Impact of COVID-19 on Breast Cancer Treatment: A Tertiary Referral Centre Experience
Article Information
Kirsty Balachandran1, Jennet Williams1, David Bell1, Anna Brown1, Phawan Hurhangee2, Rathi Ramakrishnan2, Susan Cleator1, Raoul Charles Coombes1, 3, Olivia Hatcher1, Farah Rehman1, Justin Stebbing1, 3, Laura Kenny1, 3*
1Department of Oncology, Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, UK
2Department of Histopathology, Imperial College Healthcare NHS Trust, Charing Cross Hospital, London, UK
3Department of Surgery and Cancer, Imperial College, London, UK
*Corresponding Author: Laura Kenny, Department of Oncology, Imperial College Healthcare NHS Trust, Charing Cross Hospital, Fulham Palace Road, London, W6 8RF, UK
Received: 23 July 2021; Accepted: 20 August 2021; Published: 03 September 2021
Citation: Kirsty Balachandran, Jennet Williams, David Bell, Anna Brown, Phawan Hurhangee, Rathi Ramakrishnan, Susan Cleator, Raoul Charles Coombes, Olivia Hatcher, Farah Rehman, Justin Stebbing, Laura Kenny. Impact of COVID-19 on Breast Cancer Treatment: A Tertiary Referral Centre Experience. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 389-404.
View / Download Pdf Share at FacebookAbstract
Background: The effect of the COVID-19 on breast cancer treatment is unknown. The pandemic’s impact on treatment delivery was assessed in patients undergoing chemotherapy treatment at a major London oncology centre.
Methods: Treatment and medical records for all patients attending the chemotherapy unit and on outpatient treatment for breast cancer over an 8 week period (during first lockdown; 23rd March-17th May 2020) were compared to a similar time period in 2019.
Results: Breast cancer diagnosis referrals fell by 38% mainly due to screening services halting which had a knock-on effect on patient numbers starting their first treatment (reduced by 34%). Neoadjuvant, adjuvant, and palliative patients were all affected, mostly at the start of lockdown. On-site chemotherapy and supportive treatments fell by 39% compared to 2019, mainly due to fewer number of bone-modifying agents. Towards the end of 8 week period, treatment numbers had nearly returned to normal. The system adapted by modifying treatment regimens, using telemedicine, increased use of supportive medications and less frequent blood tests.
Conclusion: COVID-19’s global impact has significantly reduced breast cancer treatments given during the first lockdown. Whilst recovery is now evident, cancer services, patients, and clinical cancer research must be prioritised in future waves.
Keywords
COVID-19; Breast cancer; Surgery and cancer
COVID-19 articles; Breast cancer articles; Surgery articles, cancer articles
COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Breast cancer articles Breast cancer Research articles Breast cancer review articles Breast cancer PubMed articles Breast cancer PubMed Central articles Breast cancer 2023 articles Breast cancer 2024 articles Breast cancer Scopus articles Breast cancer impact factor journals Breast cancer Scopus journals Breast cancer PubMed journals Breast cancer medical journals Breast cancer free journals Breast cancer best journals Breast cancer top journals Breast cancer free medical journals Breast cancer famous journals Breast cancer Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals cancer articles cancer Research articles cancer review articles cancer PubMed articles cancer PubMed Central articles cancer 2023 articles cancer 2024 articles cancer Scopus articles cancer impact factor journals cancer Scopus journals cancer PubMed journals cancer medical journals cancer free journals cancer best journals cancer top journals cancer free medical journals cancer famous journals cancer Google Scholar indexed journals chemotherapy unit articles chemotherapy unit Research articles chemotherapy unit review articles chemotherapy unit PubMed articles chemotherapy unit PubMed Central articles chemotherapy unit 2023 articles chemotherapy unit 2024 articles chemotherapy unit Scopus articles chemotherapy unit impact factor journals chemotherapy unit Scopus journals chemotherapy unit PubMed journals chemotherapy unit medical journals chemotherapy unit free journals chemotherapy unit best journals chemotherapy unit top journals chemotherapy unit free medical journals chemotherapy unit famous journals chemotherapy unit Google Scholar indexed journals Neoadjuvant articles Neoadjuvant Research articles Neoadjuvant review articles Neoadjuvant PubMed articles Neoadjuvant PubMed Central articles Neoadjuvant 2023 articles Neoadjuvant 2024 articles Neoadjuvant Scopus articles Neoadjuvant impact factor journals Neoadjuvant Scopus journals Neoadjuvant PubMed journals Neoadjuvant medical journals Neoadjuvant free journals Neoadjuvant best journals Neoadjuvant top journals Neoadjuvant free medical journals Neoadjuvant famous journals Neoadjuvant Google Scholar indexed journals oncologists articles oncologists Research articles oncologists review articles oncologists PubMed articles oncologists PubMed Central articles oncologists 2023 articles oncologists 2024 articles oncologists Scopus articles oncologists impact factor journals oncologists Scopus journals oncologists PubMed journals oncologists medical journals oncologists free journals oncologists best journals oncologists top journals oncologists free medical journals oncologists famous journals oncologists Google Scholar indexed journals malignancy articles malignancy Research articles malignancy review articles malignancy PubMed articles malignancy PubMed Central articles malignancy 2023 articles malignancy 2024 articles malignancy Scopus articles malignancy impact factor journals malignancy Scopus journals malignancy PubMed journals malignancy medical journals malignancy free journals malignancy best journals malignancy top journals malignancy free medical journals malignancy famous journals malignancy Google Scholar indexed journals progesterone receptor articles progesterone receptor Research articles progesterone receptor review articles progesterone receptor PubMed articles progesterone receptor PubMed Central articles progesterone receptor 2023 articles progesterone receptor 2024 articles progesterone receptor Scopus articles progesterone receptor impact factor journals progesterone receptor Scopus journals progesterone receptor PubMed journals progesterone receptor medical journals progesterone receptor free journals progesterone receptor best journals progesterone receptor top journals progesterone receptor free medical journals progesterone receptor famous journals progesterone receptor Google Scholar indexed journals abemaciclib articles abemaciclib Research articles abemaciclib review articles abemaciclib PubMed articles abemaciclib PubMed Central articles abemaciclib 2023 articles abemaciclib 2024 articles abemaciclib Scopus articles abemaciclib impact factor journals abemaciclib Scopus journals abemaciclib PubMed journals abemaciclib medical journals abemaciclib free journals abemaciclib best journals abemaciclib top journals abemaciclib free medical journals abemaciclib famous journals abemaciclib Google Scholar indexed journals
Article Details
1. Introduction
At the outset of the COVID-19 pandemic, the effects of COVID-19 infection in cancer patients were uncertain. Liang et al had published a study in the Lancet Oncology suggesting that mortality would be higher in cancer patients, however only 18 patients were included in that study and most oncologists thought it would be difficult to draw direct inferences, particularly as none of the patients in that study reflected an active breast cancer population [1]. The specific data on individual treatment regimens and COVID-19 related safety was not yet available. Guidelines were produced by the European Society of Medical Oncology (ESMO) about treatment prioritisations and assumptions were made based on the degree of specific drug or regimen-related neutropenia shown in previous studies [2]. However still uncertainties remained about the individual risk to patients undergoing treatment, and the ability to deliver safe and effective treatments in a health system that is under pressure. Other than infection related morbidity and mortality, the impact of the COVID-19 pandemic on individuals undergoing systemic treatment for breast cancer in terms of completion of planned treatment has not been measured, hence the reason for our study.
Breast cancer is the commonest malignancy in females and will affect 1 in 8 women in their lifetime. The treatment options include surgery, endocrine therapy (for those tumours that express the oestrogen receptor ER), and chemotherapy (indicated for higher-risk tumours which can be ER positive, or triple negative (negative for ER, the progesterone receptor (PR), and HER2), or HER2 positive. Treatment outcomes for patients with advanced (stage 4) disease have improved significantly in the past 10 years with a recent study showing that overall survival of 56 months can be seen in HER2 positive subtypes (Cleopatra trial) supporting aggressive treatment options [3]. In the past 4 years there has been a switch from intravenous chemotherapy to oral systemic anti-cancer therapy (SACT) options with the development of CDK4/6 inhibitors such as abemaciclib, palbociclib, and ribociclib for the first or/and second line treatment of metastatic breast cancer that is ER positive [4]. These agents are effective and well tolerated and patients can be managed in an outpatient clinic with regular blood monitoring.
In this study we evaluated the impact of COVID-19 on chemotherapy administration in breast cancer patients. We decided early on that treatment decisions needed to be made on a case-by-case basis, taking into account patient choice, co-morbidities, risk of hospital attendances, risk of progression and cancer-related mortality in the absence of treatment and NICE guidelines. Anxiety was a common feature amongst patients and physicians alike. Some patients were too afraid to leave home and come to hospital for treatment or monitoring. As oncologists we strive to used evidence-based practise but were in an arena where the evidence was absent. Rapid changes occurred in the way we practiced, many consultations were switched to video and telephone, oral medication was delivered by post, and there was support from general practitioners and district nurses in administration of subcutaneous injections such as denosumab. Face-to-face consultations were reserved for those patients with a new cancer diagnosis or life-threatening condition. Conducting a face-to-face consultation with a surgical mask on hinders the direct communication that we are used to. At an early stage there was concern regarding theatre space beyond a 2 week window so some neoadjuvant patients were directed to early surgery to be followed by adjuvant chemotherapy. This was in the light of national guidance that encouraged primary surgical rather than neoadjuvant therapy for all non-metastatic, operable breast cancer. The aim of this study was to assess the impact of COVID-19 on the systemic treatment of breast cancer patients in a real-world setting and compare it to normal practise, based on evaluating treatment patterns over an 8 week period from the start of the first lockdown and comparing this to the same period in 2019.
2. Methods
2.1 Study setting and cohort
We performed this observational cohort study in a tertiary referral centre in London, UK. Approximately 500 patients receive anti-cancer treatment for breast cancer each year at this centre. Participants all had a diagnosis of breast cancer (either primary or metastatic) and received treatment for this during the study period. All patients prescribed oral or intravenous chemotherapy, monoclonal antibodies, targeted therapies, bone modifying agents and intramuscular or subcutaneous hormone therapies (collectively referred to as SACT) were included. The study was formally registered with the hospital trust audit team under the directorate of oncology and palliative care.
2.2 Data sources
Our hospital oncology-specific electronic database (ARIA Oncology Information System, Varian Medical Systems) was used to identify patients who were scheduled to receive treatment on our chemotherapy unit in the 8-week period from the start of the national lockdown in the UK (23rd March to 17th May 2020; n=312). Patients were identified retrospectively for the corresponding 8 weeks of the previous year (25th March to 19th May 2019; n=336) for comparison and assessment of the impact of covid-19 on our practice. We also used our hospital’s general electronic health record system (Cerner EHR) to collect further information on the outcomes detailed below.
2.3 Statistical analysis and outcomes
We collected the ages of all the patients in the 2019 and 2020 cohorts and calculated the range. Paired sample t-tests were performed on patient age categories for both of the study years. We recorded the specific treatments patients were scheduled to receive and categorised these according to intravenous chemotherapy, oral, HER2 targeting, bone modifying and parenteral endocrine treatments for both years. Data was collected for 2019 and 2020 regarding the intention of treatment (neoadjuvant, adjuvant or metastatic). The primary outcomes were whether treatment was given as scheduled, modified, delayed or stopped and the indication for this (covid-19 related or otherwise). For patients who had neoadjuvant or adjuvant chemotherapy stopped, the indication for stopping and number of cycles of treatment patients received before this cessation was also recorded. Prior to the covid-19 pandemic, patients receiving oral cdk4/6 inhibitors had routine blood tests performed every 4 weeks at our centre to assess for treatment toxicity. The location and frequency of these blood tests changed for many patients who received oral treatments during the pandemic and this was analysed in the 2020 patient cohort.
Separate analyses were also performed on patients planned to receive bone modifying, HER2 targeting or parenteral endocrine therapies either adjuvantly or palliatively during the study periods. Again, the main outcomes measures included were whether treatment proceeded unaffected or was modified, delayed or stopped and the indication for this. The actual number of attendances on our chemotherapy unit were calculated for each of the 8 weeks in the 2019 and 2020 periods and compared. We also identified patients commencing a first cycle of treatment during both study periods to assess the impact of covid-19 on instigating new treatments. Finally, we compared the number of new breast cancer referrals (both primary and recurrences) to our hospital trust in the 8-week periods from 2019 and 2020 and the referral pathway taken. The software package Prism (version 8.4.3) was used for generating graphical outputs of the data.
2.4 Patient and public involvement
Due to challenges associated with lockdown and additional clinical pressures during this time, patients were not actively involved in the design or completion of this study. However, we fully acknowledge the importance of public involvement and will ensure that our patients remain updated about the progress and findings of this study
3. Results
336 patients had a breast cancer treatment requiring hospital attendance planned in the 8 week period between 25th March and 19th May 2019, compared to 312 patients in a similar period in 2020 (23rd March to 17th May). Patient age did not differ significantly between these 2 cohorts who had treatments planned (2019 mean 59.2 years, 2020 mean 57.2 years, p>0.05, t-test), Figure 1. Treatment setting (neoadjuvant, adjuvant or palliative) distribution was also not significantly different in 2019 compared to 2020 (p>0.05, chi-squared) Figure 1. Table 1 lists the systemic anti-cancer therapies (SACTs) administered during the study periods, divided into 5 categories (chemo, HER2 targeted and parenteral endocrine therepies, bone modifying agents (BMAs) and oral SACT). Of note oral endocrine therapies (aromatase inhibitors, and Tamoxifen) were not included in this study as they are usually prescribed by GPs and do not therefore require hospital attendance. As Table 2 demonstrates, referrals to the breast oncology service between March and May 2020 were 38.2% lower than during the equivalent period in 2019 (123 referrals in 2019 compared with just 76 in 2020). Hospital referrals fell by 53.3%, GP referrals by 8.8% and screening service referrals by 60.8%. Accordingly, the number of patients receiving their first cycle of treatment during the 2020 period was 33.9% lower (62 in 2019 compared to 41 in 2020) however the proportion of patients starting treatment during the 2 periods was not significantly different (22.6% in 2019 and 15.1% in 2020, p>0.05, chi-squared).
Although the numbers of planned treatments were similar
between the 2 cohorts (336 in 2019 v 312 in 2020), the numbers of treatments given and therefore treatment unit attendances were 39.1% lower in 2020, Figure 2. There were a total of 369 attendances (mean 46.1/week) in 2020 compared to 606 (mean 75.8/week) in 2019. Weekly attendance increased week on week during the study period in 2020 as so was down 57.8% in week 1 but only 16.9% by week 8 as the situation improved. There were no in hospital deaths due to COVID-19 in our cohort, one patient was admitted and successfully discharged, one with leptomeningeal disease on olaparib died elsewhere which is likely to have been due to progressive disease, we had one neoadjuvant positive and then surgery delayed, we had some with symptoms that weren’t tested but were delayed and then recovered.
3.1 Chemotherapy
3.1.1 Neoadjuvant: Neoadjuvant chemotherapy was planned for 24 patients during the study period in 2019 and 11 of them (45.8%) had their first cycle. Over the equivalent period in 2020, 33 patients were scheduled to receive neoadjuvant chemotherapy and 8 of them (24.2%) cycle 1. Whereas 1 patient (4.2%) had their chemotherapy altered in 2019 (surgery was expedited due to poor response), 28 (84.8%) had their treatment changed during 2020, all as a result of COVID-19, Figure 3A. The 5(15.2%) patients whose treatment was unaffected in 2020 all started chemotherapy in May, towards the end of the study period. Of those patients that had their treatment altered due to COVID-19, 24 (72.7%) stopped treatment early to proceed to surgery; 2 (6%) had chemotherapy omitted from their last cycle of treatment (but proceeded with dual anti-HER2 antibodies, trastuzumab and pertuzumab); 1 (3%) declined early surgery and requested to postpone neoadjuvant chemotherapy; and a further 1 had early surgery cancelled as she had a positive COVID-19 swab and proceeded with neoadjuvant chemotherapy after recovery. Of the 24 patients that underwent early surgery 29.2% (7/24) had HER2 positive breast cancer and 70.8% (17/24) triple negative breast cancer (TNBC). Of the 7 HER2 positive patients who had their neoadjuvant chemotherapy interrupted, 3 restarted chemotherapy in the adjuvant setting; 2 had docetaxel omitted from their final 2 cycles of docetaxel, trasutuzumab and pertuzumab,DTP, (1 had a pathological complete response [pCR] at surgery, the other had had an admission with neutropenic sepsis following neoadjuvant DTP); and 2 had Docetaxel omitted from their last 3 cycles of DTP due to age and co-morbidities and so perceived risk from COVID-19. Of the 17 patients with TNBC that had their neoadjuvant chemotherapy interrupted to proceed with surgery. Eight restarted adjuvantly; 4 had a pCR and so didn’t have further treatment; another 4 who had completed the anthracycline component of their neoadjuvant chemotherapy but had only received between 2 and 9 weeks (from 12) of Carboplatin/Paclitaxel switched to Capecitabine post-operatively; and the final patient declined further chemotherapy in the adjuvant setting.
3.1.2 Adjuvant: 23 patients in 2019 and 31 in 2020 were scheduled to receive adjuvant chemotherapy during the study period. Twelve (52.2%) and 15 (48.4%) respectively were due to start treatment. The majority (78.3%, 18/23) of patients’ treatment was unaffected in 2019, compared with only 25.8% (8/31) that were unaffected in 2020, Figure 3B. Of the 5 patients who had their treatment altered in 2019, 3 were delayed for infection and 2 stopped chemotherapy (1 due to toxicity and the other patient preference). Only 3 (9.7%) patients had their treatment altered in 2020 for non-COVID-19 reasons (1 delay for port thrombus; 1 stopped as new diagnosis of non-breast malignancy, treatment of which took priority; and 1 discontinued due to toxicity). Of the remaining 20 patients 6 (19.4%) had their treatment modified (dose reduction or protocol reversal i.e. they received the taxane component of their chemotherapy first) without delay; a further 6 had their treatment delayed; and the rest (25.8%) had their treatment stopped. Two thirds of the delays (4/6) were less than 1 month. The 2 patients who had longer treatment delays had asthma (9 week delay) and COPD (13 week delay). Three of the 8 patients who stopped adjuvant chemotherapy due to COVID-19, did so at the patients’ request. The other 5 had co-morbidities and predicted benefit chemotherapy of £7%.
3.1.3 Palliative: In 2019, 20 patients were due palliative intravenous chemotherapy during the study period, compared with 21 patients during the equivalent period in 2020, Figure 3C. Three patients (15%) had a change to their chemotherapy in 2019 (2 stopped due to PD and 1 changed treatment following admission with infection), compared to 12 (57.1%) in 2020. Of the twelve patients whose treatment was affected in 2020, 2 (9.5%) had dose reductions which would likely have been made regardless of COVID-19; 7 (33.3%) had their treatment delayed an average of 13.6 weeks (range 11-17 weeks) to avoid hospital attendance during the first COVID-19 peak; and 3 (14.3%) stopped chemotherapy (1 continued dual antibodies only; 1 declined to restart; and 1 was not well enough to restart).
3.2 Oral systemic anti-cancer therapy
Ninety-one and 110 patients were due to be on oral SACT for metastatic breast cancer during the study periods in 2019 and 2020 respectively. Oral SACT included Capecitabine (27/91 or 29.7% in 2019, 33/110 or 30% in 2020) CDK4/6 inhibitors (58/91 or 63.7% in 2019, 72/110 or 65.5% in 2020), PARP inhibitors (1/91 or 1.1% in 2019, 1/110 or 0.9% in 2020) and Everolimus (5/91 or 5.5% in 2019, 4/110 or 3.6% in 2020). Twelve patients (13.2%) had their treatment changed in 2019 (4 had their treatment stopped and 8 delayed). In 2020 30 patients (27.3%) were affected. Six (5.5%) for non-COVID reasons (2 modified, 3 delayed and 1 stopped) and 24 (21.8%) due to COVID-19 (9 modified, 7 delayed and 8 stopped), Figure 3D. Many of the patients on CDK4/6 inhibitors were offered less frequent blood tests, or had blood tests done locally with a GP, and had medication posted to them at hope to reduce hospital attendances (Figure 4).
3.3 HER2 targeted treatments
Thirty-two patients in 2019 and 37 in 2020 were due adjuvant HER2 targeted therapies (dual antibodies Trastuzumab and Pertuzumab, single agent Trastuzumab, or Kadcyla) without additional chemotherapy, during the trial periods. None of the patients in 2019 had their treatment altered, compared to 75.7% (28/37) of patients in 2020. Of the 9 affected in 2020, 5 (13.5%) stopped adjuvant single agent Trastuzumab early (all of whom had had at least 10/18 cycles); 3 (8.1%) chose to delay their treatment by 5-7 weeks to avoid hospital attendance during the first peak of the pandemic; and 1 patient (2.7%) had single agent Herceptin without induction chemotherapy, Figure 3E. With regards the use of these HER2 targeted treatments in the palliative setting, 29 patients in 2019 and 35 in 2020 had treatment planned during the study periods. Five patients (17.2%) had their treatment altered in 2019 (4 delayed, 1 stopped), compared to 17 (48.6%) in 2020. Two (5.7%) of those whose treatment was impacted in 2020 stopped due to progressive disease, so 42.9% (15/35) of patients had their treatment changed in 2020 due to COVID-19. Of these 15 patients, 8 (22.9%) switched to 4 weekly treatments, 5 (14.3%) had their treatment delayed by an average of 11 weeks (range 3-16 weeks); and 2 (5.7%) stopped HER2 targeted treatment and continued with single agent endocrine therapy, Figure 3F.
3.4 Parental endocrine treatments
Eighteen patients were due to receive adjuvant goserelin during the 2019 study period and 21 patients were scheduled for treatment during the equivalent period in 2020. One patient (5.6%) stopped goserelin in 2019 as they were trying to conceive. Two patients (9.5%) chose to delay their treatment in 2020 in order to avoid hospital attendance. Thirty patients were scheduled to receive parental endocrine treatment (goserelin, fulvestrant or both) in 2019 and none of them had their treatment altered. In comparison 49 patients were due goserelin (17/49, 34.7%) Fulvestrant (30/49, 61.2%) or both (2/49, 4.1%) in 2020 and 12 of them (24.5%) had their treatment altered in some way. Nine patients (18.4%) had their treatment deferred: 2 because they were unwell, not COVID-19; 2 because they were self-isolating following close contact with a COVID-19 positive individual; 3 whilst they arranged for their GP to administer treatment; and 2 to avoid hospital attendance. Three patients (6.1%) stopped treatment: a 91 year old with co-morbidities and low volume disease; a patient who moved away and a lady that died (Figure 5).
3.5 Bone modifying agents
Sixty-six patients were scheduled to receive adjuvant zolendronic acid during the study period in 2019, compared with 55 in 2020. Only 4 treatments (6%) were altered (all stopped) in 2019 whereas this number was 45 (81.8%) in 2020, all due to COVID-19. In 2020 thirty-seven patients (67.3%) had their treatment delayed an average of 14.9 weeks (range 6 – 38 weeks) in order to avoid hospital attendance during the first peak of the pandemic, Figure 5. The remaining 8 (14.5%) have yet to resume treatment. 86 patients in 2019 and 81 in 2020 were due to receive bone modifying agents (BMAs) for metastatic disease during the study periods. BMAs included denosumab (48/86 or 55.8% in 2019, 43/81 or 53.1% in 2020) and zolendronic acid (38/86 or 44.2% in 2019, 38/81 or 46.9% in 2020). Only 39.5% of palliative BMA treatments were unaffected in 2020 compared to 90.7% in 2019. Of the 49 patients whose treatment was altered in 2020, 5 (10%) were affected for non-COVID reasons (PD, unwell, dental issues), 34 (42%) were delayed an average of 12 weeks (range 4-21 weeks); and 10 (12.3%) stopped treatment. Seven of the 34 patients delayed received ibandronate in the interim.
3.6 GPs administration of drugs
Arrangements were made for a number of patients to have their SC or IM breast cancer treatments (goserelin, fulvestrant, denosumab and trastuzumab) with their GPs, in order to limit hospital attendances. Three patients (14.3%) changed to having their GP administer their adjuvant goserelin without a delay in their treatment. Ten patients (20.4%) chose to self-administer or have their GP or friend administer their palliative parental endocrine treatment (6 fulvestrant and 4 goserelin), in 7 cases without a delay in treatment. Six patients (14%) receiving Denosumab in the palliative setting, switched to having their GP administer treatment, 2 without a delay. And 1 lady had palliative SC trastuzumab administered by her distric nurse team due to severe anxiety about attending hospital.
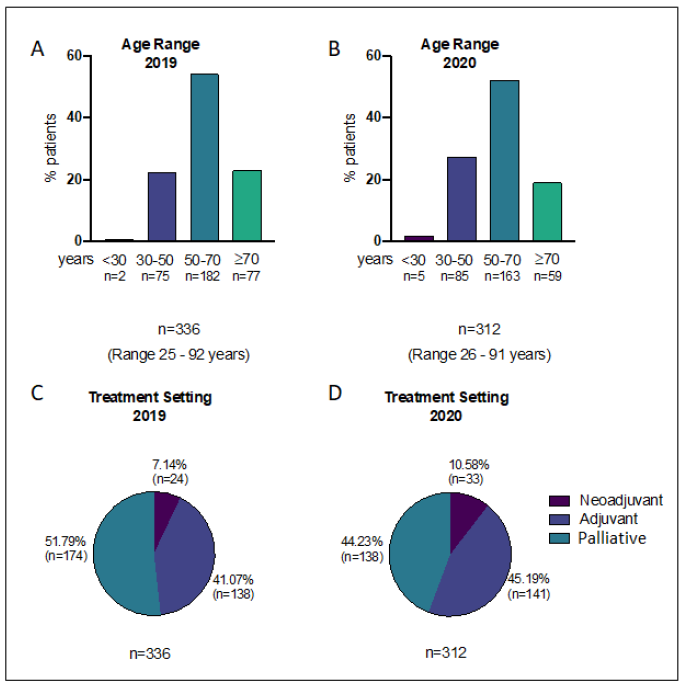
Figure 1: A) Age distribution of patients attending hospital for treatment during study period in 2019 and; B) 2020; C) Treatment setting distribution of patients attending hospital for treatment during study period in 2019 and; D) 2020.
|
Chemotherapy Based Treatments |
2019 n (%) |
2020 n (%) |
|
Nab-paclitaxel |
1 (0.23) |
3 (0.63) |
|
Nab-paclitaxel & Herceptin |
0 (0) |
1 (0.21) |
|
AC |
22 (5.21) |
22 (4.65) |
|
Carboplatin |
2 (0.47) |
0 (0) |
|
CMF |
1 (0.24) |
2 (0.42) |
|
Docetaxel |
1 (0.24) |
1 (0.21) |
|
EC |
2 (0.47) |
0 (0) |
|
Eribulin |
5 (1.18) |
1 (0.21) |
|
FEC |
1 (0.24) |
0 (0) |
|
Gemcitabine & Carboplatin |
2 (0.47) |
3 (0.63) |
|
Paclitaxel |
12 (2.84) |
27 (5.71) |
|
Paclitaxel & Carboplatin |
0 (0) |
11 (2.33) |
|
Pertuzumab & Trastuzumab & Docetaxel |
11 (2.61) |
13 (2.75) |
|
Trastuzumab & Paclitaxel |
7 (1.66) |
1 (0.21) |
|
Total |
67 (15.88) |
85 (17.97) |
|
Oral Treatments |
2019 n (%) |
2020 n (%) |
|
Capecitabine |
27 (6.40) |
33 (6.98) |
|
Everolimus |
5 (1.18) |
4 (0.85) |
|
Abemaciclib |
9 (2.13) |
17 (3.59) |
|
Palbociclib |
42 (9.95) |
45 (9.51) |
|
Ribociclib |
7 (1.66) |
10 (2.11) |
|
Olaparib |
0 (0) |
1 (0.21) |
|
Talazoparib |
1 (0.24) |
0 (0) |
|
Total |
91 (21.56) |
110 (23.26) |
|
HER2 Targeting Treatments |
2019 n (%) |
2020 n (%) |
|
TDM1 |
8 (1.9) |
9 (1.9) |
|
Pertuzumab & Trastuzumab |
17 (4.03) |
35 (7.4) |
|
Trastuzumab |
39 (9.24) |
28 (5.92) |
|
Total |
64 (15.17) |
72 (15.22) |
|
Bone Modifying Treatments |
2019 n (%) |
2020 n (%) |
|
Zoledronic Acid |
104 (24.64) |
93 (19.66) |
|
Denosumab |
48 (11.37) |
43 (9.09) |
|
Total |
152 (36.02) |
136 (28.75) |
|
Parenteral Endocrine Treatments |
2019 n (%) |
2020 n (%) |
|
Fulvestrant |
16 (3.79) |
30 (6.34) |
|
Goserelin |
31 (7.35) |
38 (8.03) |
|
Fulvestrant & Goserelin |
1 (0.24) |
2 (0.42) |
|
Total |
48 (11.37) |
70 (14.8) |
Table 1: Treatments requiring hospital attendance in 2019 and 2020 (percentages are of all treatments requiring hospital attendance: n = 422 in 2019 and n = 473 in 2020).
|
Source of Referral |
2019 n (%) |
2020 n (%) |
||
|
Primary |
Recurrence |
Primary |
Recurrence |
|
|
A&E Department |
1 (0.85) |
- |
- |
- |
|
Consultant, other than A&E Department |
11 (9.32) |
2 (40.00) |
7 (9.59) |
- |
|
Following Emergency Admission |
1 (0.85) |
- |
- |
- |
|
General Practitioner |
34 (28.81) |
- |
30 (41.10) |
1 (33.33) |
|
National Screening Programme |
50 (42.37) |
1 (20.00) |
20 (27.40) |
- |
|
Other |
21 (17.8) |
2 (40.00) |
16 (21.92) |
2 (66.67) |
|
Total |
118 (100) |
5 (100) |
73 (100) |
3 (100) |
Table 2: Referral numbers and their sources during study periods 2019 and 2020.
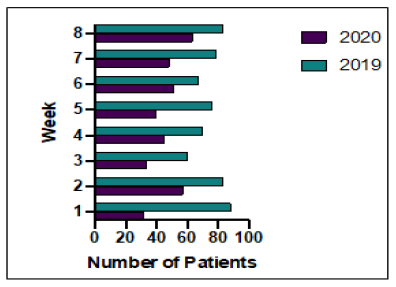
Figure 2: Outpatient treatment unit weekly attendance during study periods 2019 and 2020.
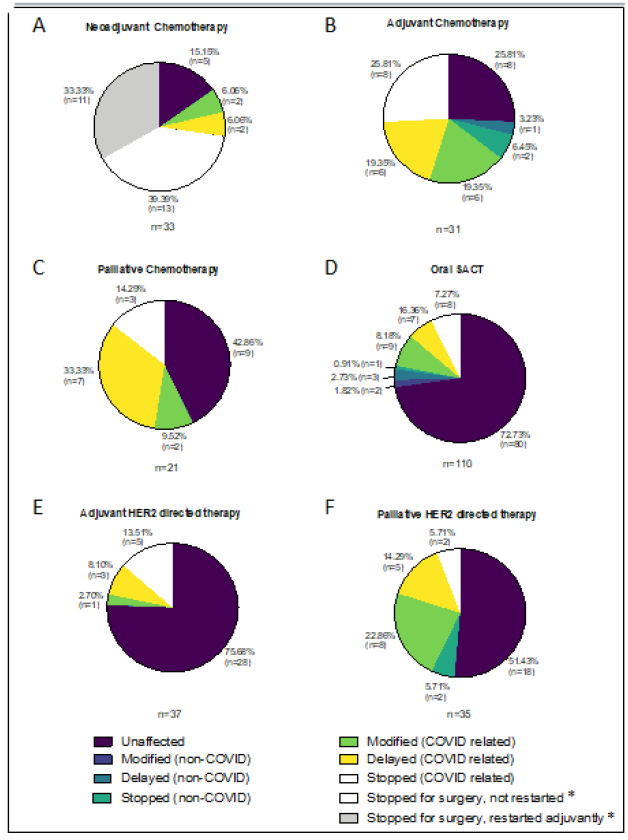
Figure 3: Changes to planned treatment for patients on A,B&C) chemotherapy, D) oral systemic anti-cancer therapy (SACT) and E&F) HER2 targeted therapy, during 2020 study period. *All stops for surgery were COVID related.
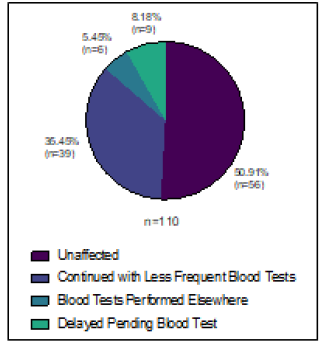
Figure 4: Changes to blood test monitoring for oral SACT during 2020 study period.
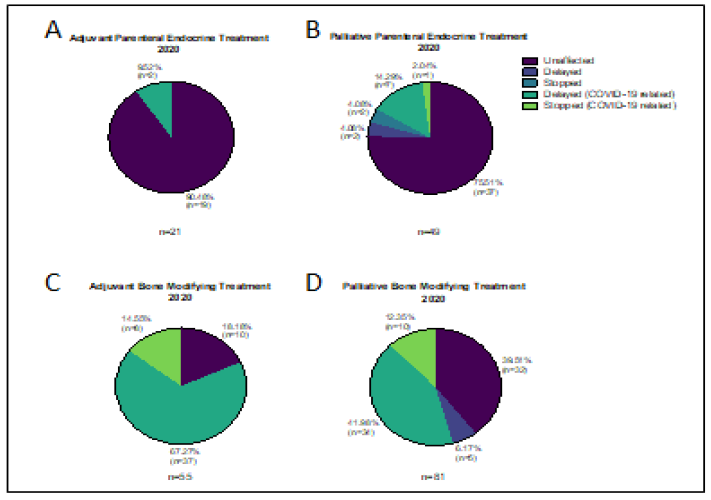
Figure 5: Changes to planned treatment for patients on A&B) endocrine therapy and C&D) bone modifying agents during 2020 study period.
4. Discussion
This study shows that during the first lockdown cancer treatment for breast cancer patients was affected by the Covid-19 pandemic with reductions in the numbers of cancers being diagnosed, the number of hospital attendances for treatment, and changes in the treatment paths for patients with newly diagnosed breast cancer. Our data clearly shows that the impact was felt most during the early weeks of lockdown, with recovery towards the later stages (Figure 2). The Covid-19 pandemic is not over. Cancer patients have had major anxieties about the ability to start and continue their treatment, and faced increased isolation due to shielding. We have shown here that it is possible to continue oncological treatment for breast cancer patients and this will hopefully not have major impacts on longterm outcomes. Oncologists were advised to prioritise treatments, however given the improved survival rates seen in metastatic breast cancer, this was assessed on an individual basis with patient’s preferences and co-morbidities being taken into account.
In March 2020, NHS England advised hospital trusts that “Essential and urgent cancer treatments must continue. Cancer specialists should discuss with their patients whether it is riskier for them to undergo or to delay treatment at this time” (Source NHS England publications reference 001559). Subsequently in June a second document was produced stating three guiding principles for cancer treatment during the pandemic “1) Capacity: there needs to be sufficient capacity to ensure anyone referred with suspected cancer can be diagnosed and treated promptly; 2) Fairness: access to cancer diagnostic and treatment services should be equitable and based on clinical priority; and 3) Confidence: patients need to have confidence their diagnosis and treatment will take place in an environment and manner that is safe.” A list of six cancer treatment priority categories was included based on chance of success with treatment. What is a serious concern is our finding that the number of referrals with suspected breast cancer has dropped significantly - by 38.2% in our cohort (see table 2). This was an expected outcome, as there have been major impacts on cancer screening which was suspended. This will translate into delayed diagnoses and worse outcomes for patients, as larger more advanced tumours are more likely to need mastectomies and chemotherapy. 34% fewer patients started a new treatment in 2020 than in 2019 (41 v 62), reflecting fewer referrals, and both patient and clinician concern regarding risk of Covid-19 in the treatment population. Government-backed advertising campaigns are now encouraging patients with possible cancer-related symptoms to seek medical help. Gatthani et al compared the number of patients referred with suspected breast cancer in the first 6 months of 2019 to the same period in 2020 and found that the number in 2020 was 28% lower (N=231,765 versus N=322,994) [5]. The difference in numbers here most likely reflects the longer duration of that study (6 months v 8 weeks).
The main group of patients that were affected were those that were undergoing neoadjuvant chemotherapy, where 85% were affected. There was concern that we would not have operating space so in most cases we decided to expedite early surgery, which was then followed by the most appropriate systemic therapy and or radiotherapy. Scheduling of surgery was altered in most of the neoadjuvant patients with the assistance of private hospitals so that they could be operated on in a clean hub. Some of the patients who didn’t complete the entire course of neoadjuvant chemotherapy chose to do so themselves, and in others where there was a complete pathological response treatment was stopped. It is likely that these changes were mirrored throughout the UK due to guidelines avoiding primary systemic therapy [6, 7]. The effects on breast cancer surgery have been investigated by others [8-11]. However as confidence grew with the effect of chemotherapy the situation reverted back to normal within a short time span soon after our study. This impact was closely followed by the adjuvant group where 75% saw changes to their treatment plans. In patients where the benefit of chemotherapy was not thought to be worth the risk, patients were offered endocrine therapy as an alternative. Recent data has shown that genetic prediction assays may mean that we are able to avoid chemotherapy in larger groups of patients than before [12], and these were used to aid decision-making [13, 14].
Modifications to treatment were common in our study. One of the commonest was to add granulocyte colony stimulating factor (GCSF) for all patients on intravenous chemotherapy to prevent neutropenia and or neutropenic sepsis. Another approach was to administer the taxane prior to the anthracycline to avoid severe neutropenia. Some authors have suggested that the use of metronomic chemotherapy is a possible option for managing patients during the pandemic, but whether this corresponds to the same outcomes for patients as standard treatment schedules remains to be tested [15]. Many of our patients who were on established treatment with stable disease (some for several years) were offered 4 weekly schedules instead of 3 weekly visits during this intense period. Some patients chose to have “treatment holidays” due to age and underlying comorbidities and restarted treatment at later dates. In some cases patients on longterm treatments for metastatic disease with no signs of active disease for >12 months, stopped treatment, these patients were continued on close monitoring. Our service has adapted to change with more telemedicine, fewer hospital attendances for patients, and some treatments have been given in the community. In terms of specific drugs, the bone modifying agents given in the adjuvant setting were the ones that were altered most commonly by treatment delays, this was because of the pressure on the chemotherapy unit and the fact that alternative agents oral agents such as ibandronate were available for use in the short term, and that they have lower benefits compared to other treatments (Figure 5).
Other studies have looked at the effect of COVID-19 on morbitidy and mortality of cancer patients, but that was not the primary objective of our study [16]. The authors of the UK Coronavirus Cancer Monitoring Project (UKCCMP) did not find a significant effect on mortality for patients with immunotherapy, hormonal therapy, targeted therapy, radiotherapy use within the past 4 weeks compared to the general population. The risk of COVID-19 related mortality was similar to other studies with age, male sex, hypertension, and cardiovascular disease being significant. A retrospective study by Pinato et al found that cancer patients infected with COVID-19 UK had worse mortality than those from Spain or Italy, and corroborated the UKCCMP study results in terms of risk of treatment [17]. The limitations of this study are that it was performed in a single centre, and we have not studied what has happened with further tier restrictions. Our study does represent a major treatment hub in London with a screening service on-site within the setting of an acute general hospital, and this is likely to have been a factor in our results as we would propose less impact may have been seen in stand-alone cancer treatment units. The size of the cohort means that similar results are likely to have been reflected throughout the UK. With the advent of mass asymptomatic testing we now test all patients prior to each chemotherapy appointments and this, together with regular staff testing, should minimise the risk of cross-infection within the treatment unit providing reassurance for patients. Our treatment centre is currently working at full capacity, back to pre-pandemic levels. We were fortunate that chemotherapy administration was not affected by staff shortages, but this may not be the same for smaller units. Outpatient visits are still reduced where possible and converted to telephone clinics to minimise travel and exposure to patients. The development of successful vaccines will hopefully enable us to return to normal.
Many clinical trials were halted to new patient recruitment during the which will mean that the results of studies that could have large clinical benefits for patients have been postponed. The charities that fund much of the vital new drug developments in cancer have had severe reductions in funding, clinical research must continue to drive improvements in outcomes. With the third wave of the pandemic ongoing, even though vaccination of different communities have commenced, it will take a while before patients feel confident about attending hospital for treatment and investigations. Staff and patients are all now tested regularly, and priority vaccination should help protect patients. There are still some uncertainties around whether vaccination will provide the same level of protection in immunocompromised patients, and how to manage issues such as patients who persistently test positive weeks after infection. The past 12 months have seen a paradigm shift in the management of patients, their care must be protected so that they do not become the unforgotten casualties of the pandemic. Cancer can have major psychological effects, and the additive burden of social isolation necessitated by shielding needs to be considered. Policy makers should note the impact of the pandemic on cancer patients and provide a protected service for the future, as we are likely to see rises in cancer morbidity and mortality as a result. Clinical cancer research and it’s funding by charities have been severely affected, this needs attention so that better outcomes for patients can be achieved in future.
Conflict of Interest Statement
The authors have no conflicts of interest to declare related to this manuscript.
Acknowledgements
The authors would like to thank the staff from Imperial College Healthcare NHS Trust who contributed to this effort to preserve treatment during COVID. We did not receive any funding for the project.
References
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 21 (2020): 335-337.
- Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID-19 pandemic: an ESMO multidisciplinary expert consensus. Ann Oncol 31 (2020): 1320-1335.
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21 (2020): 519-530.
- Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 395 (2020): 817-827.
- Gathani T, Clayton G, MacInnes E, et al. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer (2020).
- Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat 181 (2020): 487-497.
- Curigliano G, Cardoso MJ, Poortmans P, et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast 52 (2020): 8-16.
- Tsang-Wright F, Tasoulis MK, Roche N, et al. Breast cancer surgery after the COVID-19 pandemic. Future Oncol 16 (2020): 2687-2690.
- Ahmed M. Optimizing breast cancer surgery during the COVID-19 pandemic. Breast Cancer 27 (2020): 1045-1047.
- Tasoulis MK, Roche N, MacNeill F. Rationalizing breast cancer surgery during the COVID-19 pandemic. Eur J Surg Oncol 46 (2020): 1192-1193.
- Yang J, Fu Z, Du L, et al. Time to surgery in patients with breast cancer during the COVID-19 pandemic. Br J Surg 107 (2020): e419-e421.
- Crolley VE, Marashi H, Rawther S, et al. The impact of Oncotype DX breast cancer assay results on clinical practice: a UK experience. Breast Cancer Res Treat 180 (2020): 809-817.
- Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med 380 (2019): 2395-2405.
- Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol 26 (2008): 4063-4071.
- Fedele P, Sanna V, Fancellu A, et al. De-escalating cancer treatments during COVID 19 pandemic: Is metronomic chemotherapy a reasonable option?. Crit Rev Oncol Hematol 157 (2020): 103148.
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 395 (2020): 1919-1926.
- Pinato DJ, Zambelli A, Aguilar-Company J, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov (2020).
