Implications of High Levels of Activin B in Human Keloid: A Case Report
Article Information
Seungmin Ham, PhD1,3, Craig Harrison, PhD2, Peter Temple-Smith, PhD1#*, Graeme Southwick, MD1,4#
#Authors equally contributed to this work as last authors
1Department of Obstetrics and Gynaecology, Monash University, Melbourne, Victoria, 3168, Australia
2Department of Physiology, Monash University, Melbourne, Victoria, 3168, Australia
3Hudson Institute of Medical Research, Melbourne, Victoria, 3168, Australia
4Melbourne Institute of Plastic Surgery, Malvern, Victoria 3144, Australia
*Corresponding Author: Prof. Peter Temple-Smith, Department of Obstetrics and Gynaecology, School of Clinical Sciences at Monash Health, Monash University, Level 5, Monash Medical Centre, Clayton VIC 3168 Australia
Received: 15 July 2022; Accepted: 25 July 2022; Published: 25 August 2022
Citation: Seungmin Ham, Craig Harrison, Peter Temple-Smith, Graeme Southwick. Implications of High Levels of Activin B in Human Keloid: A Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 591-594.
View / Download Pdf Share at FacebookKeywords
Keloid; Activins; Human dermal fibroblasts; Gene expression; Protein expression
Keloid articles; Activins articles; Human dermal fibroblasts articles; Gene expression articles; Protein expression articles
Keloid articles Keloid Research articles Keloid review articles Keloid PubMed articles Keloid PubMed Central articles Keloid 2023 articles Keloid 2024 articles Keloid Scopus articles Keloid impact factor journals Keloid Scopus journals Keloid PubMed journals Keloid medical journals Keloid free journals Keloid best journals Keloid top journals Keloid free medical journals Keloid famous journals Keloid Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Activins articles Activins Research articles Activins review articles Activins PubMed articles Activins PubMed Central articles Activins 2023 articles Activins 2024 articles Activins Scopus articles Activins impact factor journals Activins Scopus journals Activins PubMed journals Activins medical journals Activins free journals Activins best journals Activins top journals Activins free medical journals Activins famous journals Activins Google Scholar indexed journals Encephalitis articles Encephalitis Research articles Encephalitis review articles Encephalitis PubMed articles Encephalitis PubMed Central articles Encephalitis 2023 articles Encephalitis 2024 articles Encephalitis Scopus articles Encephalitis impact factor journals Encephalitis Scopus journals Encephalitis PubMed journals Encephalitis medical journals Encephalitis free journals Encephalitis best journals Encephalitis top journals Encephalitis free medical journals Encephalitis famous journals Encephalitis Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Protein expression articles Protein expression Research articles Protein expression review articles Protein expression PubMed articles Protein expression PubMed Central articles Protein expression 2023 articles Protein expression 2024 articles Protein expression Scopus articles Protein expression impact factor journals Protein expression Scopus journals Protein expression PubMed journals Protein expression medical journals Protein expression free journals Protein expression best journals Protein expression top journals Protein expression free medical journals Protein expression famous journals Protein expression Google Scholar indexed journals Gene expression articles Gene expression Research articles Gene expression review articles Gene expression PubMed articles Gene expression PubMed Central articles Gene expression 2023 articles Gene expression 2024 articles Gene expression Scopus articles Gene expression impact factor journals Gene expression Scopus journals Gene expression PubMed journals Gene expression medical journals Gene expression free journals Gene expression best journals Gene expression top journals Gene expression free medical journals Gene expression famous journals Gene expression Google Scholar indexed journals Human dermal fibroblasts articles Human dermal fibroblasts Research articles Human dermal fibroblasts review articles Human dermal fibroblasts PubMed articles Human dermal fibroblasts PubMed Central articles Human dermal fibroblasts 2023 articles Human dermal fibroblasts 2024 articles Human dermal fibroblasts Scopus articles Human dermal fibroblasts impact factor journals Human dermal fibroblasts Scopus journals Human dermal fibroblasts PubMed journals Human dermal fibroblasts medical journals Human dermal fibroblasts free journals Human dermal fibroblasts best journals Human dermal fibroblasts top journals Human dermal fibroblasts free medical journals Human dermal fibroblasts famous journals Human dermal fibroblasts Google Scholar indexed journals
Article Details
1. Introduction
Keloid is a benign tumour caused by an injury to skin with resultant abnormal proliferation of fibrous tissues that grow outside the region of injury leading to a symptomatic disfiguring scar. Studies have shown that patient ethnicity directly influences the incidence of keloids; individuals with pigmented skin have more than five times higher incidence of developing keloids compared to non-pigmented skin [1]. The average onset of keloids is in the mid-20s for both males and females, with females having a higher incidence than males [2]. Despite numerous studies, there are no effective treatments currently. Activins are members of the transforming growth factor-β (TGF- β) superfamily and were originally found to regulate follicle-stimulating hormone biosynthesis [3]. Although different subtypes of activins exist, activin A and activin B are the best studied of these dimeric proteins. Activin A and B genes are strongly expressed during wound healing in mice and are known to be important during skin wound repair [4]. Activin A is highly expressed in keloid fibroblasts [5, 6], but in contrast activin B gene expression is usually very low or undetectable and, as a consequence, there has been little interest in activin B and keloids. However, a recent study that explored the molecular mechanism of hypertrophic scar formation in the bile duct showed that activin B had an important role in transforming normal fibroblasts to scar fibroblasts [7]. In this case, we report supraphysiological levels of activin B gene and protein expression in dermal keloid fibroblasts from a patient with bilateral ear keloids and discuss the potential significance of this finding.
2. Case Report
A 21-year-old Caucasian woman with an unremarkable medical history presented with benign tumour on the right ear lobe and to a lesser extent on the left ear lobe. Six years earlier she had undergone uneventful bilateral earlobe piercing and 12 months prior to presentation she noted the onset of an itchy, tender and sometimes painful nodule growing from the piercing site in both ear lobes, which was worse on the right side (Figure 1a). A diagnosis of ear lobe keloid was made and surgical excision was undertaken to remove 13 x 7 x 3 mm anterior and 15 x 4 x 9 mm posterior keloid growths from the right earlobe respectively (Figure 1b). No recurrence of the earlobe keloid has been reported by the patient since surgery. Other relevant history included the presence of a normal back scar following the surgical removal of a benign naevus, other helical rim and umbilical piercings without keloid formation and the presence of tattoos which had healed normally.
Keloid tissues were fixed in 10% formalin, embedded in paraffin wax, sectioned at a thickness of 5μm and stained with either haematoxylin and eosin (H&E) or Masson's trichrome stain. Histological examination revealed a thickened, flattened epidermis (Figure 1c, black arrow) with a very thick papillary dermis (Figure 1c, blue arrow) compared to normal (Figure 1e and Figure 1f) and no evidence of malignancy. In these sections, an increased cell number was observed in the papillary dermis which correlated with the presence of high levels of collagen in keloid tissue as shown by extensive Masson trichrome staining in papillary dermis (Figure 1d).
Gene expression studies of in vitro cultured keloid fibroblasts from the patient showed high levels of both activin A (INHBA) and B (INHBB) genes compared to fibroblasts from normal skin samples (n=4) (Figure 2a and 2b, respectively). High INHBA and INHBB were directly related to a high content of activin A and B proteins in cell lysate (Figure 2c and 2d, respectively). Moreover, high activin A expression in these keloid fibroblasts was correlated with high levels of activin A in serum-free medium (Figure 2e). Fibroblasts from the keloid patient also secreted unusually large amounts of activin B into serum-free medium (Figure 2f), but activin B was not detectable in medium from cultured normal fibroblasts.
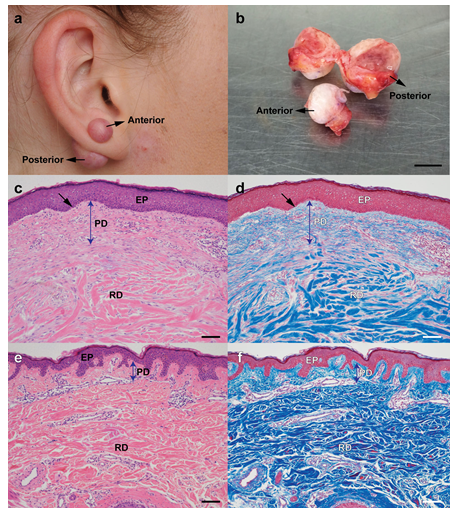
Figure 1: Keloid tissues and morphological analysis. A patient demonstrates right earlobe keloid (a) and keloid tissues were taken out by surgical methods (b). After removing tissues, Haematoxylin and Eosin (H&E) (c) or Masson's trichrome stain (d) were performed on the tissue sections and compared with similarly stained normal non-keloid adjacent tissue (e, f) [{Epidermis (EP), Papillary Dermis (PD), and Reticular Dermis (RD), Scale bar represents 100 μm].
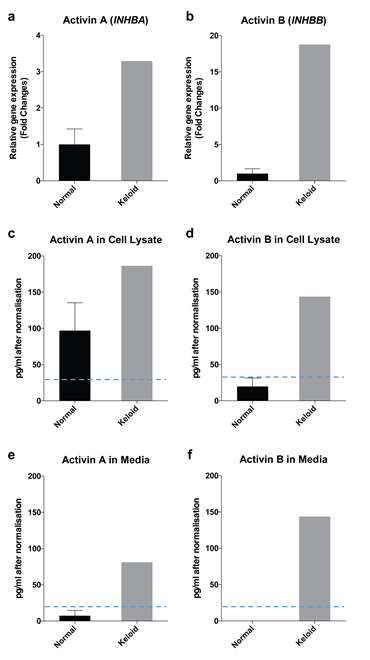
Figure 2: In vitro assessment of fibroblasts for activin expression. Activin A gene (INHBA) (a) and activin B gene (INHBB) (b) were highly expressed in keloid fibroblasts. Consistent with gene expressions, activin A (c, e) and activin B (d, f) proteins were highly expressed in keloid fibroblasts compared to normal fibroblasts in both cell lysate (c, d) and cultured media (e, f). Normal data (n=4) were presented as mean ± standard error of the mean (SEM). [dashed lines =detection levels of ELISA].
3. Discussion
We report the case of a 21 year old Caucasian girl with ear lobe keloids whose fibroblasts produce the expected high levels of activin A, but unusually also produce significant amounts of activin B. Activins have many functions in wound healing and fibrosis and activin A has been shown to stimulate cell proliferation and differentiation during wound repair. Keloid fibroblasts have been reported to produce 29 times higher activin A levels than normal fibroblasts [6]. In mice, activin A and B mRNA were significantly upregulated within seven days of wound healing [4] and transgenic mice, which overexpress activin A, show increased wound healing [8]. This high level activin A gene expression was also found in keloid fibroblasts from our patient and the high INHBA gene expression was positively correlated with high levels of activin A protein in the lysate of fibroblasts and in activin A protein secreted in vitro in cultured fibroblast medium [9]. Importantly, activin A secreted by fibroblasts may also affect other cells located in human skin. These observations show a clear and important link between the fibrotic and wound repair activity of keloid fibroblasts and activin A.
Although activin B has similar functions to activin A, few studies have focused on the relationship of activin B to fibrotic diseases [3]. This may be due to the very low levels of activin B gene and protein expression measured previously in normal and keloid dermal fibroblasts [5]. This study confirmed extremely low basal levels of activin B in cell lysates of normal fibroblasts from our keloid patient but showed unusually high and significantly elevated levels of activin B in cell lysates from her keloid fibroblasts, which were positively associated with markedly upregulated expression of the activin B gene (INHBB). Previous work has shown that high expression of activin B can affect keratinocytes in the epidermis, as well as fibroblasts in the dermis and that activin B is involved in epithelial wound healing in vivo [10]. A recent observation showed that activin B levels were higher in cancer tumours through the SMAD signalling pathway [11], which may explain why keloids have been shown to have tumour-like characteristics. Further evidence for the importance of activin B in promoting tumour growth comes from the activin B knock-out mouse model in which tumour growth is inhibited [12]. In addition, Deng et al. [7] recently showed that siRNA-Act B treatment of scar fibroblasts significantly decreased Act B mRNA expression and significantly increased early apoptosis in these cells suggesting that Activin B has an important role in the transformation and expansion of scar fibroblasts. Therefore, activin B appears to have an important role, with activin A, in the initial development and expansion of keloid tissues and that activin A is important in continued maintenance of keloid tumour expansion. This suggests the possibility that tissues from established keloids, rather than early developing and expanding keloid tumours, have been the focus of most past studies.
An interesting question for this case report is why our patient developed keloids in her ear lobes 5 years after they were pierced, and why other piercings in her umbilicus as well as her back scar and tattoo perforations did not produce similar keloid reactivity? This suggests that there was an external influence on the development of her ear lobe keloids. The cause of this remains unknown but possibilities include a low grade subclinical infection, a local reaction to an allergenic metal, such as nickel, used in posts or earrings, or a skin response or sensitivity to some other reactive agent. Exhaustive questioning of the patient failed to elicit an obvious cause.
4. Conclusions
The findings in this report emphasise the possibility that keloid response to injury is likely to be multifactorial and that the high activin B gene and protein expression in this patient may be due to the keloid initiation response from an extrinsic promoter rather than, or in addition to, a hereditary response to local injury. The results from this study suggest that blocking the activity of activins using a powerful activin inhibitor, like follistatin, may have an important role in future keloid treatment to suppress, or even reverse, keloid progression.
Acknowledgments
We thank Oxford Brookes University for kindly supplying reagents used in the activin assays and Professor David de Kretser for advice and providing assay services. We also acknowledge use of facilities at Monash University (Monash Histology Platform and Monash Health Translation Precinct Medical Genomics Facility, Australia). We thank Simone Goddard (Melbourne Institute of Plastic Surgery, Australia) for organising patient samples and information and Dr. Sarah Meachem for critical reading of the manuscript.
Data Availability
All data generated or analysed during this study are included in this published article.
Author Contributions Statement
SH contributed acquisition, analysis and interpretation of data, writing, reviewing and editing manuscript; CH contributed to manuscript drafting and supervision; PTS conceived the project, contributed to data analysis, manuscript drafting and supervision; GS conceived the project, collected human samples, contributed to manuscript drafting and supervision.
Funding sources
This research was supported by funding from the Advanced Plastic Surgery Education Foundation (Australia). We also acknowledge support for this project from the Glitter Ball Committee (Australia). SH was a recipient of Monash International Postgraduate Research Scholarship and Monash Graduate Scholarship through Australian Government Research Training Program Scholarship (Australia) and a Postgraduate Publications Award from Monash University.
Conflict of Interest
The authors declare no conflict of interest. This research is purely science-based work without bias. The funding bodies had no role in conceptualising, designing, conducting, data analysis or manuscript preparation for this study.
References
- LeFlore IC. Misconceptions regarding elective plastic surgery in the black patient. J Natl Med Assoc 72 (1980): 947-948.
- Cosman B, Crikelair GF, Ju DMC, et al. The surgical treatment of keloids. Plastic and Reconstructive Surgery 27 (1961): 335-358.
- Hedger MP, Winnall WR, Phillips DJ, et al. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm 85 (2011): 255-297.
- Hubner G, Hu Q, Smola H, et al. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev Biol 173 (1996): 490-498.
- Ham S, Harrison C, de Kretser D, et al. Potential treatment of keloid pathogenesis with follistatin 288 by blocking the activin molecular pathway. Exp Dermatol 30 (2021): 402-408.
- Mukhopadhyay A, Chan SY, Lim IJ, et al. The role of the activin system in keloid pathogenesis. Am J Physiol Cell Physiol 292 (2007): C1331-1338.
- Deng SK, Tang JZ, Jin Y, et al. Activin B signaling may promote the conversion of normal fibroblasts to scar fibroblasts. Medicine (Baltimore) 99 (2020): e20253.
- Munz B, Smola H, Engelhardt F, et al. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis dermal fibrosis and wound repair. EMBO J 18 (1999): 5205-5215. 188
- Ham S, Harrison C, de Kretser D, et al. Potential treatment of keloid pathogenesis with follistatin 288 by blocking the activin molecular pathway. Exp Dermatol (2020).
- Zhang M, Liu NY, Wang XE, et al. Activin B promotes epithelial wound healing in vivo through RhoA-JNK signaling pathway. PLoS One 6 (2011): e25143.
- Xiong S, Klausen C, Cheng JC, et al. Activin B induces human endometrial cancer cell adhesion migration and invasion by up-regulating integrin beta3 via SMAD2/3 signaling. Oncotarget 6 (2015): 31659-31673.
- Wacker I, Sachs M, Knaup K, et al. Key role for activin B in cellular transformation after loss of the von Hippel-Lindau tumor suppressor. Mol Cell Biol 29 (2009): 1707-1718.
