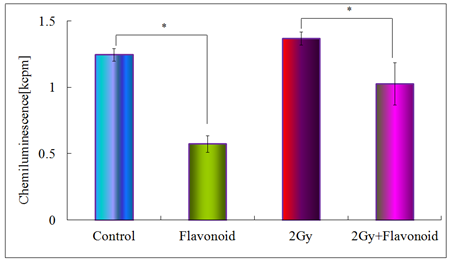
Figure 6: Antioxidant activity of flavonoid in the blood plasma taken from the heart of mice. Each bar represents the mean value ±SE from 10 mice (M). Significantly different *p<0.05 Control vs flavonoid, 2Gy vs flavonoid +2Gy, respectively.
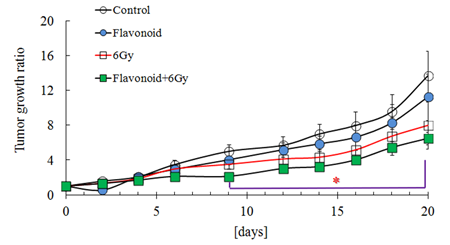
Figure 7: Effect of flavonoid on the tumor growth in mice inoculated with Sarcoma-180 cells. Groups of ten mice each were subjected to each treatment. Results represent means ± S.D for 10 mice (M).
*Statistically significant (P<0.05) from the control group.
4. Discussion
Whole-body irradiation is known to cause free radical-induced DNA damage and carcinogenesis, and to compromise the immune system due to its effect on leukocytes [11]. To evaluate the effects of flavonoid on irradiation-induced damage, we investigated changes of hemocytes in the peripheral blood, inhibition of oxidation induced by AAPH, a radical generator, and changes in tumor growth of Sarcoma-180 following flavonoid administration. The effects of irradiation and flavonoid on hemocytes (i.e., lymphocytes, monocytes, granulocytes and leukocytes), which are directly associated with the immune system, is shown with time in Figure 2. Administration of flavonoid prior to irradiation increased the hemocyte count significantly. Hemocytes were markedly reduced by irradiation, but flavonoid administration inhibited this reduction and increased the minimum level. Nada et al. have reported that single intraperitoneal administration of flavonoid at a dose of 50 mg/kg significantly increases leukocytes 3 and 7 days after administration, with a concomitant increase in the weight of the spleen [1], and also reported that leukocyte count and spleen weight increased after repeated oral administration of flavonoid at a dose of 50 mg/kg 5, 10 and 15 days before determination [12]. Kimoto et al. showed both in vivo and in vitro inhibitory effects of artepillin C, which is a component of flavonoid, on the growth of various tumors, and reported that cytotoxicity and adjuvanticity increased in lymphocytes [13].
Hydroxyl radical (×OH) and superoxide radical (O2-) are free radicals that are induced by irradiation. The OH radical is known to oxidize lipids to peroxy radicals (ROO×) and to increase lipid peroxide levels [11]. AAPH, a soluble azo compound, is a free radical initiator that generates radicals through simultaneous thermolysis, which results in oxygen inclusion into peroxy radicals [14]. These radicals can then abstract a hydrogen atom from various molecules, combining with the hydrogen to stabilize themselves while inducing a radical chain reaction in the substances undergoing hydrogen loss. In this study, under conditions with and without irradiation, flavonoid inhibited AAPH-induced peroxy radical formation (Figure 6). Nagai et al. have reported the antioxidant and radical scavenging effects of flavonoid on DPPH and hydroxyl radicals [15], and Cos et al. showed similar effects for a caffeic acid ester extracted from flavonoid [16]. El-Ghazaly et al. have reported that flavonoid suppresses inflammation caused by whole-body irradiation [10], and also showed that superoxide dismutase (SOD) activity is increased in hemocytes following flavonoid treatment of both irradiated and non-irradiated rats. SOD inhibits oxygen toxicity by catalytic scavenging of free radicals that would otherwise cause tissue damage [17]. Hence, flavonoid is a natural antioxidant that enhances SOD activity and inhibits production of oxides from lipid oxides [18, 19], and Kobayashi et al. showed the O2-scavenging effect of flavonoid at super critical state (40ºC, 350 atm pressure), and suggested that flavonoid contains vitamin C [20]. Collectively, these results suggest that flavonoid has both an antioxidant effect and a free-radical scavenging effect.
Figure 6 shows the tendency for inhibition growth of the Sarcoma 180 tumor by combined administration of flavonoid and irradiation. Similar antitumor effects have been reported previously; hence, Gu et al. showed an effect of flavonoid on tumorigenesis of colorectal cancer, and showed that flavonoid regulates DNA damage, using a Comet assay [21]. It has also been reported that flavonoid and its components, including flavonoids, aromatic carboxylic acids and esters, prevent oncogenesis [22]. Matsuno et al. showed that PRF-1 extracted from flavonoid has toxicity for human hepatocellular carcinoma [23], and Kimoto et al. showed that flavonoid suppresses FeNTA-induced renal adenocarcinoma in CD-1 and ddY mice [24]. Clinical treatment methods using flavonoid have yet to be clearly established, but many pathological studies have shown biological effects of flavonoid [25]. It has been reported that leukocytes and CD8+ and CD4+ cells are significantly increased in Flavonoid-treated mice [26], and flavonoid and its components have been shown to have mobility and bactericidal properties in vivo and in vitro, while enhancing tumorigenic activity and producing factors activating IL-1, TNF and lymphocytes in mammals [27]. It is well known that irradiation of tumors suppresses tumor growth: irradiation has a direct effect on tumor DNA, thereby suppressing cell growth and inducing apoptosis [28], and can delay tumor growth by an oxygenic effect (reoxygenation) on tumor blood vessels [29]. The combined antitumor effects of flavonoid and irradiation-inhibited tumor growth appear to be stronger than the effects of each treatment alone.
In conclusion, prior administration of flavonoid enhances the immune systems and suppresses irradiation-induced damage to hemocytes in the peripheral blood. Flavonoid also shows an antioxidant effect in reducing irradiation-induced free-radical damage, and can inhibit the growth of sarcoma cells, with increased inhibition occurring in combination with irradiation.
Acknowledgments
We thank Dr & Professor YK Park (Department of Food Science, College of Food Engineering, State University of Campinas, P.O. Box 6177, Campinas, SP, Brazil) for he excellent technical assistance in flavonoid.
References
- Nada O, Ivan B. Immunomodulation by water-soluble derivative of flavonoid: a factor of anititumor reactivity. J Ethnopharmacol 84 (2003): 265-273.
- Jin UH, Song KH, Motomura M, et al. Caffeic acid phenethyl ester induces mitochondria-mediatedapoptosis in human myeloid leukemia U937 cells. Mol Cell Biochem 310 (2007): 43-48.
- Yeun-Hwa Gu, Yasuyuki Takagi, Takashi Nakamura, et al. Enhancement of radioprotection and anti-tumor immunity by yeast-derived beta-glucan in mice. J Med Food 8 (2005): 154-158.
- Gu YH, Takagi Y, Nakamura T, et al. Enhancement of radioprotection and anti-tumor immunity by yeast-derived beta-glucan in mice. J Med Food 8 (2005): 154-158.
- Jin UH, Chung TW, Kang SK, et al. Caffeic acid phenyl ester in propolisis a strong inhibitor of matrix metalloproteinase-9 and invasion inhibitor:Isolationand identification. Clinica Chemica Acta 362 (2005): 57-64.
- Takagi Y, Choi IS, Yamashita T, et al. Immune Activation and Radioprotection by Propolis. Am J Chi Med 33 (2005): 231-240.
- Figueiredo SM, Nogueira-Machado JA, Almeida B, et al. Immunomodulatory properties of green propolis. Recent Pat Endocr Metab Immune Drug Discov 8 (2014): 85-94.
- Khayyal MT, el-Ghazaly MA, el-Khatib AS. Mechanisms involved in the anti-inflammatory effect of flavonoid extract. Drugs Exp Clin Res 19 (1993): 197-203.
- Strehl E, Volpert R, Elstner EF. Biochemical activities of flavonoid extracts: III. Inhibition of dehydrofolate reductase. Z Naturforsch [C] 49 (1994): 39-43.
- Volpert R, Elstner EF. Interactions of different extracts of flavonoid with leucocytes and leucocytic enzymes. Arzneimittelforschung 46 (1996): 47-51.
- Mirzoeva OK, Calder PC. The effect of flavonoid and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids 55 (1996): 441-449.
- Dobrowolski JW, Vohora SB, Sharma K , et al. Antibacterial, antifungal, antimoebic, anti-inflammatory, and antipyretic studies on flavonoid bee products. J Ethnopharmacol 35 (1991): 77-82.
- Krol W, Czuba Z, Scheller S , et al. Anti-oxidant property of etanolic extract of flavonoid (EEP) evaluated by inhibiting the chemiluminescence oxidation of luminol. Biochem Int 21 (1990): 593-597.
- Scheller S, Krol W, Swiacik J, et al. Antitumoral property of etanolic extract of flavonoid in micebearing Ehrlich carcinoma, as compared to bleomycin. Z. Naturforsch [C] 44 (1989): 1063-1065.
- Grunberger D, Banerjee R, Eisinger K , et al. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from flavonoid. Experientia 44 (1988): 230-232.
- El-Ghazaly MA, Khayyal MT. The use of aqueous flavonoid extract against radiation-induced damage. Drugs Exp Clin Res 21 (1995): 229-236.
- Riley PA. Free radical in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65 (1994): 27-33.
- Orsolic N, Knezevic AH, Sver L, et al. Immunomodulatory and antimetastatic action of flavonoid and related polyphenolic compounds. J Ethnopharmacol 94 (2004): 307-315.
- Kimoto T, Arai S, Kohguchi M, et al. Apoptosis and suppression of tumor growth by artepillin C extracted from Brazilian flavonoid. Cancer Detect Prev 22 (1998): 506-515.
- Olsher M, Yoon SI, Chong PL. Role of Sterol Superlattice in Free Radical-Induced Sterol Oxidation in Lipid Membranes. Biochemistry 44 (2005): 2080-2087.
- Nagai T, Nagashima T, Myoda T , et al. Preparation and functional properties of extracts from bee bread. Nahrung 48 (2004): 226-229.
- Cos P, Rajan P, Vedernikova I, et al. In vitro antioxidant profile of phenolic acid derivatives. Free Radic Res 36 (2002): 711-716.
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23 (1983): 239-257.
- Fuliang HU, Hepburn HR, Xuan H, et al. Effects of flavonoid on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res 51 (2005): 147-152.
- Matsui T, Ebuchi S, Fujise T, et al. Strong antihyperglycemic effect of water-soluble fraction of Brazilian flavonoid and its bioactive consitituent, 3,4,5-tri-O-caffeoylqunic acid. Biol Pharm Bull 27 (2004): 1797-1803.
- Kobayashi N, Unten S, Kakuta H, et al. Diverse Biological Activities of Healthy Foods. In Vivo 15 (2001): 17-23.
- Rodrigo O. Modifying Effect of Propois on Dimethlhydrazine-Induced DNA Damage But Not Colonic Aberrant Crypt Foci in Rats. Enviromental and Molecular Mutagenesis 45 (2005): 8-16.
- Bazo AP, Rodrigues MA, Sforcin JM , et al. Protective action of flavonoid on the rat colon carcinogenesis. Teratog Carcinog Mutagen 22 (2002): 183-194.
- Xiao J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit Rev Food Sci Nutr 57 (2017): 1874-1905.
