Incidence and Risk Factors of Hypophosphatemia in Patients with HIV Infection Receiving Tenofovir Disoproxil Fumarate; a real practice report from a Hospital Belonging to a Medical Service Department
Article Information
Praewa Netsuk1, Jirapat Jullabath1, Pornchita Lakum1, Passara Phuetthanyakij1, Pongsri Phuawaranukhroh2, and Karunrat Tewthanom1, *
1Department of Pharmaceutical care, Silpakorn University, Meaung, Nakhon Pathum, Thailand
2Department of Pharmacy, Ratchaphiphat Hospital, Phutthamonthol, Nakhon Pathom, Thailand
*Corresponding author: Karunrat Tewthanom, Department of Pharmaceutical care, Silpakorn University, Meaung, Nakhon Pathom, Thailand.
Received: 07 October 2022; Accepted: 12 October 2022; Published: 26 October 2022
Citation: Praewa Netsuk, Jirapat Jullabath, Pornchita Lakum, Passara Phuetthanyakij, Pongsri Phuawaranukhroh, and Karunrat Tewthanom. Incidence and Risk Factors of Hypophosphatemia in Patients with HIV Infection Receiving Tenofovir Disoproxil Fumarate; a real practice report from a Hospital Belonging to a Medical Service Department. Archives of Microbiology and Immunology 6 (2022): 242-246.
View / Download Pdf Share at FacebookAbstract
This retrospective cross-sectional study aimed to estimate the incidence and risk factors of hypophosphatemia in patients receiving TDF-containing anti-HIV regimens. Data of patients with HIV infection who received TDF between July 1, 2018, and June 30, 2020, at a hospital belonging to a medical service department were reviewed. Data such as serum creatinine and serum phosphate levels were collected from medical records and electronic medical records and then transferred through a data record form. Hypophosphatemia was defined as serum phosphate value lower than 2.5 mg/dL. As a result, from 798 cases of patients with HIV infection who received TDF, 26 patients met the inclusion criteria and five patients had hypophosphatemia (19.2%), and the standard drug dose was used (300 mg/day) or was properly adjusted according to patients’ renal function. The median duration of TDF use was 10 (1–63) months. Other factors that may contribute to the development of hypophosphatemia are comorbidities and other drugs; there was one patient who used antacids longer than 1 week before onset of hypophosphatemia. This study may help develop a risk assessment tool for monitoring hypophosphatemia in patients who received TDF.
Keywords
Tenofovir Disoproxil Fumarate, Hypophosphatemia, HIV Infection, Incidence
Tenofovir Disoproxil Fumarate articles, Hypophosphatemia articles, HIV Infection articles, Incidence articles
Article Details
1. Introduction
Tenofovir disoproxil fumarate (TDF) is one of most commonly used antiretroviral medication in patients with HIV infection. Owing to its widespread application, studies have reported its side effects [1-2]. The most common adverse effect of TDF is nephrotoxicity [3-6]. Hypophosphathemia also occurred in most cases with TDF before nephrotoxicity was detected. Appearance can demonstrate the relationship between hypophosphatemia and nephrotoxicity [7]. As a result, the use of hypophosphatemia as a monitoring tool in routine practice is limited, but results of previous studies were controversial [8]. The incidence of hypophosphatemia ranged from 9% to 30% [5-10]. To our knowledge, no study in Thailand has examined the incidence of hypophosphatemia, but only a study on the incidence of tenofovir isoproxil fumarate-induced proximal tubulopathy in patients with human immunodeficiency virus (HIV) infection found that 9.2% of patients had hypophosphatemia [5]. Therefore, this study aimed to determine the incidence of hypophosphatemia in patients with HIV infection receiving TDF and to explore the risk factors of TDF-induced hypophosphatemia.
2. Materials and methods
2.1 Research design
This retrospective cross-sectional study was carried out to determine the incidence of hypophosphatemia in patients with HIV infection receiving TDF.
2.2 Population
The study population comprised patients with HIV infection who received TDF and visited the hospital between July 1, 2019, and June 30, 2020.
2.3 Inclusion criteria
- Receiving TDF at least 1 month
- Age >18 years
- Assessment of SCr before and after 1 month of receiving TDF as baseline values
- Serum phosphate levels were monitored before and after 1 month of receiving TDF as baseline value
- Continuous monitoring of SCr and serum phosphate at least once after receiving TDF
- Antiretroviral therapy with dose modified according to renal function by considering creatine clearance
Exclusion criteria
- Patients who withdraw from the study or died of causes other than hypophosphatemia.
- Patients who stopped using TDF for reasons other than hypophosphatemia.
The cutoff value of hypophosphatemia was serum phosphate below 2.5 mg/dL.
2.4 Data collection
The data collection form consisted of the following information:
- Demographic data
- Treatment information
- Laboratory information before and after receiving TDF, Scr, and serum phosphate
- Other factors that affect serum phosphate level (such as alcoholic cirrhosis, malnutrition, Crohn’s disease, severe vomiting, steatorrhea, chronic diarrhea, diabetic ketoacidosis, and infection) and use of drug-induced hypophosphatemia (increase phosphate excretion: adefovir, ifosfamide, cefepime, and ceftolozane/tazobactam; increase phosphate absorption: antacid, high-dose niacin, and acetazolamide) were recorded.
For data collection, Google Forms was used via QR code (https://forms.gle/vGY3vgyTnhxFAM4y9).

2.5 Data analysis
The incidence of hypophosphatemia was calculated using the following formula:
Incidence of hypophosphatemia =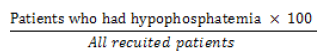
Factors associated with hypophosphatemia were analyzed by multivariate regression with level of significance set at 0.05.
This study was approved by the ethics committee of Silpakorn University, Nakhon Pathom, Thailand (REC 631012-1205118), and the local hospital (Ratphiphat Hospital ethic committee.
3. Results
3.1 Demographic data
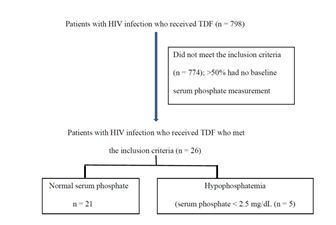
A total of 26 patients were recruited, and most of the patients were male (76.9) with median weight of 59 kg. The median duration of using TDF was 28.5 months. The median serum creatinine and serum phosphate levels at baseline were 0.8 mg/dL and 4.1 mg/dL, respectively. Only one patient had concomitant drug that can cause hypophosphatemia (Table 1).
|
Characteristics |
Results |
|
Mean Age (SD) |
46.65 (13.23) years |
|
Range |
21 - 70 years |
|
Gender |
|
|
- Male (%) |
20 (76.9) |
|
- Female (%) |
6 (23.1) |
|
Weight [median (IQR)] |
59.0 (48.5-73.6) kg |
|
Range |
32.3 - 95 kg |
|
Duration of using TDF, [Median (IQR)] |
28.5 (18.3-64.0) months |
|
Range |
2-102 months |
|
serum creatinine baseline [Median, (IQR)] |
0.8 (0.7-1.0) mg/dL |
|
Range |
0.41 - 2.14 mg/dL |
|
Serum Phosphate baseline [Median, (IQR)] |
4.1 (3.6-4.7) mg/dL |
|
Range |
1.1 - 5.6 mg/dL |
|
Number of patients who use concurrent drugs that caused hypophosphatemia - Antacid |
1 (3.8) |
Table 1: Demographic data (n = 26)
3.2 Incidence of hypophosphatemia
In the data collection, 798 patients with HIV infection received TDF. However, 774 patients did not meet the inclusion criteria. Approximately 50% of these patients did not have serum phosphate measurement at baseline. Therefore, 26 patients were recruited, of which five patients had hypophosphatemia (Figure 1). The calculated incidence is 19.2 and median duration of receiving TDF until hypophosphatemia occurred is 10 months as presented in Table 2
|
Include patients (cases) |
26 |
|
Patients with hypophosphatemia (cases) |
5 |
|
Incidence of hypophosphatemia |
19. 2 |
|
Median duration of receiving TDF until hypophosphatemia occurred (range) in month |
10 (1-63) |
Table 2: Incidence of hypophosphatemia and duration
3.3 Cases of hypophosphatemia
Table 3 summarizes data of five patients who received TDF and developed hypophosphatemia.
All patients did not have comorbidity. The suspected comedication that could have caused hypophosphatemia was not identified, except in patient 2 who received antacid with TDF for 8 weeks, following which hypophosphatemia occurred. All patients took 300 mg of TDF at bedtime, or the dose was adjusted according to their renal function. The serum phosphate level returned to normal values in patients 1, 3, and 4 within 1 week, 3 days, and 1 month, respectively, without any intervention.
|
Factors |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
|
Comorbidity |
- |
- |
- |
- |
- |
|
Medication |
- |
Antacid for 8 weeks |
- |
- |
- |
|
Baseline |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
|
Serum Phosphate (mg/dL) |
4.4 |
2.7 |
3.5 |
4 |
3.7 |
|
Scr (mg/dL) |
0.8 |
0.9 |
0.86 |
0.72 |
0.82 |
|
Visit 1 |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
|
Serum Phosphate (mg/dL) |
2.1 |
1.9 |
2.4 |
2.2 |
2.4 |
|
Scr (mg/dL) |
1.15 |
1.8 |
0.75 |
0.58 |
1.18 |
|
Dosage adjustment |
- |
TDF 300 mg q 48 hr |
- |
- |
- |
|
Monitoring |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
|
Serum Phosphate (mg/dL) |
3.1 |
- |
2.7 |
2.0 then 3.1 |
- |
|
Scr (mg/dL) |
1.01 |
- |
0.95 |
2.0 then 3.1 |
- |
Table 3: Characteristics of patients with HIV infection who developed hypophosphatemia
4. Discussion
4.1 Incidence of hypophosphatemia
The incidence of hypophosphatemia in this study was similar to that in a previous study [6] of 145 patients with HIV infection, which also reported a relationship between nephrotoxicity and increase risk of severe renal tubular damage despite normal serum creatinine level. The mean duration of using TDF until hypophosphatemia occurred was 11+ 9 months, and the incidence of hypophosphatemia was 26%. Hypophosphatemia could be explained by the mechanism of TDF that induces renal tubular damage by decreasing mitochondria function, which leads to Fanconi syndrome and disrupted phosphate absorption because of phosphaturia [10, 12].
4.2 Risk factors of hypophosphatemia
Previous studies have investigated risk factors of hypophosphatemia [13-17], and factors are classified as comorbidity factors and comedication factors [15-17]. Comorbidities that affect serum phosphate levels are alcoholism, malnutrition, Crohn’s disease, severe vomiting, steatorrhea, diabetic ketoacidosis, and sepsis. Comedications that influence serum phosphate level include adefovir, ifosfamide, cefepime, and ceftolozane/tazobactam which can increase phosphate excretion. Nevertheless, some medications interfere with phosphate absorption, such as antacids, high-dose niacin, and acetazolamide [15-17]. In this study, only one patient had one risk factor; the patient was also taking antacid. Given the small sample size, this study could not use statistical methods to evaluate or explore significant factors that influence hypophosphatemia. Although nearly all cases did not have evidence of risk factors that increase the risk of hypophosphatemia, undetermined risk factors may have existed, such as poor nutrition status which is not usually evaluated in routine practice.
This study has some limitations. First, data may have been insufficient and incomplete owing to the retrospective collection of data. Therefore, the calculated incidence and onset of events were only estimated from existing data. To confirm this value, a prospective study is warranted. Second, given the small sample size, further statistical analysis was not performed to determine significant factors associated with hypophosphatemia regardless of TDF.
Acknowledgement
The researchers thank the kind cooperation of all staffs. In addition, the researchers acknowledge the financial support of the Faculty of Pharmacy, Silpakorn University.
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics committee of Silpakorn University, Nakhon Pathom, Thailand, and the local hospital (Ratphiphat Hospital) (REC 631012-1205118).
Author Contribution
Conceptualized: Karunrat Tewthanom, Pongsri Phuawaranukhroh
Data curation: Praewa Netsuk, Jirapat Jullabath, Pornchita Lakum, Passara Phuetthanyakij
Investigation: Praewa Netsuk, Jirapat Jullabath, Pornchita Lakum, Passara Phuetthanyakij
Formal analysis preparing Tables and Figures: Praewa Netsuk, Jirapat Jullabath, Pornchita Lakum, Passara Phuetthanyakij
Writing and manuscript preparations and submission: Karunrat Tewthanom
Reviewing and Editing manuscript: Praewa Netsuk, Jirapat Jullabath, Pornchita Lakum, Passara Phuetthanyakij, Karunrat Tewthanom, Pongsri Phuawaranukhroh
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
ORCID
Karunrat Orchid ID: https://orcid.org/0000-0001-8496-3848
References
- Wassner C, Bradley N, Lee Y. A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide. Journal of the International Association of Providers of AIDS Care (JIAPAC) 19 (2020): 232595822091923.
- Fernandez-Fernandez B, Montoya-Ferrer A, Sanz A, et al. Tenofovir Nephrotoxicity: 2011 Update. AIDS Research and Treatment (2011): 1-11.
- Bunpeth W, Supasyndh O, Satirapoj B. et al among HIV Positive Patients Receiving Tenofovir Based Anti-Retroviral Therapy. J Southeast Asian Med 1 (2017): 6-11.
- Dauchy F, Lawson-Ayayi S, de La et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney International 3 (2011): 302-309.
- Bunpeth W, Supasyndh O, Satirapoj et al among HIV Positive Patients Receiving Tenofovir Based Anti-Retroviral Therapy. J Southeast Asian Med Res 1 (2017): 6-11.
- Badiou S, Merle De Boever C, Terrier N, et al Is tenofovir involved in hypophosphatemia and decrease of tubular phosphate reabsorption in HIV-positive adults?. Journal of Infection 52 (2006): 335-338.
- Casado J, Bañón S, Santiuste C, et al. Prevalence and significance of proximal renal tubular abnormalities in HIV-infected patients receiving tenofovir. AIDS 30 (2016): 231-239.
- Day S, Leake Date H, Bannister A, et al. Hypophosphatemia in Tenofovir Disoproxil Fumarate Recipients Is Multifactorial in Origin, Questioning the Utility of Its Monitoring in Clinical Practice. J Acquir Immune Defic Syndr 38 (2005): 301-304.
- Kichloo A, Chugh S, Gupta S et al. Tenofovir and Severe Symptomatic Hypophosphatemia. Journal of Investigative Medicine High Impact Case Reports 7 (2019): 232470961984879.
- Buchacz K, Brooks J, Tong T et al. Evaluation of hypophosphataemia in tenofovir disoproxil fumarate (TDF)-exposed and TDF-unexposed HIV-infected out-patients receiving highly active antiretroviral therapy. HIV Medicine 7 (2006): 451-456.
- Cochran W. G., Cochran W. G. Sampling Techniques. 3rd ed. New York: John Willey and Son; 1977.
- Jotwani V, Atta M, Estrella M. Kidney Disease in HIV: Moving beyond HIV-Associated Nephropathy. Journal of the American Society of Nephrology 28 (2017): 3142-3154.
- Ritz P. Acute hypophosphatemia. Kidney International 22 (1982): 84-94.
- Liamis G, Milionis H, Elisaf M. Medication-induced hypophosphatemia: a review. QJM. 103 (2010): 449-459.
- Hypophosphatemia: Causes of hypophosphatemia [Internet]. Uptodate.com. 2021 [cited 19 May 2021].
- Shields H. Rapid fall of serum phosphorus secondary to antacid therapy. Gastroenterology 75 (1978): 1137-1141.
- Maccubbin D, Tipping D, Kuznetsova O, et al . Hypophosphatemic Effect of Niacin in Patients without Renal Failure: A Randomized Trial. Clinical Journal of the American Society of Nephrology. 5 (2010): 582-589.