Influence of Recipients’ Sex Differences on the Growth in Grafts after Rat Fetal Metanephros Transplantation
Article Information
Kotaro Nishi1, Shozo Okano1, Takashi Yokoo2, Satomi Iwai1,*
1Laboratory of Small Animal Surgery 2, School of Veterinary Medicine, Kitasato University, Towada, Aomori, Japan
2Division of Nephrology and Hypertension, Department of Internal Medicine, The Jikei University School of Medicine, Minato-ku, Tokyo, Japan
*Corresponding Author: Dr. Satomi Iwai, DVM, PhD, Laboratory of Small Animal Surgery 2, School of Veterinary Medicine, Kitasato University, 35-1, Higashi 23, Towada-city, Aomori, Japan
Received: 24 October 2020; Accepted: 05 November 2020; Published: 08 December 2020
Citation: Kotaro Nishi, Shozo Okano, Takashi Yokoo, Satomi Iwai. Influence of Recipients’ Sex Differences on the Growth in Grafts after Rat Fetal Metanephros Transplantation. Archives of Veterinary Science and Medicine 3 (2020): 109-120.
View / Download Pdf Share at FacebookAbstract
We have attempted a regeneration method to promote a kidney growth by transplanting a fetal metanephros with bladder (MNB) into the recipient’s body. In many studies regarding regeneration, males were used, but the development of treatment for chronic kidney disease must be considered regardless of sex in clinical practice. In this study, we transplanted two MNBs to male or female recipient rats and evaluated their growth. The first MNB (MNB1) was transplanted to male (n=12: male group) and female (n=14: female group) Lewis rats (Day 0). The morphology of MNB1 in every seven days was evaluated using ultrasonography, and the second MNB (MNB2) was transplanted on Day 28. On Day 56, all MNBs were collected and used for histopathological examination. The size of MNBs in the male group was significantly larger than that of the females on every measurement day (p<0.05). Furthermore, there were no marked changes in the volume of MNB1 in the female group on Day 28. In the male group, the ratio of urine retention of MNBs were significantly higher than that of the female group. Female MNBs were found the mononuclear cell infiltration and tubular atrophy in the histopathological examination. This study investigated MNBs transplanted to male or female rats over time, and clarified that the size and urine volume of MNBs in the male group were larger than those of the female. Therefore, we suggested that recipients’ sex differences have an influence on the growth in grafts after the rat fetal metanephros transplantation.
Keywords
Sex differences of recipients; Rat; Kidney regeneration therapy; Chronic kidney disease; Ultrasonography
Sex differences of recipients articles; Rat articles; Kidney regeneration therapy articles; Chronic kidney disease articles; Ultrasonography articles
Article Details
1. Introduction
Patients with both nephropathy persisting for ≥3 months and a decrease in the glomerular filtration rate or either one is regarded as having chronic kidney disease (CKD). Especially in cats, CKD is an important disease because reportedly present in approximately 80% of elderly cats aged ≥15 years [1]. CKD treatments include fluid therapy, dietary therapy, symptomatic therapy for proteinuria, hypertension, uremia, renal anemia, and dialysis therapy with blood or peritoneum. However, histological changes related to CKD are irreversible, and kidney transplantation is the only radical treatment [2]. Furthermore, in cats, it is shown that once the kidney is damaged, its histological changes progress in comparison with other animals [3]. Therefore, donor cats for living kidney transplantation, with the remaining kidney, frequently show a chronic progressive kidney disease after the onset of acute nephropathy, such as ureteral obstruction, of which the incidence has recently increased, so a risk of donors may be high [4, 5].
As radical treatment replacing kidney transplantation, kidney regeneration therapy has been emphasized. In particular, we have adopted the method in which the fetus-derived metanephros with bladder (MNB) is transplanted to a recipient [6]. It was indicated that the transplanted MNB became mature in the recipient body, having urine-synthesizing and -concentrating capacities. The use of the Stepwise Peristaltic Ureter (SWPU) system in which the recipient’s ureter is anastomosed with the bladder region of the MNB promotes hydronephrosis-free metanephric growth [6]. Therefore, it is clinically considered to be one of the most realistic kidney regeneration methods. However, the metanephros tissue is small, and an experiment in which the metanephros alone was transplanted showed that its function corresponded to only a few percent of the normal individual’s kidney function. Therefore, the kidney function should be more increased.
CKD progresses regardless of animal species, age, or sex. Its risk differs among individuals. In humans, the age and sex are prognostic factors for CKD [7, 8]. In particular, the sex is an important factor that influences various diseases, and sex steroid hormones and sex-specific tissues/cells influence the biological body [9]. Furthermore, sex differences have been investigated from the viewpoint of tissue regeneration/transplantation [10, iue97511]. However, many studies reported the influence of donor-side factors on grafts. To our knowledge, few studies have examined recipient-side factors. In addition, for metanephros/MNB transplantation, only males were used as recipients. No study has investigated the influence of sex on metanephros/MNB transplantation. In this study, we examined the influence of sex on the growth of the transplanted MNB as a basic experiment for clinical kidney regenerative medicine in cats.
2. Materials and Methods
2.1 Animals and groups
To obtain MNBs, 7 female Lewis rats (Japan Charles River Laboratories, Kanagawa, Japan) on Day 15 of pregnancy were used. As recipient rats, 11-week-old male (n=12; male group) and female (n=14; female group) Lewis rats (Japan Charles River Laboratories) weighing 179 to 333 g (245.7±59.5 g) were used. For acclimation, food and water were given ad libitum. These rats were acclimated in a room where the temperature and humidity were constant, with a lighting cycle of 12 hours. Prior to this animal experiment, its protocol was approved by the Animal Experiment Guideline and Laboratory Animal Ethics Review Board of Kitasato University School of Veterinary Medicine (approval number: 17-127).
2.2 Isolation and preservation of MNB
In rats on Day 15 of pregnancy, anesthesia was induced with 5% isoflurane, and maintained with 3% isoflurane. The uterus was aseptically extracted through abdominal median section, and a fetus was isolated in Hanks’ Balanced Salt Solution (Hank’s Balanced Salt Solution: HBSS, gibco®, Life Technologies, CA, America), which was preserved in cooled HBSS. Under a microscope for surgery (Leica 320 F12®, Leica Microsystems, Tokyo, Japan), MNBs were isolated from the fetus of rats. HBSS containing MNB was preserved on ice until transplantation.
2.3 Transplantation and collection of MNB
In this study, second MNB was transplanted on 28 days after initial MNB transplantation, as previously reported [12]. The recipient rats were anesthetized with isoflurane. After the abdominal median was incised, the intestinal tract was pulled out of the body to expose the retroperitoneum and abdominal aorta. Under a microscope for surgery, a minor incision of the retroperitoneum was made, and the first MNB (MNB1) was transplanted into the subretroperitoneal space adjacent to the abdominal aorta (Day 0). After transplantation, the retroperitoneum was sutured by a single stitch with 6-0 non-absorbable suture thread. After returning the intestinal tract into the abdominal cavity, the abdominal wall and skin were closed by continuous suture with 3-0 non-absorbable suture thread. The left kidney was extracted on Day 28 after MNB1 transplantation, and the second MNB (MNB2) was transplanted as described above. MNB2 was distinguished by transplanting it on the cranial side of MNB1. Intra-MNB1 urine was obtained on Day 28. On Day 56, intra-MNB2 urine was obtained, and the rats were sacrificed by anesthesia with isoflurane (5%) and the intraperitoneal administration of pentobarbital sodium (100 mg/kg). After that, both MNB1 and MNB2 were collected. On Day 0, 28, and 56, a blood was obtained through the tail artery or vein under anesthesia to measure the blood urea nitrogen (BUN) level, blood creatinine (Cr) level, and hematocrit (Hct). The two collected MNBs were fixed in tissue fixative (ALTFiX Meiji Seika Pharma Co., Ltd., Tokyo, Japan), paraffin-embedded, and cut into 2-mm sections for hematoxylin and eosin (H-E) staining.
2.4 Measurement of the MNB volume using ultrasonography
Ultrasonography was performed every seven days from Day 7 until Day 56 after MNB1 transplantation to examine its morphology and size. For imaging, a diagnostic ultrasonography (LOGIQ S8, GE Healthcare Japan K.K., Tokyo, Japan was used. A 3-11MHz linear-array-type probe was used. As a display mode, B-mode was adopted, and gain and depth were established as 90 and 2.3 to 2.5, respectively. Furthermore, we searched for the transplanted MNB to an area adjacent to the posterior abdominal aorta by adopting the color Doppler mode. For examination, rats were anesthetized as described above, and fixed in the supine position after shaving the abdominal hair. Assuming that MNB may be a spheroid, the cloaca volume was calculated by inserting the length (L), width (W), and height (H) of MNB to a formula for calculating the spheroid volume: ∏/6 (≒0.52) x L x W x H.
2.5 Statistical analysis
The results of blood examination and the volume of MNBs between both groups were compared using Student’s t-test. The volume of MNBs on each days were compared using Kruskal-Wallis test. The urine volume on Day 28 after transplantation was evaluated using the Mann-Whitney U-test. To examine the association between the body weight/anesthesia time and MNB volume, Spearman’s rank correlation coefficient was used.
3. Results
The results are expressed as the mean±standard deviation. The rat information and results of blood examination are presented in Table 1. On Day 0, 28, and 56, the mean body weight in the male group was significantly heavier than that of the female group. There was a positive correlation between the weight in all individuals and the volume of MNB1 on Day 28, but there was no correlation with the body weight in the male group and females, respectively. There was no difference in the total anesthesia time, and it was not correlated with the volume of MNBs (r=-0.11). Furthermore, there was no correlation between the body weight and anesthesia time (r=0.03). On Day 28 and 56, urine could be obtained from MNB1 and MNB2 in 75% (9/12) and 69.7% (8/12) of the male group, respectively, and in 14.3% (2/14) each of female group. The BUN and Cr levels on Day 0 and 28 in the male group were higher than those of the female group, but they were within the normal ranges. The Hct value was also within the criteria range.
|
|
Male (n=12) |
Female (n=14) |
P value
|
|
|
Body Weight (g) |
Day 0 |
309.3±11.9 |
193.6±8.7 |
< .01 |
|
Day 28 |
347.9±14.3 |
207.0±8.3 |
< .01 |
|
|
Day 56 |
369.0±14.1 |
207.7±8.9 |
< .01 |
|
|
Total anesthesia time (min) |
- |
254.8±18.6 |
250.1±20.1 |
.56 |
|
Number of Rats which urine could be collected |
MNB 1 |
75% (9/12) |
14.3% (2/14) |
- |
|
MNB 2 |
66.7% (8/12) |
14.3% (2/14) |
- |
|
|
BUN (mg/dL) |
Day 0 |
13.1±1.6 |
11.0±1.3 |
< .01 |
|
Day 28 |
16.3±1.9 |
14.3±2.7 |
< .05 |
|
|
Day 56 |
18.8±1.9 |
17.9±5.4 |
.57 |
|
|
Cr (mg/dL) |
Day 0 |
0.13±0.03 |
0.16±0.02 |
< .01 |
|
Day 28 |
0.10±0.02 |
0.14±0.03 |
< .01 |
|
|
Day 56 |
0.27±0.02 |
0.30±0.11 |
.33 |
|
|
Hct (%) |
Day 0 |
46.8±3.9 |
46.6±1.3 |
.87 |
|
Day 28 |
46.9±1.3 |
43.9±1.1 |
< .01 |
|
|
Day 56 |
46.1±2.7 |
43.9±3.7 |
.10 |
|
Table 1: Rat information and results of blood examination.
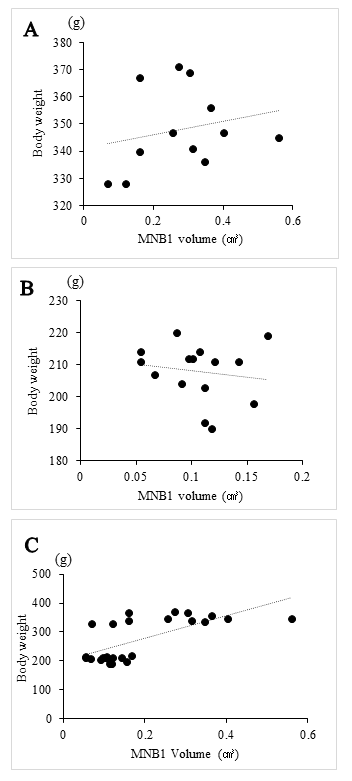
As shown in Table 2, the volume of MNBs was evaluated every seven days after transplanted MNB1. In the male group, the volume of MNBs was significantly larger than in the female group in all Days. Furthermore, there were increases the volume of MNB1 and MNB2 in the both groups, but the volume of the female group was smaller than that of the male group, and there were no marked differences the growth in MNB1 after Day 28. The correlation between the volume of MNB1 and body weight of each group was low (Figures 2A and 2B). However, the correlation between MNB1 volume and body weight for both sexes showed a positive correlation (Figure 2C).
|
MNB volume in each seven days by ultrasonography (cm3) |
|||||||||
|
Day7 |
Day14 |
Day21 |
Day28 |
Day35 |
Day42 |
Day49 |
Day56 |
||
|
Male (n=12) |
MNB 1 |
ND |
0.073± 0.04† |
0.139± 0.09* |
0.277± 0.13†, ‡ |
0.270± 0.08†, § |
0.396± 0.14† |
0.361± 0.15† |
0.381± 0.25† |
|
MNB 2 |
- |
- |
- |
- |
0.016± 0.01† |
0.098± 0.09* |
0.145± 0.08†, || |
0.214± 0.16† |
|
|
Female (n=14) |
MNB 1 |
ND |
0.039± 0.03 |
0.073± 0.03 |
0.107± 0.03‡ |
0.116± 0.05 |
0.114± 0.05 |
0.122± 0.04§ |
0.105± 0.05 |
|
MNB 2 |
- |
- |
- |
- |
0.004± 0.01 |
0.026± 0.02 |
0.051± 0.05|| |
0.039± 0.04 |
|
ND: no date for cannot observe, ✲: vs. Female, p<0.05, †: vs. Female, p<0.01
‡: vs. Day14, p<0.01, §: vs. Day21 p<0.05, ||: vs. Day35, p<0.05
Table 2: MNB volume by ultrasound in each seven days.
The correlation between MNB1 volume and body weight on Day 28 was shown. A, B: No correlation was recognized between volume and body weight for the male group and female group (male: r = 0.23, female: r = -0.15). C: There was a positive correlation between volume and body weight in both groups (r = 0.69).
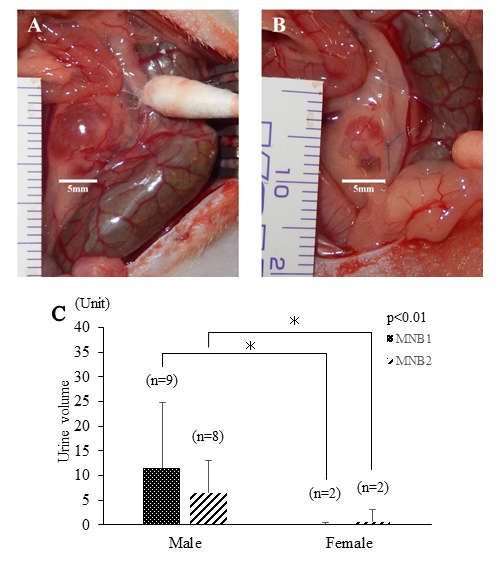
On macroscopic image of MNB1 on Day 28 after transplantation, its size in the male group was markedly larger than that of the female group, and the number of MNBs with urine retention was larger (Figures 3A and 3B). Furthermore, the rates of rats in which urine collection from MNB1 or MNB2 was possible on Day 28 and 56 were more than that of the male group. The urine volume was also significantly larger in the male group (p<0.01) (Figure 3C).
The images are indicated the typical MNB1 of each group on Day 28. A: The MNB of males was large and had urine retention. New blood vessels were easily observed. B: The MNB of females appeared to be small, and no urine retention was observed. C: The graph is the volume of obtained urine in MNB1, 2 on 28 days after transplantation of each MNB. The amount of urine volume was significantly larger in MNB of the male group than that of female group on both MNB1 and MNB2.
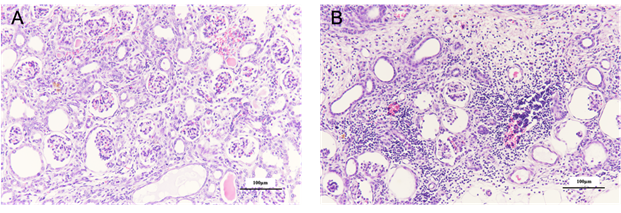
Histopathological examination with H-E staining showed tubular dilation related to the absence of a urine-excreting route, dilation of Bowman’s space, and interstitial fibrosis with respect to MNBs in the male group (Figure 4A). In MNBs of the female group, the diffuse interstitial infiltration of mononuclear cells, tubular atrophy, and interstitial fibrosis were observed (Figure 4B).
Figure 3: Histopathological images of MNB.
A: The histopathological image of MNBs in the male group is shown. It was confirmed that dilation of the renal tubules and Bowman's capsule are observed. In addition, hemorrhage in the interstitium is observed in some area. B: The MNBs of female were observed diffuse fibrosis and renal tubular atrophy in the tissue. Many mononuclear cells could be found in the blood vessels and infiltrate into the interstitium.
4. Discussion
The purpose of this study was to examine the MNBs transplanted to male and female recipient rats over time and evaluate over time the influence of sex on the growth of MNBs.
As a result, hydronephrosis was histologically noted due to the absence of a urine-excreting route in the MNBs of the male rats, but the urine-synthesizing capacity could be confirmed. As previous studies reported hydronephrosis or fibrosis on 4 weeks from transplantation, hydronephrosis may have been prevented in the male rats in this study, as previously indicated, assuming the use of the SWPU system [6]. However, the volumes of MNB1 and MNB2 in the female group were small, and urine synthesis was achieved in a small number of individuals. This reason was possibly associated with the relationship between the body weight and organ weight. In both sexes, the body weight slightly increased, and it was positively correlated with the volume of MNBs. In this study, 11-week-old (juvenile) recipient rats were used. A study indicated that weight gain in the male group was more marked than it of the female group at 60 to 100 days of age [13]. Thus, MNB transplantation and growth observation were conducted at the timing of body weight change; the results of this study suggested an association between the body weight and MNB growth. Furthermore, the kidney weight in the male group is reportedly heavier than in the female group, and androgen may be involved. Androgen significantly increases the kidney weight in castrated rats, compensatory glomerular and tubular growth have been shown to be amplified when androgen is administered to female rats who have undergone sterilization and have had one kidney removed [14, 15]. Therefore, as a factor involved in the favorable urine-synthesizing capacity of MNB and an increase in the MNB volume in male group, the influence of androgen was considered.
As described above, MNB essentially acquires a urine-excreting route through anastomosis with the recipient’s ureter. The MNB tissue volume is still small, and it may be necessary to transplant several MNBs; therefore, we have adopted a method to transplant two MNBs step by step. In some animals, like to mice and rats, the number of glomeruli rapidly increases in a few weeks after birth. In addition, unilateral nephrectomy increases the contralateral kidney size regardless of sex [16, 17]. Therefore, in this experiment, the unilateral kidney was extracted on Day 28, assuming the actual SWPU system. Based on our experience, growth of the MNBs transplanted after nephrectomy in male rats tended to be promoted than that transplanted at first (data not published). Therefore, we expected that MNB2 growth might also be accelerated in the female group, leading to urine synthesis, but the results differed. In this study, female kidney tissue caused fibrosis and atrophy of the renal tubules, despite little urine production. There was a possibility of foreign body reaction or rejection since mononuclear cells were infiltrated extensively. Since the MHC haplotypes between Lewis rats are the same and the rejection is not severe, generally, Lewis rats are used in transplantation research [18, 19]. Male animals are used not only in transplantation research but also in various animal study fields. It has been considered that the sexual cycle of female animals may have an influence on the experimental results. Estrogen is the most important factor associated with the estrus cycle of normal female rats. There are many reports which suggested estrogen has the renoprotective effects and contributes to compensatory kidney growth [20-22]. However, in these studies, mature animals were used, and tissues may differ from those in the process of organ formation. In addition, estrogen relieves ischemia with an increase in the expression of vascular endothelial growth factor (VEGF), but VEGF may promote fibrosis [23, 24]. The presence of estrogen is considered to be one of the factors that have a stronger immune response in the female group than in the male group. It has also been reported that either systemic or organ-specific autoimmune diseases are 2 to 10 times higher in women of humans [25, 26]. Apart from sex hormones, initial-phase compensatory kidney growth depends on growth hormones in the male group, but not in the female group. Its association with insulin-like growth factor was also indicated [27]. Therefore, in addition to sex hormones, growth factor may be involved in tissue maturation. Therefore, as an etiological factor for MNB growth suppression in the female group, some hormone may have influenced growth of the transplanted metanephros. Furthermore, the isolation of MNBs and transplantation were randomly conducted, and the growth of most MNBs in the male group was favorable; therefore, the quality of isolated MNBs from a fetal may be not involved.
This study showed that there was a sex difference in MNB growth, which is considered to be a very important finding for actual clinical application. It has limitations in this study: no sex hormone level was measured; actual metanephros growth must be further investigated although the volume of MNB was measured. In the future, various factors, such as the influence of various hormones on the growth of MNB, should be performed.
5. Conclusion
The results of this study showed that the growth of MNB in the female group was less favorable than in the male group. This may contribute to the future application of regenerative transplantation research with MNB. As CKD develops regardless of animal species, age, or sex, a study targeting further MNB maturation in the female group should be conducted.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 16 (2014): 465-472.
- Aronson LR. Update on the Current Status of Kidney Transplantation for Chronic Kidney Disease in Animals. Vet Clin North Am Small Anim Pract 46 (2016): 1193-1218.
- Sugisawa R, Hiramoto E, Matsuoka S, et al. Impact of feline AIM on the susceptibility of cats to renal disease. Sci Rep 6 (2016): 35251.
- Picavet P, Detilleux J, Verschuren S, et al. Analysis of 4495 canine and feline uroliths in the Benelux. A retrospective study: 1994-2004. J Anim Physiol Anim Nutr (Berl) 91 (2007): 247-251.
- Lulich JP, Berent AC, Adams LG, et al. ACVIM Small Animal Consensus Recommendations on the Treatment and Prevention of Uroliths in Dogs and Cats. J Vet Intern Med 30 (2016): 1564-1574.
- Yokote S, Matsunari H, Iwai S, et al. Urine excretion strategy for stem cell-generated embryonic kidneys. Proc Natl Acad Sci U S A 112 (2015): 12980-12985.
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int 69 (2006): 375-382.
- Kattah AG, Garovic VD. Understanding sex differences in progression and prognosis of chronic kidney disease. Ann Transl Med 8 (2020): 897.
- Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res 33 (2010): 383-392.
- Jones KJ. Recovery from Facial Paralysis Following Crush Injury of the Facial Nerve in Hamsters: Differential Effects of Gender and Androgen Exposure. Experimental Neurology 121 (1993): 133-138.
- Pham TL, Kakazu A, He J, et al. Mouse strains and sexual divergence in corneal innervation and nerve regeneration. FASEB J 33 (2019): 4598-4609.
- Fujimoto E, Yamanaka S, Kurihara S, et al. Embryonic kidney function in chronic renal failure model in rodents. Clin Exp Nephrol 21 (2017): 579-588.
- Tadokoro S, Kurihara Y, Kurihara N, et al. Body weight and organ weight in rats. The Kitakanto medical journal 12 (1962): 250-265.
- Shortliffe LM, Ye Y, Behr B, et al. Testosterone changes bladder and kidney structure in juvenile male rats. J Urol 191 (2014): 1913-1919.
- Zeier M, Schönherr R, Amann K, et al. Effects of testosterone on glomerular growth after uninephrectomy. Nephrol Dial Transplant 13 (1998): 2234-2240.
- Bonvalet JP, Champion M, Courtalon A, et al. Number of glomeruli in normal and hypertrophied kidneys of mice and guinea-pigs. J Physiol 269 (1977): 627-641.
- Mulroney SE, Woda C, Johnson M, et al. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int 56 (1999): 944-953.
- Zhang Y, Yang Y, Li X, et al. Thalidomide ameliorate graft chronic rejection in an allogenic kidney transplant model. Int Immunopharmacol 71 (2019): 32-39.
- Deng J, Xia Y, Zhou Q, et al. Protective effect of rosiglitazone on chronic renal allograft dysfunction in rats. Transpl Immunol 54 (2019): 20-28.
- Kang DH, Yu ES, Yoon KI, et al. The impact of gender on progression of renal disease: potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. Am J Pathol 164 (2004): 679-688.
- Sun J, Langer WJ, Devish K, et al. Compensatory kidney growth in estrogen receptor-alpha null mice. Am J Physiol Renal Physiol 290 (2006): F319-F323.
- Wu CC, Chang CY, Chang ST, et al. 17β-Estradiol Accelerated Renal Tubule Regeneration in Male Rats After Ischemia/Reperfusion-Induced Acute Kidney Injury. Shock 46 (2016): 158-163.
- Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J 29 (2007): 976-985.
- Kariya T, Nishimura H, Mizuno M, et al. TGF-β1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am J Physiol Renal Physiol 314 (2018): F167-f180.
- Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev 11 (2012): A479-A485.
- McCarthy M. The gender gap in autoimmune disease. Lancet 356 (2000): 1088.
- Mulroney SE, Pesce C. Early hyperplastic renal growth after uninephrectomy in adult female rats. Endocrinology 141 (2000): 932-937.