Incidence of Peripheral Neuropathy and Cardiovascular Adverse Effects in β-Thalassemia Patients Following Off-Label Thalidomide Therapy
Article Information
Muhammad Azaz Ali Khan*,1, Tanzeel Imran2, Asad Ullah Amjad1, Ume Amin1, Uroosa Hasan Khan1, Muhammad Usama Tahir1, Areesha Fatima1
1Medical officer Jamila Sultana Foundation
2Consultant hematologist and incharge Lab Jamila sultana foundation
*Corresponding Autho: Muhammad Azaz Ali Khan, Medical officer Jamila Sultana Foundation
Received: 23 November 2025; Accepted: 28 November 2025; Published: 10 December 2025
Citation: Dr. Muhammad Azaz Ali Khan, Dr. Tanzeel Imran, Dr. Asad Ullah Amjad, Dr. Ume Amin, Dr. Uroosa Hasan Khan, Dr. Muhammad Usama Tahir, Dr. Areesha Fatima. Incidence of Peripheral Neuropathy and Cardiovascular Adverse Effects in β-Thalassemia Patients Following Off- Label Thalidomide Therapy. Fortune Journal of Health Sciences. 8 (2025): 1153-1155.
View / Download Pdf Share at FacebookAbstract
Background: Thalidomide, used off-label as an HbF inducer, is associated with significant neurological and cardiovascular toxicity. This study examines the incidence of peripheral neuropathy and cardiovascular adverse effects among β-thalassemia patients using externally sourced thalidomide.
Methods: A retrospective review was conducted at JSF (jan 2024 to oct 2025) including patients who reported independent thalidomide use. Clinical data were analyzed to identify neurological and cardiovascular complications.
Results: Peripheral neuropathy and generalized body aches were the most prevalent neurological complications, while arrhythmias represented the most significant cardiovascular toxicity.
Conclusion: Off-label thalidomide poses substantial risks when used without clinical supervision. Improved regulation and patient education are required to prevent avoidable morbidity. However it has beneficial effect in thalassemic patient having allo antibodies.
Keywords
β-Thalassemia, Thalidomide, Periphral neuropathy, Cardiovascular toxicity, Arrythmias, Adverse drug reactions, Off label drug use
Article Details
Introduction
β-thalassemia is a hereditary hemoglobin synthesis disorder requiring lifelong transfusion therapy. Thalidomide has gained renewed interest as a low-cost fetal hemoglobin inducer in resource-limited settings without being approved by authorities for thalasemic patient. Despite reported hematological benefits, its safety profile remains concerning.
Recent observations at JSF identified a pattern of neurological and cardiovascular toxicities among patients who obtained thalidomide from off label external sources. Given the known risks of thalidomide-induced peripheral neuropathy (TIPN), coagulopathy etc and its effects on cardiac conduction, evaluating these adverse reactions is crucial. This study aims to quantify and describe the incidence of these complications in our patient population.
Methods
A retrospective review was conducted at JSF. Inclusion criteria:
- • Confirmed β-thalassemia major/intermedia
- • Patient-reported thalidomide use not prescribed by JSF
- • New neurological or cardiovascular symptoms plus other generalized and non specific symptoms
Data parameters included duration of thalidomide intake, onset of symptoms, neurological evaluation, ECG findings, and associated systemic complaints.
Results
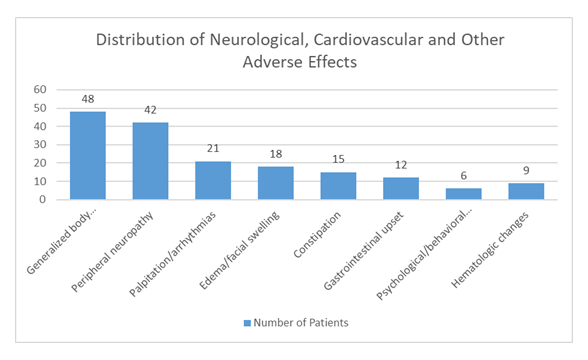
A total of 72 out of 90 symptomatic patients were confirmed to be using thalidomide obtained externally. Neurological complications were most frequently observed, followed by cardiovascular symptoms including palpitations and documented arrhythmias. Below is the distribution of adverse effects:
Table 1: Systemic adverse effects associated with thalidomide therapy
|
Adverse Effect |
Number of Patients |
|
Generalized body ache/fatigue |
48 |
|
Peripheral neuropathy |
42 |
|
Palpitation/arrhythmias |
21 |
|
Edema/facial swelling |
18 |
|
Constipation |
15 |
|
Gastrointestinal upset |
12 |
|
Psychological/behavioral changes |
6 |
|
Hematologic changes |
9 |
This table shows the number of patients developed differet side effects after taking off-label thalidomide
Discussion
Peripheral neuropathy is a well-established adverse effect of thalidomide therapy, often dose-dependent and irreversible. Our findings align with global data showing high incidence of TIPN among unmonitored users. Cardiovascular toxicity, although less recognized, poses serious risks particularly arrhythmias in thalassemia patients already predisposed to iron-overload cardiomyopathy. Lack of baseline cardiac assessment and ECG monitoring increases vulnerability. These findings highlight the consequences of unregulated thalidomide distribution in Pakistan.
Conclusion
Off-label use of thalidomide for thalassemia, especially when based on limited early studies, carries significant risks, including peripheral neuropathy, thromboembolic events, and other systemic toxicities. These dangers are heightened by inconsistent dosing and lack of clinical monitoring. Despite this, clinical evidence shows that thalidomide can offer meaningful benefits in selected patients particularly those with transfusion-dependent thalassemia complicated by alloantibodies and individuals with thalassemia intermedia by improving hemoglobin levels and reducing transfusion needs. Thus, while therapeutically promising, thalidomide requires strict medical supervision and regulatory oversight to ensure safe use.
Reference
- Bhurani D, Kapoor J, Yadav N, et al. Thalidomide has a significant effect in patients with thalassemia intermedia. Ann Hematol. (2021).
- Yang K, Wu Y, Zhou Y, et al. Thalidomide for patients with β-thalassemia: A multicenter experience. Mediterr J Hematol Infect Dis. (2020).
- Begum M, Moslem MHM, Fatema NN, et al. Outcome of treatment with thalidomide in transfusion-dependent thalassemia patients: A prospective study, Am J Pediatr, (2020).
- Khan MTM, Rahman IU, Shah H, et al. Efficacy and safety of low-dose thalidomide in transfusion-dependent thalassemia: A preliminary study, Int J Pathol, (2022).
- Li T, Chen D, Zhou Y, et al. Effects of thalidomide on erythropoiesis and iron homeostasis in transfusion-dependent β-thalassemia, Hematology, (2023).
- Al-Khalidi HA, Mohammed SA, Rashid AK. Promising response to thalidomide in symptomatic β-thalassemia: A single-center experience from Iraqi Kurdistan, Hematol Oncol Stem Cell Ther, (2020).
- Karim R, Rahman MQ, Sultana R, et al. Efficacy and safety of thalidomide in children with transfusion-dependent thalassemia: A prospective study, Pediatr Blood Cancer, (2021).
- Zhang Y, He S, Wen J, et al. Long-term clinical efficacy and safety of thalidomide in transfusion-dependent β-thalassemia: A multicenter cohort study, Sci Rep, (2023).
- Shamim F, Arif F, Haider M. Clinical patterns of thalidomide toxicity in children and adolescents with transfusion-dependent β-thalassemia, J Pediatr Hematol Oncol, (2024).
- Guo R, Li Q, Chen C, Luo M. Thalidomide for β-thalassemia: A systematic review and meta-analysis, Front Pharmaco, (2021).
- Sarwar S, Javed M, Ali N. Safety and efficacy of thalidomide versus hydroxyurea in transfusion-dependent β-thalassemia: A systematic review, Pharmacy Practice, (2025).
- Khan A, Zafar S, Javid M. Long-term follow-up of patients undergoing thalidomide therapy for transfusion-dependent β-thalassemia, Int J Gen Med, (2023).
- Li C, Fang Y, Zhou Q. Clinical efficacy of thalidomide among different β-thalassemia genotypes: A two-year evaluation, BMC Med Genomics, (2024).
- Rahman M, Hoque S, Islam K. Long-term thalidomide therapy’s efficacy and safety in β-thalassemia major: A systematic review, KMUJ, (2024).
- Haque S, Bano R, Sheikh S. Clinical efficacy and adverse-effect profile of thalidomide in transfusion-dependent β-thalassemia, Int J Biomed Res, (2023).
- Brown JD, Smith WB. Thalidomide-induced peripheral neuropathy: Mechanisms and clinical features, Clin Neuropharmacol (2019).
- Miguel L, Perez-Julian J, Cox M. Cardiovascular complications associated with thalidomide therapy: A review, Cardiovasc Toxicol (2020).
- Kumar A, Gupta S. Thalidomide-related thrombotic events in chronic anemias: Clinical patterns and risk predictors, Ann Vasc Med Res (2022).
- McGowan J, Perlman A. Systemic toxicities of immunomodulatory agents: Focus on thalidomide, Drug Saf (2018).
- Teo SK. Properties of thalidomide and its analogues: Implications for clinical safety, Drug Dev Res, (2020).
- Sammarco P, Leone G. Anti-angiogenic drug toxicities: Lessons from thalidomide, J Hematol Oncol, (2019).
- Mendes E, Bezerra I. Gastrointestinal and metabolic adverse effects of thalidomide: A clinical review, Ther Adv Drug Saf, (2021).
- Govindasamy P, Tan G, Norbaya S. Neurological complications in β-thalassemia patients receiving thalidomide: Observational findings from Malaysia, Asian Hematol J, (2023).
- Saleh A, Abdullah M, Hassan R. Cardiovascular and thrombotic toxicity of thalidomide in hemoglobinopathies: A regional cohort analysis, Middle East J Hematol, (2024).