In Vitro Proof of Concept Evaluation of the Radial Force Generated by a Novel Mechanical Thrombectomy Device that Incorporates Controlled, Expandable Wire Spheres
Article Information
Michael Bruce Horowitz1*, Jack B. Sattell2, Brandon Repko3, Hieu Le4, Benjamin Bobo4
1HCA Florida First Coast Neurosurgery HCA Florida-Orange Park Hospital 1825 Kingsley Avenue, Suite 170 Orange Park, FL 32073, United States
256 Portsmouth Street Cambridge, MA. 02141, United States
3Department of Radiology Butler Medical Center, 1 Hospital Way, Butler, PA 16001, United States
4Retriever Medical, 6016 Agatha Christie Ave, Las Vegas, NV 89131, United State
*Corresponding author: : Michael Bruce Horowitz, MD. HCA Florida First Coast Neurosurgery HCA Florida-Orange Park Hospital 1825 Kingsley Avenue Suite 170, Orange Park, FL 32073, United States
Received: 18 January 2024; Accepted: 23 January 2024; Published: 31 January 2024
Citation: Michael Bruce Horowitz, Jack B. Sattell, Brandon Repko, Hieu Le, Benjamin Bobo. In Vitro Proof of Concept Evaluation of the Radial Force Generated by a Novel Mechanical Thrombectomy Device that Incorporates Controlled, Expandable Wire Spheres. Cardiology and Cardiovascular Medicine. 8 (2024): 43-47.
View / Download Pdf Share at FacebookAbstract
Background: This study measured the radial force (RF) imparted upon a vessel wall when a manually controlled, expandable wire sphere that was incorporated into a mechanical thrombectomy device was expanded to oppose the vessel wall. Results would help determine the device’s safety profile with regards to risk of vessel rupture during device deployment.
Methods: A novel proprietary manually controlled, expandable, wire sphere (Retriever Medical, Las Vegas, NV, USA) was advanced in its collapsed form into an MSI RX 650 RF Tester (Machine Solutions Inc., Flagstaff, AZ, USA). The device cylinder iris was adjusted to provide cylinder test diameters of 5 mm, 7.5 mm and 10 mm. RF exerted by the expandable sphere was measured at each of the above diameters. The testing was performed with the thrombectomy device delivered along both a straight and tortuous course to determine whether or not pathway complexity influenced the terminal RF generated.
Results: Maximum RF generated by the wire sphere was 22 Newtons (N). RF increased as cylinder and wire sphere diameter increased from 5 – 10 mm. No significant differences in generated RF were detected when the straight and tortuous device delivery pathway were compared.
Conclusion: The RF generated by the novel, proprietary, manually controlled, expandable wire sphere designed to perform mechanical thrombectomy fell far below the established N required to cause rupture of the saphenous vein and pulmonary artery. This information, combined with our prior published work showing no venous endothelial damage following porcine In Vivo sphere manipulation, provides additional evidence supporting the device’s favorable safety profile. Trial Registration: This article does not report the results of a health care intervention on human subjects.
Keywords
Thrombectomy; Radial Force; Chronic Outward Force; Radial Resistive Force; Mechanical; Venous
Thrombectomy articles; Radial Force articles; Chronic Outward Force articles; Radial Resistive Force articles; Mechanical articles; Venous articles.
Article Details
Abbreviations:
Newtons; N: Millimeters; mm: Meters; M: Mercury; Radial Force, RF: Radial Resistive Force (RRF); Chronic Outward Force ; COF
1. Introduction
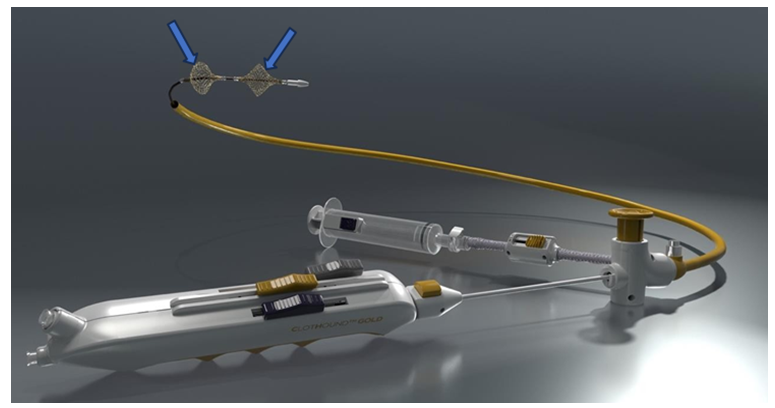
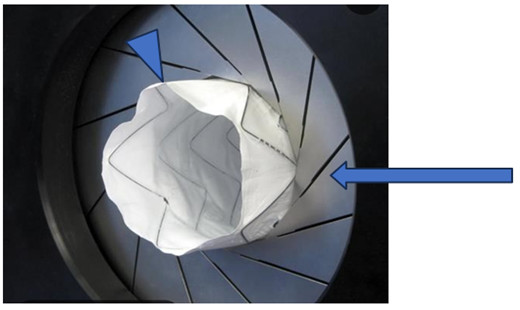
The authors have recently reported results after testing a novel mechanical thrombectomy device (Figure 1) aimed towards removing acute, subacute, and chronic thrombus from peripheral and more central (pulmonary) porcine venous conduits [1]. This study demonstrated no histologic damage nor angiographic abnormalities in veins after a mechanically expandable wire thrombectomy sphere was expanded and slid along the vessel wall (Table 1). Lack of injury was noted despite repeatedly expanding the device 200% of the angiographically measured vein’s normal diameter (Figure 2).
Table 1: Venous histologic findings post vessel manipulations using DVT or PE mechanical thrombectomy device (1)
When evaluating devices (stents, balloons) expanded within a geometric volume, the terms RF, Radial Resistive Force (RRF), Chronic Outward Force (COF) and hoop force (HF) are often used interchangeably [2]. While these terms are not necessarily mathematically equivalent, they are in essence expressions of the forces generated perpendicularly outward from the center of a sphere or from the central axis of a cylinder. This outward force resists external compressive forces. For the purposes of this study, the term RF will refer to the force generated against a vessel wall by our device’s expanding thrombectomy sphere. A Radial Force Tester (MSI RX650, MSI, Flagstaff, AZ, USA) measured the RF generated by the mechanical thrombectomy device at a variety of expanded diameters. By determining the RF generated by the expanded thrombectomy spheres, it may be possible to provide further information to clinicians regarding the likelihood (ie. risk) of vessel rupture during device manipulation (device expansion and radial translation).
2. Methods
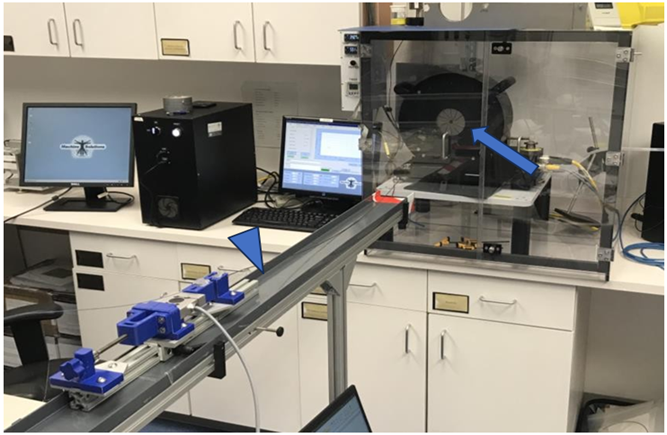
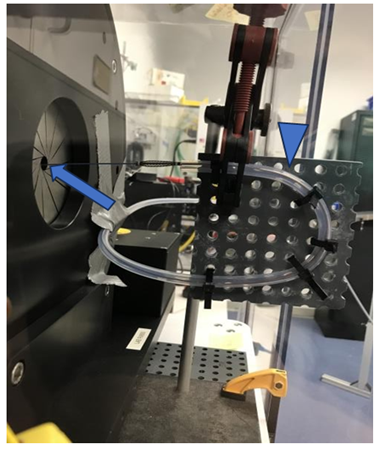
This study was performed at Machine Solutions, Inc (Flagstaff, AZ, US) using an MSI RX 650 Radial Force Tester (Figure 2). The study goal was to understand the RF generated by the mechanical thrombectomy device at a variety of expanded diameters. Vessel diameters were selected by adjusting the inner diameter of the RX650 iris. Within each diameter, the spheres were expanded until a RF plateau was reached. In order to determine how tortuosity affected these measurements, the RF was measured by first deploying the device along a straight course (Straight Course Force Testing) (Figure 3) and then deploying it after being advanced through a tortuous path (Tortuous Path Force Testing) (Figure 3). The latter required advancing the system through a 4 cm radius, 180-degree bend followed by an 8 cm radius, 90-degree bend.
The Straight and Tortuous RF Testing was performed at a variety of vessel (iris) diameters (5mm; 7.5mm; 10mm) to determine how the expanded diameter of the mechanical wire thrombectomy spheres might affect the generated RF.
3. Results
Using the above methodology, the following was determined:
1. The expanded spheres could generate up to 22N RF against the iris
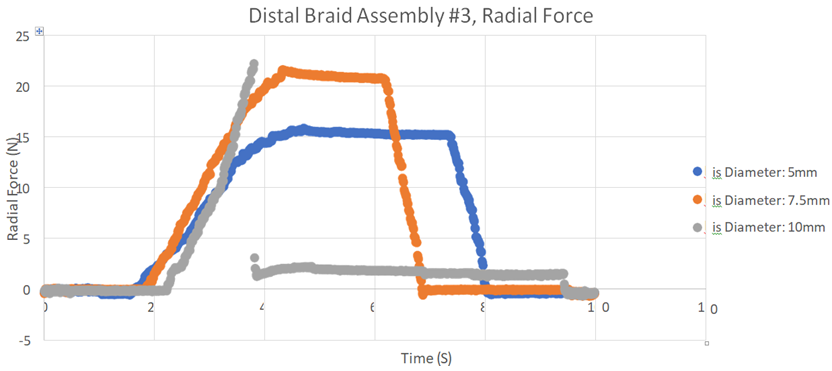
2. RF applied to the iris increased as the diameter of the iris was increased (Figure 5).
3. There was no significant difference seen in wire sphere RF generation when the straight and tortuous course models were compared.
Figure 5: This graph records the RF generated when the wire sphere was expanded within a cylinder variably adjusted to diameters of 5 mm, 7.5 mm, and 10 mm. Note that as the thrombectomy sphere is expanded against a larger diameter cylinder wall, the RF increases to a peak of 20 N at 10 mm. The curves show the RF in each diameter cylinder as the mechanical sphere is expanded and then collapsed.
4. Discussion
The authors’ initial publication [1] demonstrated that repeated use of their novel mechanical thrombectomy device (expansion and linear translation) in porcine iliac veins, jugular veins, and pulmonary arteries (even when expanded 200% beyond the vessel’s native diameter) induced no histologic vascular wall injury. Measurement of the device’s actual approximate RF generation was felt to be another important data point needed to assure clinicians that the system could safely operate within the biologic mechanical constraints of target vessels. RF was measured in Newtons (1N is the force required to accelerate a 1 kg object 1 meter per second 2; 1N = 1kg m/s2). In order to compare our values to those published by other investigators we used the following conversion factors:
1 mmHg = 133.322 N/m2
1 mmHg = 1.33322 millibars
1 millibar = 100 N/m2
According to works performed by Kirschbaum and Neufurth [3, 4] the burst pressure for the saphenous vein is at a minimum 1373 millibar or 137,000 N-m2 while the burst pressure for the porcine pulmonary artery is 140 mmHg or 18,665 N-m2. These reported burst pressures far exceed the RF generated by the described thrombectomy device’s expanded mechanical spheres (22 N), thus indicating that vessel rupture during deployment is highly unlikely. The difference in rupture force vs device RF also helps explain the paucity of vessel injury documented in our previously published animal study.
Conclusion:
This study measured the RF generated by a previously described novel expandable mechanical wire sphere designed to assist with peripheral and central venous thrombectomy. The recorded maximum RF of 22 N falls far below the published forces required to rupture the saphenous vein and pulmonary artery. Combining these findings with those of our prior histologic publication [1] seems to lend further credence to the device’s ability to atraumatically remove venous thromboemboli.
DECLARATIONS
Consent:
This in vitro study did not require consent
No individual personal data was contained in this study.
Availability of Data and Materials:
The datasets used and or analyzed during the current study are available from the corresponding author on reasonable request. All data analyzed are included.
Competing Interests:
Michael Horowitz, MD (MH) is a device inventor, Co-Founder of Retriever Medical. He holds equity in Retriever Medical.
Jack Sattell has no competing interests.
Brandon Repko, MD (BR) is a device inventor and Consultant for Retriever Medical. He holds equity in Retriever Medical.
Hieu Le (HL) is a device inventor and Vice President of Retriever Medical. He holds equitv in Retriever Medical.
Benjamin Bobo (BB) is a device inventor, Co-Founder of Retriever Medical, and CEO of Retriever Medical. He holds equity in Retriever Medical.
Funding:
This study was designed and funded by Retriever Medical, Inc. (Las Vegas, Nevada, USA) Radial force testing was performed by a Distal Solutions. (Westborough, MA, USA)
Author Contributions:
Study design: MH, BR, HL and BB. Study performance: JS
Manuscript preparation: MH, BR, BB
References
- Horowitz MB, Repko B, Hieu L, et al. Porcine testing of a second generation novel mechanical thrombectomy device to evaluate efficacy in performing experimental pulmonary embolus extraction along with effects on arterial and venous intima and vessel integrity. Journal of Biology and Today’s World 11(2022): 1-4.
- Duerig TW, Tolomeo DE, Wholey M. Overview of superelastic stent design. Minim Invasive Ther 9 (2000): 235-246.
- Kirschbaum A, Sassa T, Palade E. Burst Pressureof the Central Pullmonary Artery after Bipolar Vessel Sealing- Examination in an Ex Vivo Model. Zentralbl Chir 139 (2014): 342-345.
- Neufurth M, Wang X, Tolba E, et al. Modular Small Diameter Vascular Grafts with Bioasctive Functionalities 10 (2015): e0133632.