Impact of the “Bacteria-Parasite Interaction” in Animal Health and their Participation in the Control of Parasites
Article Information
Emmanuel Dunstand-Guzmán1*, Laura Lina-García1, Sandra Maricela Cuate-Rosas2, Guadalupe Peña-Chora2
1Biotechnology Research Center, Autonomous University of the State of Morelos, MOR, Mexico
2Biological Research Center, Autonomous University of the State of Morelos, MOR, Mexico
*Corresponding Author: Emmanuel Dunstand-Guzmán, Biotechnology Research Center, Autonomous University of the State of Morelos, Avenida Universidad 1001, Col. Chamilpa, 62209 Cuernavaca, MOR, Mexico
Received: 16 September 2019; Accepted: 01 October 2019; Published: 04 October 2019
Citation:
Emmanuel Dunstand-Guzmán, Laura Lina-García, Sandra Maricela Cuate-Rosas, Guadalupe Peña-Chora. Impact of the “Bacteria-Parasite Interaction” in Animal Health and their Participation in the Control of Parasites. Journal of Biotechnology and Biomedicine 2 (2019): 128-143.
View / Download Pdf Share at FacebookAbstract
The parasite infections of animals (production and pets) affect the health and quality of animal life, the parasites frequently present a microbiome associated which play several functions, like nutrition, reproduction or defense against the host immune system, and in some cases are opportunistic microorganisms. These bacteria might cause secondary infections to animals, so the parasite can become a vector of pathogenic bacterial, reason why the understanding of the "bacteria-parasite interaction” is important to combating of parasitic infestations. The current resistance to conventional dewormers or ixodicides, are issue in parasitology; attack a vital bacteria of parasites through antibiotics possibly can be a control alternative, at the same way, the use of bacteria capable to control to parasitic diseases by antagonist interaction, like lethal toxins or adhesion at the site invasion of the parasites, are alternatives to explore. Understand the bacteria-parasite interaction will help us to the control of parasitic diseases in an integral form, as well know as the bacterial infections that can develop from the parasitic infestation. The use of antagonist bacterial is a biotechnological option in the control of parasites as therapy on the animal biomedicine, so in this review, we have a general idea of the “bacteria-parasite interaction" and their impact on the animal’s health.
Keywords
Parasites, Probiotics, Bacillus thuringiensis, Animal health
Parasites articles Parasites Research articles Parasites review articles Parasites PubMed articles Parasites PubMed Central articles Parasites 2023 articles Parasites 2024 articles Parasites Scopus articles Parasites impact factor journals Parasites Scopus journals Parasites PubMed journals Parasites medical journals Parasites free journals Parasites best journals Parasites top journals Parasites free medical journals Parasites famous journals Parasites Google Scholar indexed journals Probiotics articles Probiotics Research articles Probiotics review articles Probiotics PubMed articles Probiotics PubMed Central articles Probiotics 2023 articles Probiotics 2024 articles Probiotics Scopus articles Probiotics impact factor journals Probiotics Scopus journals Probiotics PubMed journals Probiotics medical journals Probiotics free journals Probiotics best journals Probiotics top journals Probiotics free medical journals Probiotics famous journals Probiotics Google Scholar indexed journals Bacillus thuringiensis articles Bacillus thuringiensis Research articles Bacillus thuringiensis review articles Bacillus thuringiensis PubMed articles Bacillus thuringiensis PubMed Central articles Bacillus thuringiensis 2023 articles Bacillus thuringiensis 2024 articles Bacillus thuringiensis Scopus articles Bacillus thuringiensis impact factor journals Bacillus thuringiensis Scopus journals Bacillus thuringiensis PubMed journals Bacillus thuringiensis medical journals Bacillus thuringiensis free journals Bacillus thuringiensis best journals Bacillus thuringiensis top journals Bacillus thuringiensis free medical journals Bacillus thuringiensis famous journals Bacillus thuringiensis Google Scholar indexed journals Animal health articles Animal health Research articles Animal health review articles Animal health PubMed articles Animal health PubMed Central articles Animal health 2023 articles Animal health 2024 articles Animal health Scopus articles Animal health impact factor journals Animal health Scopus journals Animal health PubMed journals Animal health medical journals Animal health free journals Animal health best journals Animal health top journals Animal health free medical journals Animal health famous journals Animal health Google Scholar indexed journals livestock articles livestock Research articles livestock review articles livestock PubMed articles livestock PubMed Central articles livestock 2023 articles livestock 2024 articles livestock Scopus articles livestock impact factor journals livestock Scopus journals livestock PubMed journals livestock medical journals livestock free journals livestock best journals livestock top journals livestock free medical journals livestock famous journals livestock Google Scholar indexed journals B. thuringiensis articles B. thuringiensis Research articles B. thuringiensis review articles B. thuringiensis PubMed articles B. thuringiensis PubMed Central articles B. thuringiensis 2023 articles B. thuringiensis 2024 articles B. thuringiensis Scopus articles B. thuringiensis impact factor journals B. thuringiensis Scopus journals B. thuringiensis PubMed journals B. thuringiensis medical journals B. thuringiensis free journals B. thuringiensis best journals B. thuringiensis top journals B. thuringiensis free medical journals B. thuringiensis famous journals B. thuringiensis Google Scholar indexed journals bacterial interaction articles bacterial interaction Research articles bacterial interaction review articles bacterial interaction PubMed articles bacterial interaction PubMed Central articles bacterial interaction 2023 articles bacterial interaction 2024 articles bacterial interaction Scopus articles bacterial interaction impact factor journals bacterial interaction Scopus journals bacterial interaction PubMed journals bacterial interaction medical journals bacterial interaction free journals bacterial interaction best journals bacterial interaction top journals bacterial interaction free medical journals bacterial interaction famous journals bacterial interaction Google Scholar indexed journals Runimococcus articles Runimococcus Research articles Runimococcus review articles Runimococcus PubMed articles Runimococcus PubMed Central articles Runimococcus 2023 articles Runimococcus 2024 articles Runimococcus Scopus articles Runimococcus impact factor journals Runimococcus Scopus journals Runimococcus PubMed journals Runimococcus medical journals Runimococcus free journals Runimococcus best journals Runimococcus top journals Runimococcus free medical journals Runimococcus famous journals Runimococcus Google Scholar indexed journals Streptococcus articles Streptococcus Research articles Streptococcus review articles Streptococcus PubMed articles Streptococcus PubMed Central articles Streptococcus 2023 articles Streptococcus 2024 articles Streptococcus Scopus articles Streptococcus impact factor journals Streptococcus Scopus journals Streptococcus PubMed journals Streptococcus medical journals Streptococcus free journals Streptococcus best journals Streptococcus top journals Streptococcus free medical journals Streptococcus famous journals Streptococcus Google Scholar indexed journals onchocerciasis articles onchocerciasis Research articles onchocerciasis review articles onchocerciasis PubMed articles onchocerciasis PubMed Central articles onchocerciasis 2023 articles onchocerciasis 2024 articles onchocerciasis Scopus articles onchocerciasis impact factor journals onchocerciasis Scopus journals onchocerciasis PubMed journals onchocerciasis medical journals onchocerciasis free journals onchocerciasis best journals onchocerciasis top journals onchocerciasis free medical journals onchocerciasis famous journals onchocerciasis Google Scholar indexed journals
Article Details
1. Introduction
Parasitic infections are important in the livestock sector and pets because they affect the production and animal welfare, in addition to endangering human health by parasitosis called zoonoses, that generate economic losses focused on its control and prevention [1]. The resistance of ticks and helminths to a great variety of chemical substances used in the veterinary clinic, this is one of the main problems to be solved, because the resistant organisms increase the permanence of the parasitosis and its propagation [2]. Therefore, the search for new active principles or control strategies are the partial solution against the mentioned problem; micro-organisms such as the bacteria Bacillus thuringiensis (B. thuringiensis), whose basis is the production of spore-crystal complex and proteins with specific bioactivity, the toxins of the bacteria are mostly known for its ability insecticide, this has been used in the biochemical and biological control of nematodes, cestodes, trematodes, mites and ticks. However, currently B. thuringiensis is considered as a bacterium antagonistic of parasites used in investigations in vitro and in vivo therapeutic in laboratory and farm animals [3, 4].
Diverse bacteria have a symbiotic relationship with external and internal parasites, between the functions and most well-known properties of the symbiotic interaction between bacteria and parasite are the following; the nutritional role, immunomodulating property, and contribution to the pathogenesis [5-7], at the same time, the enteric helminths can increase or decrease the beneficial bacterial populations in the host, affecting the health of the animal [8]. So, it is of paramount importance to address the symbiosis and relationship that develop the bacteria with the intestinal parasites of pets and production animals by the side effects that can cause in animal health. It should be noted the keep symbiotic bacteria and parasites interaction can become an alternative method in therapies dewormers dependent on the presence or absence of the bacterial biota. For example, the appropriate use of antibiotics or probiotics in addition to pathogenic bacteria to parasites such as B. thuringiensis or bacteria capable of regulating the parasitic competition for nutrients and space that would allow the intestinal colonization; genders like Lactobacillus spp, can become part of the future treatment to conventional therapies in parasitized animals, thereby combating the emergence of resistance with conventional dewormers or ixodicides, for the benefit of animal health.
2. Helminths with bacterial interaction
Interaction of enteric helminths with bacteria can contribute to the permanence of bacterial infections, an example is the association that maintains the gastrointestinal nematode Schistosoma sp with the bacterial Salmonella sp, the parasite helps the maintenance of the bacteria in the small intestine of mice, attributed to bacterial attached to folds of the nematode [9]. However, the enteritis per Salmonella sp in chickens can decrease the number of Ascaridia gallis nematodes established when the infection is later [10], this parasitosis is sensitive to bacteria, such as Pasteurella multocida and Escherichia coli [11, 12], although this parasite is able to produce bactericide molecules [13]. The attachment of the Salmonella bacteria to the cuticle of Schistosoma sp allows it to evade of antibiotics [14]. Another similar example of adherence has referred for cestodiasis of fish Esox lucius in the intestinal tract, proteolytic and amylolytic bacterial symbionts take refuge in the tegument of the cestodes Eubothrium rugosum y Ligula intestinalis, the symbiosis presents a nutritional role, through the synthesis of amino acids and vitamins produced by the bacterial [15, 16].
In some enteric parasitosis, the parasite is capable of modulating the bacterial quorum through the segregation of bacteriostatic substances that alter the beneficial microflora in animals. For example, the nematode Trichuris suis that infects pigs, causes a low population of bacteria of the genus Runimococcus which present cellulolytic activity, in the colon of the pig [8]. The decrease is likely associated with the bactericidal activity of the excretion-secretion products of the adult parasite T. suis, the bactericidal activity of these bio-products is mentioned against Gram-negative bacteria (Campylobacter jejuni, Campylobacter coli, and Escherichia coli), as well as in the Gram-positive (Staphylococcus aureus) [17]. The ability to reduce the bacterial population has referred only to the adult stage of T. suis, because the larval stage is associated with bacteria surrounding epithelial tissue of the intestine, with spirochetes in minor infections to 35 days with T. suis, the alteration of ecological niche has come to associate with dysentery in pigs [18], thus promoting the pathogenesis of the parasitosis. Another example occurs during the parasitism by Trichuris muris, this nematode is able to modify the intestinal microbiota causing an increase of lactobacilli during chronic infection [19]. The interaction between nematodes and bacteria may exist through the adaptability related to the parasite and its environment microbial [20].
The bacteria-parasite interaction can modify the bacterial flora of the host by decreasing or increasing the number of bacteria, it is known that the nematode Heligmosomoides polygyrus bakeri modifies the bacteria populations in mice to lead to an increase in the group proteobacteria, in the intestinal regions of ileum, caecum and the increase Prevotella sp in colon; it is still not known why increased these bacterial populations, however, it has been proposed that interfere with immune processes to inhibit Th2 immune response that helps the expulsion of enteric parasites [21, 7]. The symbiotic association between bacteria and nematodes has been mentioned in the nutrition of larvae in soil in its two primary stages (rhabditiform) of development outside of egg for the genera Ancylostoma sp and Haemonchus sp [22]; in the case of the root-knot nematode Mesodiplogaster sp, is feeds of Pseudomonas cepacia in free life on fine-textured soils or thick [23], the diet can vary depending on the environment in which to develop the larval stages.
The bacteria-parasite association can be discussed even for hatching of nematode eggs, such is the case of Trichuris spp and the association that presents in the hatching in the presence of the bacteria Enterococcus caccae, Streptococcus hyointestinalis and Escherichia coli [20], concluding that the Gram-negative bacteria have an important role in the hatching of the nematode eggs. Also, some nematodes, includes bacteria endosymbionts for example, in the filariasis for the development of the nematode embryogenesis, the bacteria that take to this interaction belong to the genus Wolbachia, pathogenic bacteria for animals and humans, which is transmitted by the transovarial way in the parasite [24].The Wolbachia sp has an important role in the development of damage to cornea, in the rat model for river blindness (onchocerciasis) because of filarial worm infected with this bacteria, causes into the cornea an inflammatory response, and this infection is considered zoonosis [25].
Currently, the parasitology must encompass the microbiome of internal parasites, a recent publication refers the lack of information on internal bacteria of parasites such as tapeworms, these authors report the finding of the bacteria Polynucleobacter sp., considered as the most abundant and the prevalent in the microbiome of Schistocephalus solidus teniasis of fish, their presence in the parasite induces a change in the microbiota of the host in addition to intestinal presence, [26] so that the impact on the bacterial quorum of the fish is similar to previously mentioned on the bacteria population in mammals.
3. Protozoa with bacterial interaction
The protozoan Histomona meleagridis is a parasite of birds, affects the liver and intestine with lesions, these lesions are associated whit the presence of bacteria such as E. coli and Clostridium perfringens, the relationship between H. meleagridis with bacteria is evident in the in vitro cultures, where cells of the parasite present deformity or death to apply antibiotic in vitro [27]. The parasite Eimeria tenella increases the populations of Enterobacteriaceae and Salmonella spp [28, 29], while the Lactobacillus spp and Streptococcus spp decrease in the intestine [30], in addition to the injuries caused by Eimeria spp the viscosity of the intestinal mucosa is affected and possibly the provision of nutrients for the bacteria [31].
4. Ectoparasites with bacterial interaction
In this section addresses to the interaction between external parasites and their bacterial flora, between the parasites are mentioned: mites, ticks, fleas and tsetse flies, considered important in the clinic veterinary by compromising the animal health. The bacteria-parasite interaction in ectoparasites, such as mites and ticks, is linked to opportunistic bacteria that can affect the animal health, for example, the mite Psoroptes ovis, etiologic agent of sheep scab, has been associated with the bacilli Serratia marcescens that contribute to the nutrition of the mite and the process of pathogenesis [32-34]. S. marcescens can affect the rabbit eye by keratitis of cornea [35], so scabies induced by mites of the genus Psoroptes sp frequently caused complications by secondary bacterial infections [36]. In addition to pathogenic bacteria, in P. ovis have been isolated various bacterial that have features that focus on the digestion of the mite, Pseudomonas spp have proteolytic activity and Staphylococcus spp is able to hydrolyze protein of animal origin [37], they can produce irritation of the dermis in animals, and the psoroptic mange with chronic otitis can be aggravated by meningitis caused by opportunistic bacteria [36]. The riketsia Anaplasma marginale is a bovine bacterial pathogen transmitted by ticks, such as Dermacentor andersoni, Dermacentor variabilis and Rhipicephalus microplus, considered important vectors in tropical and subtropical regions, the disease is characterized by damage to erythrocytes triggering anemia, fever, and death of the animal [38]. The importance of endosymbionts in ticks focus in the fact that both Anaplasma marginale as Coxiella sp. are pathogenic to animals so the bacteria-parasite interaction compromises the health of animals and humans who can be able to develop Q fever and riquetsiosis [39, 40], on the other hand, Wolbachia persica has been isolated from tick Argas arboreus (Persicargas), bacteria capable of infect cattle [41].
Another ectoparasite of importance in cats and dogs is the flea, this arthropod can harbor bacterial symbionts of the classes Firmicute and Proteobacteria capable of colonizing the species Ctenocephalides felis and Xenopsylla cheopis. It is important to mention that the fleas do not present peritrophic matrix, so that the epithelium of the midgut is vulnerable to the contact with microbes ingested during feeding, so that is believed the bacterial flora is necessary to the flea as a defense of the arthropod. The bacterium Bartonella henselae has been isolated from fleas, it is able to affect the health of domestic cat colonizing the erythrocytes and vascular endothelial cells and is the causal agent of “cat scratch” disease [42]. In the tissue of tsetse flies from the salivary glands, Gram-negative populations have been detected, the secondary bacteria commonly found in old flies, symbionts isolated by intra and extracellular way, among these the strain GP01 symbiont, provides a possible nutritional function [43].
5. Control of parasites with probiotic bacteria
The bacteria used as probiotics have the ability to stimulate the immune system and that this generates antibodies or cells that help decrease the parasitic infection [44]. The role of probiotics against parasitic diseases in animals is the utmost importance to be an alternative safe, organic and environmentally friendly in addition to help for a good bowel function, the probiotics have preventive and prophylactic antiparasitic effect, in Table 1 are listed certain parasitosis and use the of probiotics that showed an effect on the parasite population before and after in in vivo treatments. The use of probiotic bacteria against parasites are described in vitro and in vivo, for example, the in vitro study of Lactobacillus strains isolated from chicken shows that these are capable of preventing the invasion of Eimeria tenella in MDBK cell culture (Madin-Darby bovine kidney), the authors speculate that the inhibitory effect possibly is due to steric interference or competitive exclusion; Lactobacillus salivarius strain Lb16c6 produces extracellular elements in the supernatant that present significant activity by inhibiting the invasion by coccideas Eimeria spp, however, the mode of action is unknown [52]. An in vivo study a mixture was used, biomin® Poultry Star composed; Bifidobacterium animals (DSM16284), Lactobacillus salivarius (DSM16351) and Enterococcus faecium (DSM 21813), in the diet of broilers commercial chickens (Cobb 500) parasitized with Eimeria spp, the mixture caused to decrease the parasitic oocyst in feces by more than 40% [53]. It has also been referred to the evaluation of probiotics against nematodes, the compounds released in the supernatant by Lactobacillus casei has confirmed the anthelmintic efficiency in mice treated causes decreasing the Trichinella spiralis infection in 32.5%, the reduction was associated with the stimulation of IL-2 and nitric oxide production by L. casei [49].
Note: NH/EH: natural host/experimental host in the study, D.P.I-days post infection; (A): after infection; (B): before infection.
Table 1: Probiotics used for the control of animal parasites.
Other bacteria used in the control of nematodes in animals is Bacillus laterosporus, the toxins produced by this bacillus are able to inhibit the hatching of eggs and larval development of the nematode Trichostrongylus colubriformis [54].
6. Control of parasites with B. thuringiensis
Another bacterium that has taken an important role in the control of parasites is B. thuringiensis, this microorganism is capable of producing a sub-apical spore and one or several parasporal bodies: inclusions composed of proteins that present specific insecticide activity, even to specie level, this microorganism is known such a potent bioinsecticide [55, 56]. The symptoms that develop the insects (larvae of Lepidoptera) after the ingestion of the spore-crystal complex are the following: the host presents inanition and diarrhea, intestinal paralysis and eventually death [57].
B. thuringiensis, is a viable alternative for the control of animals and plants parasites, because the Cry proteins produced by these bacteria are harmless to humans, vertebrates and plants, in addition to be biodegradable [57, 58]. B. thuringiensis presents toxic activity in parasites of animals as in the case of mites, cestodes, and nematodes with broad effects on different stages of the parasite (egg, larva, and adult). The strains GP123, GP138, GP130 and GP140 of B. thuringiensis was tested in vitro against R. sanguineus ticks, the strains presented a mortality rate of 75.15 to 95.8% [59]. In the same way, the strain GP532 of B. thuringiensis, proved to kill in vitro near of 50% after 72 h of ectoparasite P. cuniculi by contact and besides was able to damage intestinal tissue of the mite and generate vacuoles in ventricles [60].
In the case of gastrointestinal parasites, B. thuringiensis is toxic against larvae of H. contortus (Rudolphi), T. colubriformis and Ostertagia circumcincta, nematodes of livestock [61] Within the group of Cry proteins that possess nematicide action we have the following; Cry 5A, 5B, 6A, 6B, 12A, 13A, 14A and 21A [62]. So far there are few reports of activity of B. thuringiensis against platyhelminths, GP526 strain is presumed to be highly toxic against Centrocestus formosanus [63], so it also acts on the egg and the tegument of the Dipylidium caninum cestode of dogs [64]. The ovicidal activity of B. thuringiensis can be used in the treatment of wastewater, the contamination for helminths eggs is a problem because the wastewater is using for the vegetal culture in agriculture. And the dual activity of GP526 can disrupt the life cycle of the parasite, for example in their viability on intermedia host.
The high effectiveness of B. thurinigiensis against parasites in vitro and in vivo possibly might be used by pharmaceutical industry to develop next-generation anthelmintics from this bacterium, due to their toxic function against multiple parasitic infections such as those cited in Table 2. It is expected that B. thuringiensis will contribute to strengthening the health programs against parasitosis [3]. The toxicity of Cry 5B protein, has been studied on H. contortus nematode of ruminants [69], and nematode of pigs Ascaris suum with positive results, [66] as well as in Ancylostoma ceylanicum, parasite present in humans and hamsters, the toxin Cry 5B in vitro causes diminution on the motility in larvae (L1/L2) and adult death [65]. The application of B. thuringiensis in the veterinary clinic, is a current topic, the biodegradable proteins can be used in the production of organic meat, by replacing chemical dewormers that extends the time of sacrifice of the animal before being of consumption, so the use of this bacterium is a possible solution to the problem of chemical pollution of the meat.
Note: TP/NH: type of parasite/natural host, *adult stage,**larvae stage and ***egg stage, C-D: concentration or dosage, SC: Spore-Crystal complex, CL50: 50% Lethal Concentration, DL50: 50% Lethal Dosage, EF:effect or afficacy parasitocidal.
Table 2: Use of B. thuringiensis against parasites of domestic animals in vitro.
The use of B. thuringiensis in ectoparasites has allowed the treatment of tick infestations of livestock through the implementation of the GP543 spore and crystal complex in cattle, it should be noted that this strain was isolated from the corpse of a tick, it is mentioned that the decrease of the parasite on day 14 came to be up to 99.56% and 99.21% at day 21, with only two applications [4]. In addition, other studies have evaluated three subspecies of B. thuringiensis (kurstaki, thuringiensis and israeliensis) spraying of spore-crystal complex in the ticks Argas persicus and Hyalomma dromedarii [70, 71]. The mite P. cuniculi is a parasite of veterinary importance, it is the cause of psoroptic scabies, the strain GP532 decreased the cutaneous damage caused by the mite P. cuniculi, with only two applications of bacterial treatment the decrease is 76.38% of clinical signs against natural infestations [72], so that in the future a mixture of toxins with acaricidal activity of broad- spectrum on the basis of B. thuringiensis can be used in ixodicidal shower by immersion. In the case of enteric parasites, the administration of B. thuringiensis in vivo for the treatment of helminthiasis has been extensively evaluated, the oral administration of Cry 5B for the control of A. ceylanicum, on the model of the golden hamster, had a powerful anthelmintic action by reducing the infection in the number of eggs released in feces [65].
In another study, the same protein was evaluated in vivo on mice against Heligmosomoides bakeri with positive results in chronic infection with 70% of reduction in worms and 98% in eggs. The authors mention the need to prepare a formulation of this protein to resist gastric fluids for best results, however, the effect of this protein compared with other chemical and natural alternatives, is considered as dewormer powerful [73]. The Cry 5B tested in hamsters infected with A. ceylanicum with different concentrations of protein in co-administration with anthelmintic drugs as pyrantel and tribendimidine, is able to completely eliminate the intestinal parasitosis. The therapeutic applications of Cry5B have been a positive effect anthelmintic in dogs infected with Ancylostoma caninum [74] so that this protein is considered to be highly effective against roundworms and a candidate anthelmintic on the pharmacology veterinary medicine. Also, the role of bacterial endosymbionts in the promotion of paratransgenesis is important with the use of genetically modified bacteria to induce damage to the host (for control of parasite).
7. Bacteria of parasites susceptible to antibiotics
An alternative in the parasitosis control is the use of antibiotics that diminish or cancel the presence of the symbionts bacteria of the parasite to control, an example of this tendency is the decrease of the bacteria of the Wolbachia spp genus, an obligate mutualist of stable coexistence with filarial nematodes. The elimination of Wolbachia with antibiotics has lethal consequences for the nematode, causing alterations in the development of worms, in response to the bacteria decrease, causes the blockage of the embryogenesis and eventual death of the worm [75]. Consequently, Wolbachia spp represents a new important pharmacological objective for the control of diseases caused by filariasis. This has led to the possibility of the use of tetracycline for the control of this parasite [76], until now the use of tetracycline in parasitized animals has achieved an attenuated and poor growth in larvae [77], while clinical trials with doxycycline in Africa and Asia are reported as positive [78]. The 47% of nematodes of the onchocercarie family have a relationship with Wolbachia spp as an endosymbiont [79], onchocercariae are filarial that affect cattle, other ungulates and are considered zoonoses [80], so the control of this parasitosis is important.
The use of doxycycline for the control of the adult stage of Onchocerca gutturosa has been shown in vitro to be successful [81]. However, the administration of antibiotics in some parasites is not clinically efficacy, for example, in the schistosomiasis, which can cause bladder cancer associated with uribacteremias [82], and liver abscess due to secondary infections with Staphylococcus aureus [83], in this parasitosis the antibiotics, not reduce the charge of schistosomes. So far the effect of antibiotic therapy has not been reported to control these bacterial infections associated with the parasite, possibly because the resistance of the bacteria to be eliminated with antibiotics happens when they adhered to the surface of the parasite, the adherence is due to the presence of a fimbrial protein (FimH) that recognizes the surface of the parasite, this union confers to the bacterium survival up to eight antibiotics [84].
The antibiotics can be used for the control of ectoparasites, for example the ticks of the genus Dermacentor sp, Haemaphysalis sp and Amblyomma sp [85] present Coxiella sp as a symbiont, the administration of antibiotics in the adult tick of Amblyomma americanum decreased the reproduction of the parasite, because the bacteria is transmitted transovarially [86,87], it is important to mention that Coxiella sp is potentially transmissible to humans and animals [85].
8. Disadvantages at the use of antibiotics in animals for parasites control
The intestinal bacterial flora in mammals plays a role in homeostasis, in the regulation of activation of the cito-protective genes, and have a stimulant function of the immune system [88, 89]. The diminution or increment of the intestinal bacteria causing health problems in the host [90]. So the application of antibiotics and dewormers is related to the appearance of diarrhea [91]. And the use of antibiotics in short periods generate resistant bacteria intestinal populations for years [92], and the deteriorate the native biota of vertebrates, causing digestive problems. In addition, the relationship of microflora (bacterial) and macroflora (parasites) their achieves a symbiotic control that favors the healthy state of the intestine. It should also consider that administration of antibiotics in therapies dewormers are not a very addressed, however, must be studied in order to understand the dosage of the antibiotic that is administered to the animal and antibiotic to amount gets really the parasite and their vital bacteria (symbionts), ensuring a more appropriate parasites control using dewormers in form integral and antibiotics in the control of parasitic diseases.
9. Conclusions
The bacteria-parasite interaction represents a vital role in some parasitic diseases since it is essential in the life cycle of the parasites and its survival, so this interaction can be an alternative target for the control using parasitic fighting bacteria, implementing the use of antibiotics and in opposite case, probiotic bacteria that compete for nutrients or same space of parasites in addition to enzymes or lethal effect toxins produced by B. thuringiensis Figure 1. The above highlights the importance of knowing the symbionts bacteria in the parasitic diseases and the use of bacteria agents versus parasitic diseases. Therefore, the anthelmintic effect of the "bacteria-parasite interaction" should be considered as a biotechnological application in animal biomedicine.
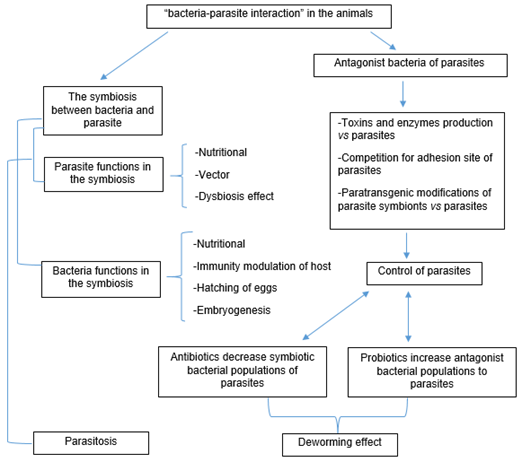
Figure 1: The “bacteria-parasite interaction” in the parasitosis of animals. The diagram shows the symbiont and antagonist bacterial of parasites in animals and the role of the decrease of symbiont bacteria in the parasite by antibiotics, as well as the increase of the parasite antagonist bacterial population as deworming therapy.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
EDG acknowledge the fellowships received from Consejo Nacional de Tecnología (305290).
References
- Watson JD, Slifko FHTR, Smith HV, et al. Emerging parasite zoonoses associated with water and food. International journal for parasitology 30 (2000): 12-13.
- Nari A, Hansen JW. Resistance of ecto-and endoparasites: current and future solutions. In Conference of the Office International des Epizooties (OIE), Paris, France (1999): 13-22.
- Vázquez-Pineda A, Bravo-de-la-Parra, A. Mendoza-de-Gives P, et al. Uso de productos derivados de Bacillus thuringiensis como alternativa de control en nematodos de importancia veterinaria Revisión. Revista mexicana de ciencias pecuarias 3 (2012): 77-88.
- Romo-Martínez A, Fernández-Ruvalcaba M, Hernández-Velázquez VM, et al. Evaluation of natural origin products for the control of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) on cattle artificially infested. Basic Research Journal of Agricultural Science and Review 2 (2013): 64-79.
- Foligné B, Dewulf J, Breton J, et al. Probiotic properties of non-conventional lactic acid bacteria: immunomodulation by Oenococcus oeni. International journal of food microbiology 140 (2010): 136-145.
- Roest HI, Ruuls RC, Tilburg JJ, et al. Molecular epidemiology of Coxiella burnetii from ruminants in Q fever outbreak, the Netherlands. Emerging Infectious Diseases 17 (2011): 668.
- Rausch S, Held J, Fischer A, et al. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PloS one 8 (2013): 74026,
- Wu S, Li RW, Li W, et al. Worm burden-dependent disruption of the porcine colon microbiota by Trichuris suis infection. PloS one 7 (2012): e35470.
- Phillips SM, Reid WA, Doughty BL, et al. The immunologic modulation of morbidity in schistosomiasis. The American journal of tropical medicine and hygiene 29 (1980): 820-831.
- Eigaard NM, Schou TW, Permin A, et al. Infection and excretion of Salmonella Enteritidis in two different chicken lines with concurrent Ascaridia galli infection. Avian Pathology 35 (2006): 487-493,
- Dahl C, Permin A, Christensen JP, et al. The effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickens. Veterinary Microbiology 86 (2002): 313-324.
- Permin A, Christensen J, Bisgaard M. Consequences of concurrent Ascaridia galli and Escherichia coli infections in chickens. Acta Veterinaria. Scandavica 47 (2006): 43-54.
- Midha A, Schlosser J, Hartmann S. Reciprocal interactions between nematodes and their microbial environments. Frontiers in Cellular and Infection Microbiology 7 (2017).
- Hsiao A, Toy T, Seo HJ, et al. Interaction between Salmonella and schistosomiasis: a review. PLoS pathogens 12 (2016): 1005928.
- Izvekova GI, Lapteva NA. Microflora associated with the digestive-transport surfaces of fish and their parasitic cestodes. Russian journal of ecology 35 (2004): 176-180.
- Plotnikov AO, Korneva ZhV. Morphological and ultrastructural characteristics of symbiotic bacteria colonizing the surface of the helminth Triaenophorus nodulosus and the intestine of pike Esox lucius. Inland Water Biology 1 (2008): 25-31.
- Abner SR, Parthasarathy G, Hill DE, et al. Trichuris suis: detection of antibacterial activity in excretory-secretory products from adults. Experimental parasitology 99 (2001): 26-36,
- Rutter JM, Beer RJ. Synergism between Trichuris suis and the microbial flora of the large intestine causing dysentery in pigs. Infection and immunity 11 (1975): 395-404.
- Holm JB, Sorobetea D, Kiilerich P, et al. Chronic Trichuris murisinfection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of lactobacilli. PLoS One 10 (2015): 0125495,
- Vejzagi? N, Adelfio N, Keiser R, et al. Bacteria-induced egg hatching differs for Trichuris muris and Trichuris suis. Parasites & vectors 8 (2015): 371.
- Locksley RM. Th2 cells: help for helminths. The Journal of experimental medicine 179 (1992): 1405-1407.
- Munn E, Munn PD. Feeding and digestion. In: Lee DL, editor. The biology of nematodes. London, UK: Taylor and Francis (2002): 211-232.
- Elliott ET, Anderson RV, Coleman DC, et al. Habitable pore space and microbial trophic interactions Oikos (1980): 327-335.
- Taylor MJ. Wolbachia endosymbiotic bacteria of filarial nematodes. A new insight into disease pathogenesis and control. Archives of medical research 33 (2002): 422-424.
- Taylor MJ, Makunde W, McGarry HF, et al. Doxycycline treatment of Wuchereria bancrofti: a double-blind placebo-controlled trial. American Journal of Tropical Medicine and Hygiene 69 (2003): 250.
- Hahn MA, Dheilly NM. Not so sterile after all: The endomicrobiome of plerocercoids of the cestode parasite Schistocephalus solidus and changes to the microbiome of its Threespine Stickleback host. bioRxiv (2018): 290015.
- Munsch M, Lotfi A, Hafez HM, et al. Light and transmission electron microscopic studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitology Research 104 (2009): 683.
- Arakawa A and Ohe O. Reduction of Clostridium perfringens by feed additive antibiotics in the ceca of chickens infected with Eimeria tenella Poultry. Science 54 (1975): 1000-1007.
- Takimoto H, Baba E, Fukata T, et al. Effects of infection of Eimeria tenella, E. acervulina, and E. maxima upon Salmonella typhimurium infection in chickens. Poultry Science 63 (1984): 478-484.
- Kimura N, Mimura F, Nishida S, et al. Studies on the relationship between intestinal flora and cecal coccidiosis in chicken. Poultry Science 55 (1976): 1375-1383.
- Hauck-R. Interactions between parasites and the bacterial microbiota of chickens. Avian Diseases 61 (2017): 428-436.
- Sinclair AN and Filan SJ. Lipid ingestion from sheep epidermis by Psoroptes ovis (Acari: Psoroptidae). Veterinary parasitology 31 (1989): 149-164.
- Mathieson BRF and Lehane MJ. Isolation of the gram-negative bacterium, Serratia marcescens, from the sheep scab mite, Psoroptes ovis. Veterinary record 138 (1996): 210-211,
- Hogg JC, Lehane MJ. Microfloral diversity of cultured and wild strains of Psoroptes ovis infesting sheep. Parasitology 123 (2001): 441-446.
- Humen MA, De Antoni GL, Benyacoub J, et al. Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infection and Immunity 73 (2005): 1265-1269.
- Ulutas B, Voyvoda H, Bayramli G, et al. Efficacy of topical administration of eprinomectin for treatment of ear mite infestation in six rabbits. Veterinary Dermatology 16 (2005): 334-337.
- Hall SA, Mack K, Blackwell A, et al. Identification and disruption of bacteria associated with sheep scab mites-novel means of control? Experimental parasitology 157 (2015): 110-116.
- Zivkovic Z, Esteves E, Almazán C, et al. Differential expression of genes in salivary glands of male Rhipicephalus (Boophilus) microplus in response to infection with Anaplasma marginale. BMC genomics 11 (2010): 186.
- McCaughey C, Murray LJ, McKenna JP, et al. Coxiella burnetii (Q fever) seroprevalence in cattle Epidemiology and. Infection 138 (2010): 21-27.
- Angelakis E, Raoult D. “Q fever”. Veterinary microbiology 140 (2010): 297-309.
- Dilbeck PM, Evermann JF, Crawford TB, et al. Isolation of a previously undescribed rickettsia from an aborted bovine fetus. Journal of. Clinical. Microbiology 28 (1990): 814-816.
- Erickson DL, Anderson NE, Cromar LM, et al. Bacterial communities associated with flea vectors of plague. Journal of Medical Entomology 46 (2009): 1532-1536.
- Beard CB, Durvasula RV, Richards FF. Bacterial symbiosis in arthropods and the control of disease transmission. Emerging Infectious Diseases 4 (1998): 581-591.
- Travers MA, Florent I, Kohl L, et al. Probiotics for the control of parasites: an overview. Journal of Parasitology Research (2011): 1-12.
- Bautista CR, Sandoval A, Aguilar BR. Effect of High?and Low?Molecular?Weight Components of Lactobacillus casei on Resistance against Babesia microti in NIH Mice Annals of the New York Academy of Sciences 1149 (2008): 152-154.
- Guitard J, Menotti J, Desveaux A, et al. Experimental study of the effects of probiotics on Cryptosporidium parvum infection in neonatal rats. Parasitology Research 99 (2006): 522-527.
- Ritzi MM, Abdelrahman W, Mohnl M, et al. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poultry Science 93 (2014): 2772-2778.
- Humen MA, De Antoni GL, Benyacoub J, et al. Lactobacillus johnsonii La1 antagonizes Giardia intestinalis in vivo. Infection and Immunity 73 (2005): 1265-1269.
- Bautista-Garfias CR, Ixta-Rodríguez O, Martínez-Gómez F, et al. Effect of viable or dead Lactobacillus casei organisms administered orally to mice on resistance against Trichinella spiralis infection. Parasite (2001): 226-228.
- McClemens J, Kim JJ, Wang H, et al. Lactobacillus rhamnosus ingestion promotes innate host defense in an enteric parasitic infection. Clinical and Vaccine Immunology 20 (2013): 818-826.
- Basualdo M, Sparo P, Chiodo M, et al. Oral treatment with a potential probiotic (Enterococcus faecalis CECT 7121) appears to reduce the parasite burden of mice infected with Toxocara canis Annals of Tropical Medicine and Parasitology 101 (2007): 559-562.
- Tierney J, Gowing H, Van Sinderen D, et al. In vitro inhibition of Eimeria tenella invasion by indigenous chicken Lactobacillus species. Veterinary Parasitology 122 (2004): 171-182.
- Eckert NH, Lee JT, Hyatt D, et al. Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. Journal of Applied Poultry Research 19 (2010): 59-67.
- Bone LW, Singer S. Control of parasitic nematode ova/larvae with a Bacillus laterosporus. U.S. Patent 5 (1991): 314.
- Lecadet M. Bacillus thuringiensis toxins: The proteinaceous crystal. Microbial toxins. Monti T, S. Kadus S, Ajl S, Academic Press (New York), U.S.A 5 (1970): 71-437.
- Schnepf EN, Crickmore J, Van Rie D, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Reviews 62 (1998): 775-806.
- Bravo A, Gill SS, Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49 (2007): 423-435.
- Soberon M, Gill SS, Bravo A. Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cellular and molecular life sciences 66 (2009): 1337-1349.
- Fernández-Ruvalcaba M, Peña-Chora G, Romo-Martínez A, et al. Evaluation of Bacillus thuringiensis pathogenicity for a strain of the tick, Rhipicephalus microplus, resistant to chemical pesticides. International Journal of Insect Science 10 (2010): 1.
- Dunstand-Guzmán E, Peña-Chora G, Hallal-Calleros C, et al. Acaricidal effect and histological damage induced by Bacillus thuringiensis protein extracts on the mite Psoroptes cuniculi. Parasites and Vectors 8 (2015): 285.
- Kotze AC, O'grady J, Gough JM, et al. Toxicity of Bacillus thuringiensis to parasitic and free-living life-stages of nematode parasites of livestock International Journal for Parasitology 35 (2005): 1013-1022.
- Wei JZ, Hale K, Carta L, et al. Bacillus thuringiensis crystal proteins that target nematodes. Proceedings of the National Academy of Sciences 100 (2003): 2760-2765.
- Mendoza-Estrada LJ, Hernández-Velázquez VM, Arenas-Sosa I, et al. Anthelmintic effect of Bacillus thuringiensis strains against the gill fish trematode Centrocestus formosanus. BioMed Research International 1 (2016): 9.
- Peña-Chora G, Aguilar-Jiménez FA, Hallal-Calleros C, et al. In Vitro Ovicidal and Cestocidal Effects of Toxins from Bacillus thuringiensis on the Canine and Human Parasite Dipylidium caninum. BioMed Research International 7 (2012): 174619.
- Cappello M, Bungiro RD, Harrison LM, et al. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proceedings of the National Academy of Sciences 103 (2006): 15154-15159.
- Urban Jr. JF, Hu Y, Miller MM, et al. Bacillus thuringiensis-derived Cry5B has potent anthelmintic activity against Ascaris suum. PLoS neglected tropical diseases 7 (2013): 2263.
- Wu C, Wu L, Xu L, et al. Parasporal crystal proteins of Bacillus thuringiensis strain BT6, originating from the faeces of Sika Deer, demonstrate anticoccidial activity against Eimeria tenella in chickens African Journal of Microbiology Research 7 (2013): 4261-4264.
- Edwards DL, Payne J, Soares GG. Novel isolates of Bacillus thuringiensis having activity against nematodes.Washington, DC: U.S. Patent and Trademark Office. U.S 4 (1990): 734.
- O’Grady J, Akhurst RJ, Kotze AC. The requirement for early exposure of Haemonchus contortus larvae to Bacillus thuringiensis for effective inhibition of larval development. Veterinary parasitology 150 (2007): 97-103.
- Hassanain MA, El Garhy MF, Abdel-Ghaffar FA, et al. Biological control studies of soft and hard ticks in Egypt: I. The effect of Bacillus thuringiensis varieties on soft and hard ticks (Ixodidae). Parasitology Research 83 (1997): 209-213.
- Ostfeld RS, Price A, Hornbostel VL, et al. Controlling ticks and tick-borne zoonoses with biological and chemical agents. AIBS Bulletin 56 (2006): 383-394.
- Dunstand-Guzmán E, Hallal-Calleros C, Morales-Montor J, et al. Therapeutic use of Bacillus thuringiensis in the treatment of psoroptic mange in naturally infested New Zealand rabbits. Veterinary Parasitology 238 (2017): 24-29.
- Hu Y, Georghiou SB, Kelleher AJ, et al. Bacillus thuringiensis Cry5B protein is highly efficacious as a single-dose therapy against an intestinal roundworm infection in mice. PLoS neglected tropical diseases 4 (2010): e614.
- Hu Y, Nguyen TT, Lee AC, et al. Bacillus thuringiensis Cry5B protein as a new pan-hookworm cure. International Journal for Parasitology: Drugs and Drug Resistance 8 (2018): 287-294.
- Taylor MJ, Bandi C, Hoerauf A. Wolbachia. Bacterial endosymbionts of filarial nematodes. Advances in parasitology, vol. 60, (2005): pp. 245-284.
- McCall JW, Jun JJ, Bandi C. Wolbachia and the antifilarial properties of tetracycline. An untold story. Italian Journal of Zoology 66 (1999): 7-10.
- Chirgwin SR, Nowling JM, Coleman SU, et al. Brugia pahangi and Wolbachia: the kinetics of bacteria elimination, worm viability, and host responses following tetracycline treatment. Experimental Parasitology 103 (2003): 16-26.
- Lepage D, Bordenstein SR. Wolbachia: Can we save lives with a great pandemic? Trends in Parasitology 9 (2013): 385-393.
- Salunkhe RC, Narkhede KP, Shouche YS. Distribution and evolutionary impact of Wolbachia on butterfly hosts. Indian journal of microbiology 54 (2014): 249-254,
- Krueger A, Fischer P, Morales-Hojas R. Molecular phylogeny of the filaria genus Onchocerca with special emphasis on Afrotropical human and bovine parasites. Acta Tropica 101 (2007): 1-14.
- Townson S, Tagboto S, McGarry HF, et al. Onchocerca parasites and Wolbachia endosymbionts: evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria Journal 5 (2006): 1.
- Lehman JS, Farid Jr.Z, Smith Z, et al. Urinary schistosomiasis in Egypt: clinical, radiological, bacteriological and parasitological correlations. Transactions of the Royal Society of Tropical Medicine and Hygiene 67 (1973): 384-399.
- Sánchez-Olmedo JI, Ortiz-Leyba C, Garnacho-Montero J, et al. Schistosoma mansoni and Staphylococcus aureus bacteremia: a deadly association. Intensive care medicine 29 (2003): 204-1204.
- Barnhill AE, Novozhilova E, Day TA, et al. Schistosoma-associated Salmonella resist antibiotics via specific fimbrial attachments to the flatworm Parasites and Vectors 4 (2011): 123.
- Maurin M, Raoult D. “Q fever”. Clinical. Of Microbiology. Review 12 (1999): 518-553.
- Zhong J, Jasinskas A, Barbour AG. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PloS one 2 (2007): 405.
- Andreotti R, De León AAP, Dowd SE, et al. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC microbiology 11 (2011): 6.
- Patel W, Lin W. Developmental biology of gut-probiotic interaction. Gut Microbes 1 (2010): 186-195.
- Hoerauf A. Microflora, helminths, and the immune system-who controls whom? New England Journal of Medicine 363 (2010): 1476-1478.
- Hart SC, De Luca TH, Newman GS, et al. Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. Forest Ecology and Management 220 (2005): 166-184.
- Bartlett JG. Antibiotic-associated diarrhea. The New England Journal of Medicine 346 (2002): 334-339.
- Jakobsson HE, Jernberg C, Andersson AF, et al. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 5 (2010): e9836.
