Immunogenic Properties and Antitumor Effects of Novel Therapeutic Dendritic Cell Vaccines Expressing hTERT and Survivin Antigens in Metastatic Prostate Cancer Patients
Article Information
Anne Merete Aaland Tryggestad1#, Iris Bigalke1#, Wolfgang Lilleby2, Karol Axcrona3, Turid Kirsti Hønnåshagen1, Lisbeth Johanne Skoge1, Stein Sæbøe-Larssen1, Guri Solum1, Richard Willfred Olaussen1, Dag Josefsen1, Dolores Schendel4, Gunnar Kvalheim1*
#First and second author and contributed equally
1Department of Cellular Therapy, Oslo University Hospital-The Norwegian Radium Hospital, Oslo 0310, Norway
2Department of Oncology and Radiotherapy, Oslo University Hospital-The Norwegian Radium Hospital, Oslo, Norway
3Department of Urology, Akershus University Hospital, 1478 Loerenskog, Norway
4Medigene Immunotherapies GmbH, Munich, Germany
*Corresponding Author: Dr. Gunnar Kvalheim, Department of Cellular Therapy, Oslo University Hospital-The Norwegian Radium Hospital, Oslo 0310, Norway
Received: 10 August 2020; Accepted: 01 September 2020; Published: 01 October 2020
Citation: Anne Merete Aaland Tryggestad, Iris Bigalke, Wolfgang Lilleby, Karol Axcrona, Turid Kirsti Hønnåshagen, Lisbeth Johanne Skoge, Stein Sæbøe-Larssen, Guri Solum, Richard Willfred Olaussen, Dag Josefsen, Dolores Schendel, Gunnar Kvalheim. Immunogenic Properties and Antitumor Effects of Novel Therapeutic Dendritic Cell Vaccines Expressing hTERT and Survivin Antigens in Metastatic Prostate Cancer Patients. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 393-407.
View / Download Pdf Share at FacebookAbstract
Objective: To investigate the clinical effects of novel therapeutic dendritic cell vaccines targeting the universal tumour antigens human telomerase reverse transcriptase and Survivin in a small exploratory group of four patients with high-risk prostate cancer who had progression after secondary therapy. In contrast to previous dendritic cell vaccine studies, we explored application of intradermal dendritic cell vaccines every month over longer periods of time to boost and maintain potential immune responses.
Methods: Dendritic cell vaccines were given in combination with androgen deprivation therapy, with and without radiotherapy and chemotherapy, until tumour progression. PSA levels were followed throughout the whole treatment period. Immune responses were assessed investigating antigen specific CD4 and CD8 T cell responses at chosen time points.
Results: Two patients remained in clinical remission at 113 and 72 months after start of dendritic cell vaccination. Time to progression for these two patients after secondary therapy, prior to dendritic cell vaccination was 3 and 7 months, respectively. A third patient obtained a stable clinical disease for 95 months with DC vaccines, androgen deprivation therapy and radiotherapy, with time to progression of only 14 months after secondary therapy but before start of vaccination. These patients mounted specific immune responses during dendritic cell vaccination as detected at several time points during treatment.
Conclusion: These results suggest that patients with low tumour burden may benefit significantly from continuous personalized dendritic cell vaccination when combined with additional therapies. Importantly, no vaccine-related toxicity was observed despite application of substantial numbers of dendritic cells over extended periods of time. These exploratory cases provide valu
Keywords
<p>Dendritic cells; Prostate cancer; Vaccine</p> <gdiv></gdiv>
Dendritic cells articles, Prostate cancer articles, Vaccine articles
Dendritic cells articles Dendritic cells Research articles Dendritic cells review articles Dendritic cells PubMed articles Dendritic cells PubMed Central articles Dendritic cells 2023 articles Dendritic cells 2024 articles Dendritic cells Scopus articles Dendritic cells impact factor journals Dendritic cells Scopus journals Dendritic cells PubMed journals Dendritic cells medical journals Dendritic cells free journals Dendritic cells best journals Dendritic cells top journals Dendritic cells free medical journals Dendritic cells famous journals Dendritic cells Google Scholar indexed journals Prostate cancer articles Prostate cancer Research articles Prostate cancer review articles Prostate cancer PubMed articles Prostate cancer PubMed Central articles Prostate cancer 2023 articles Prostate cancer 2024 articles Prostate cancer Scopus articles Prostate cancer impact factor journals Prostate cancer Scopus journals Prostate cancer PubMed journals Prostate cancer medical journals Prostate cancer free journals Prostate cancer best journals Prostate cancer top journals Prostate cancer free medical journals Prostate cancer famous journals Prostate cancer Google Scholar indexed journals Vaccine articles Vaccine Research articles Vaccine review articles Vaccine PubMed articles Vaccine PubMed Central articles Vaccine 2023 articles Vaccine 2024 articles Vaccine Scopus articles Vaccine impact factor journals Vaccine Scopus journals Vaccine PubMed journals Vaccine medical journals Vaccine free journals Vaccine best journals Vaccine top journals Vaccine free medical journals Vaccine famous journals Vaccine Google Scholar indexed journals radiotherapy articles radiotherapy Research articles radiotherapy review articles radiotherapy PubMed articles radiotherapy PubMed Central articles radiotherapy 2023 articles radiotherapy 2024 articles radiotherapy Scopus articles radiotherapy impact factor journals radiotherapy Scopus journals radiotherapy PubMed journals radiotherapy medical journals radiotherapy free journals radiotherapy best journals radiotherapy top journals radiotherapy free medical journals radiotherapy famous journals radiotherapy Google Scholar indexed journals chemotherapy articles chemotherapy Research articles chemotherapy review articles chemotherapy PubMed articles chemotherapy PubMed Central articles chemotherapy 2023 articles chemotherapy 2024 articles chemotherapy Scopus articles chemotherapy impact factor journals chemotherapy Scopus journals chemotherapy PubMed journals chemotherapy medical journals chemotherapy free journals chemotherapy best journals chemotherapy top journals chemotherapy free medical journals chemotherapy famous journals chemotherapy Google Scholar indexed journals vaccine-related toxicity articles vaccine-related toxicity Research articles vaccine-related toxicity review articles vaccine-related toxicity PubMed articles vaccine-related toxicity PubMed Central articles vaccine-related toxicity 2023 articles vaccine-related toxicity 2024 articles vaccine-related toxicity Scopus articles vaccine-related toxicity impact factor journals vaccine-related toxicity Scopus journals vaccine-related toxicity PubMed journals vaccine-related toxicity medical journals vaccine-related toxicity free journals vaccine-related toxicity best journals vaccine-related toxicity top journals vaccine-related toxicity free medical journals vaccine-related toxicity famous journals vaccine-related toxicity Google Scholar indexed journals Androgen deprivation therapy articles Androgen deprivation therapy Research articles Androgen deprivation therapy review articles Androgen deprivation therapy PubMed articles Androgen deprivation therapy PubMed Central articles Androgen deprivation therapy 2023 articles Androgen deprivation therapy 2024 articles Androgen deprivation therapy Scopus articles Androgen deprivation therapy impact factor journals Androgen deprivation therapy Scopus journals Androgen deprivation therapy PubMed journals Androgen deprivation therapy medical journals Androgen deprivation therapy free journals Androgen deprivation therapy best journals Androgen deprivation therapy top journals Androgen deprivation therapy free medical journals Androgen deprivation therapy famous journals Androgen deprivation therapy Google Scholar indexed journals hTERT antigen articles hTERT antigen Research articles hTERT antigen review articles hTERT antigen PubMed articles hTERT antigen PubMed Central articles hTERT antigen 2023 articles hTERT antigen 2024 articles hTERT antigen Scopus articles hTERT antigen impact factor journals hTERT antigen Scopus journals hTERT antigen PubMed journals hTERT antigen medical journals hTERT antigen free journals hTERT antigen best journals hTERT antigen top journals hTERT antigen free medical journals hTERT antigen famous journals hTERT antigen Google Scholar indexed journals electroporation articles electroporation Research articles electroporation review articles electroporation PubMed articles electroporation PubMed Central articles electroporation 2023 articles electroporation 2024 articles electroporation Scopus articles electroporation impact factor journals electroporation Scopus journals electroporation PubMed journals electroporation medical journals electroporation free journals electroporation best journals electroporation top journals electroporation free medical journals electroporation famous journals electroporation Google Scholar indexed journals Peripheral blood mononuclear cells articles Peripheral blood mononuclear cells Research articles Peripheral blood mononuclear cells review articles Peripheral blood mononuclear cells PubMed articles Peripheral blood mononuclear cells PubMed Central articles Peripheral blood mononuclear cells 2023 articles Peripheral blood mononuclear cells 2024 articles Peripheral blood mononuclear cells Scopus articles Peripheral blood mononuclear cells impact factor journals Peripheral blood mononuclear cells Scopus journals Peripheral blood mononuclear cells PubMed journals Peripheral blood mononuclear cells medical journals Peripheral blood mononuclear cells free journals Peripheral blood mononuclear cells best journals Peripheral blood mononuclear cells top journals Peripheral blood mononuclear cells free medical journals Peripheral blood mononuclear cells famous journals Peripheral blood mononuclear cells Google Scholar indexed journals
Article Details
1. Introduction
Norwegian men have high life expectancy with a mean survival of 81 years. From the age of 75 years, however, one in eight men will be diagnosed with prostate cancer (PC), giving the sixth highest incidence worldwide [1]. Men diagnosed with high-risk PC have increased risk of recurrences and death after primary therapy [2]. Androgen deprivation therapy (ADT) is regarded as first line therapy when biochemical relapse (BCR) occurs after primary treatment. However, cancer cells will eventually become resistant to ADT and develop castration-resistant PC [3-5]. Both second-line ADT and upfront chemotherapy generally increase the disease-free interval but the effect is not sustained over time [6-8]. Thus, novel therapeutic strategies to prevent disease progression are needed.
Acknowledging the high medical need in these patients, we investigated whether dendritic cell (DC) vaccination targeting the antigens human telomerase reverse transcriptase (hTERT) and Survivin, when combined with standard treatment, offers clinical benefit in patients with recurrences, but still having PC sensitive to androgen signalling. In previous studies we showed that hTERT and Survivin mRNA-transfected DCs are safe and mount multi-specific T-cell responses related to clinical response [9-13]. In PC, we showed clinical effects of DC vaccines expressing the hTERT antigen [14]. Survivin is highly expressed on PC cells and appears to play a biological role in development of castration resistance [15]. Based on these findings, the cases studied here included DCs expressing both hTERT and Survivin as immunizing target antigens.
Here, we report our findings on four prognostic high-risk PC patients in whom prolonged DC vaccination was initiated subsequent to disease progression after primary and secondary combination therapy. All four patients were treated with ADT and fast DCs vaccines expressing the hTERT and Survivin antigens. Booster DC vaccinations were given every month until clinical relapse.
2. Materials and Methods
2.1 Production of mRNA-transfected dendritic cells
Peripheral blood mononuclear cells (PBMCs) were harvested by apheresis. Thereafter, monocytes were separated by elutriation and were either processed and cultured immediately or frozen until later use. Fresh or thawed and washed monocytes were transferred to VueLife cell culture bags (CellGenix, Freiburg, Germany) and differentiated into mature DCs by use of two different production protocols, Oslo or Munich fast DCs, before addition of mRNA-encoding antigen by electroporation.
2.2 Oslo fast DC
Oslo fast DCs were obtained as described previously, without IL-6 as part of the maturation cocktail [16]. Briefly, immature (im)DCs were generated by culturing monocytes in CellGro DC medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 at a cell density of 1x106 cells/mL. After 48 -72 hrs imDCs were matured over 24 hrs by adding GM-CSF, IL-4, tumour necrosis factor α (TNFα), IL-1β (all cytokines from CellGenix, Freiburg, Germany), and prostaglandin E2 (PgE2; Pfizer, New York, USA) as described. Oslo fast DCs were transfected with hTERT or Survivin mRNA by electroporation, incubated for 24 hrs without cytokines before freezing at a density of 2.5, 5, or 10x106 cells/vaccine ampule.
2.3 IDO-silenced Oslo fast DC
G4 indoleamine-pyrrole 2,3-dioxygenase- (IDO) silenced Oslo fast DCs were obtained by producing mature fast DCs as described above followed by electroporation with antigen and IDO siRNA, as described previously [9]. Briefly, after maturation, aliquots of Oslo fast DC were either co-transfected with IDO siRNA and hTERT or with IDO siRNA and Survivin mRNA. After electroporation, the DCs were incubated without cytokines for 24 hrs before freezing at a density of 5x106 cells/vaccine ampule.
2.4 Munich fast DCs
Munich fast DCs were also obtained as described previously [17,18]. Briefly, monocytes were differentiated into imDCs in medium consisting of very low endotoxin (VLE)-Roswell Park Memorial Institute (RPMI) 1640 (Bio-chrome AG, Berlin, Germany) supplemented with 1.5% human serum AB (Institute für Transfusionmedizin Suhl, Suhl, Germany or PAN-Biotech GmbH, Aidenbach, Germany), GM-CSF (Leukine; Bayer HealthCare, Berlin, Germany), and IL-4 (R&D system, Minneapolis, USA). Forty-eight to 72 hrs later, a maturation cocktail consisting of GM-CSF (Leukine), IL-4 (R&D system), IL-1β (R&D system), TNFα (R&D system), interferon γ (IFN-γ [Imukin]; Böhringer Ingelheim, Vienna, Austria), Resiquimod848 (R848; 3M Pharmaceuticals, St. Paul, Minnesota, USA), and PgE2 was added. Munich fast DCs were transfected with hTERT or Survivin mRNA by electroporation and incubated for 4 hrs without cytokines before freezing at a density of 2.5 or 5x106 cells/vaccine ampule. Vaccine production and quality control were performed according to the manufacturing licence of the Department of Cell Therapy at Oslo University Hospital.
2.5 Patient characteristics and treatment prior to DC vaccination
Following approval under hospital exemption by the Norwegian Medicines Agency and signed informed consent four patients were included in the study. At diagnosis, all patients were defined as high-risk with large tumours (T3), high Gleason scores, as well as presence of lymph node (LN) metastases (N1; n=3). The age of the patients ranged from 51 to 61 years (Table 1). As primary therapy (PT), three patients (Patients 2, 3 and 4) were treated with ADT in combination with definitive radiotherapy (RT). Patient 2 received RT targeting the prostate gland, whereas the remaining patients had RT involving the pelvic LNs in addition. Patient 1 was treated with radical prostatectomy (RP) and extended pelvic LN dissection. All patients relapsed with local and distant lymph node metastasis after primary treatment (Table 1).
|
Patient |
1 |
2 |
3 |
4 |
|
|
At diagnosis |
T-stage |
pT3b |
cT3a |
cT3a |
cT3a |
|
N-stage |
pN1 |
Nx |
N1 |
pN1 |
|
|
M-stage |
M0 |
M0 |
M0 |
M0 |
|
|
PSA, μg/L |
8.5 |
8.8 |
97 |
24.7 |
|
|
Gleason score |
7b |
8 |
7b |
8 |
|
|
Age |
61 |
58 |
51 |
57 |
|
|
Primary treatment |
ADT |
0 |
1 |
1 |
1 |
|
RP |
1* |
0 |
0 |
0 |
|
|
Gland extirpation |
1 |
0 |
0 |
0 |
|
|
RT prostate |
0 |
1 |
1 |
1 |
|
|
RT pelvic area |
0 |
0 |
1 |
1 |
|
|
Relapse site |
Prostate |
0 |
1 |
0 |
1 |
|
Local recurrence |
1 |
0 |
0 |
0 |
|
|
Lymph nodes |
1 |
1 |
1 |
1 |
T-stage: primary tumour, p: stage given by histopathological examination of a surgical specimen, c: stage given by clinical examination, N-stage: regional lymph nodes, x: N-stage unknown, M-stage: distant metastasis, PSA: prostate specific antigen, ADT: androgen deprivation therapy, RP: radical prostatectomy, RT: radiotherapy. * Positive margins
Table 1: Tumour characteristics at diagnosis. Primary treatment and relapse site prior to dendritic cell vaccination.
Following biochemical relapse 12 months after PT, Patient 1 was treated with LN resection followed by radiotherapy and ADT. Twelve months thereafter, he had a clinical progression before start of DC vaccination (Table 2, Supplementary Figure S1a) that lasted 17 months. Patient 2 developed a biochemical relapse 37 months after PT, which was followed 3 months later with a local relapse (Table 2). He was treated with salvage RP and extended LN dissection. Since five of eight resected LN showed metastatic spread and PSA levels increased rapidly immediately after surgery, the patient was started on ADT combined with Oslo fast DC vaccination for 74 months, followed thereafter with Munich fast DCs for 38 months (Supplementary Figure S1b).
Twenty-six months after primary treatment, Patient 3 developed a biochemical relapse, which was followed 18 months later by a clinical relapse in paraaortic LNs (Table 2). Immediately thereafter the patient started with ADT and DC vaccination, initially using Oslo fast DCs for 38 months, followed by Oslo fast DCs + IDO siRNA for 28 months and finally using Munich fast DCs for 29 months (Supplementary Figure S1c). In addition to RT, Patient 4 was given ADT and later cyclophosphamide which was discontinued due to side effects. After a 26-month treatment-free interval, he had a biochemical relapse and 10 months later progressed with LN metastases (Table 2). Munich fast DC vaccines were given in combination with ADT for 72 months (Table 2, Supplementary Figure S1d).
|
Patient |
1 |
2 |
3 |
4 |
|
Time to biochemical relapse (months) |
12 |
37 |
26 |
59 |
|
Time from biochemical relapse to clinical progression (months) |
12 |
3 |
12 |
10 |
|
Treatment with Oslo fast DCs (months) |
17 |
74 |
38 |
na |
|
Treatment with Oslo fast DCs+ IDO siRNA (months) |
na |
na |
29 |
na |
|
Treatment with Munich fast DCs (months) |
na |
38 |
28 |
72 |
|
DTH skin reaction Oslo fast DC* |
- |
+ |
+ |
na |
|
DTH skin reaction Oslo fast DC + IDO siRNA |
na |
na |
+ |
na |
|
DTH skin reaction Munich fast DC |
na |
+++ |
+++ |
+++ |
|
CD4 immune response baseline† |
nd |
+ |
+ |
+/- |
|
CD4 immune response Oslo DCs |
++ |
+++ |
+ |
na |
|
CD4 immune response IDO DCs |
na |
na |
+++ |
na |
|
CD4 Immune response Munich DCs |
na |
+ |
+/- |
+ |
|
CD8 immune response baseline± |
nd |
++ |
++ |
- |
|
CD8 immune response Oslo DCs |
++ |
+ |
+/- |
na |
|
CD8 immune response IDO DCs |
na |
na |
+ |
na |
|
CD8 Immune response Munich DCs |
na |
+ |
+++ |
+ |
|
Remission status during DC vaccination |
PD |
CR |
SD |
CR |
|
Remission duration during DC vaccination (months) |
18 |
113 |
95 |
72 |
|
Overall survival after vaccination |
§ |
CR |
PD |
CR |
*DTH: delayed-type hypersensitivity reaction in relation DC formulation. †: CD4 and ±: CD8 immune responses related to DC formulations given to individual patients. Immune responses and DTH reactions stratified as strong (+++), weak (+) or no response (-). CR: complete remission, SD: stable disease, PD: progressive disease, na: not applicable, nd not determined, §: patient deceased
Table 2: Time to biochemical relapses and time from biochemical relapse to progression with time of treatment with different DC formulations in individual patients.
2.6 Vaccination
Thawed and washed DC vaccines were suspended in 200 μL saline and administered as intradermal injections, irrespective of DC formulation. Aliquots of DCs transfected separately with hTERT and Survivin mRNA were injected at separate sites. Booster vaccines were given at monthly intervals, except for Patient 2 who received monthly boosters for four years, followed by DC boosters every second month. The number of DCs per injection varied from 2.5 to 10x106 cells but was the same for each antigen applied separately for a given patient at a given time. Delayed-type hypersensitivity (DTH) skin tests were performed at week six and thereafter at each boost vaccination. A positive skin reaction was defined as erythema and induration of >5 mm diameter 2 days after injection.
2.7 Quality tests of large-scale production of DCs
All quality control testing of DCs was performed on frozen samples by thawing representative vials from the production batches.
2.7.1 Fluorescence-activated cell sorting analysis: For phenotyping different DC formulations, fluorescence-activated cell sorting (FACS) analysis was performed using the following fluorescence conjugated antibodies for staining: anti-cluster of differentiation (CD)80-phycoerythrin (PE), anti-CD14-allophycocyanid (APC)-cyanine 7 (Cy7) from Becton Dickinson (Franklin Lakes, New Jersey, USA), anti-CD86-APC, anti-CD274-fluorescein isothiocyanate (FITC), anti-chemokine receptor 7 (CCR7)-PE-Cy7 (all from BD Pharmingen, San Diego, USA), anti-CD40-PE, (Beckman Coulter, Brea, USA), anti-human leukocyte antigen (HLA)-DR-PE-Cy7 (BioRad, Kidlington, UK), anti-CD83-APC (BD Pharmingen). 7 aminoactinomycin D (7-AAD, BD Pharmingen) was added to distinguish living from dead cells. After washing, acquisition was performed using FACS Canto (BD Biosciences) and post-acquisition analysis was done with FlowJo software (BD Bioscience). For DC characterization, the gate was set to include all large cells in the FCS/SSC plot. Further gating was done in histograms of the single colours to determine MFI for each antigen.
2.7.2 Signal 3 assay of IL-10 and IL-12 secretion by mature DCs: The signal 3 assay used has been described previously [18-20] as a means to assess cytokine secretion by DCs after stimulation through CD40 by CD40L. In short, DCs were thawed and seeded in 96-well plates at 2x104 DCs/well in the presence of 5×104 L-cells/well. As controls, CD40L-expressing L-cells and medium were seeded alone. After 24 hrs culture supernatant media were transferred into 96-well V-bottom plates and stored at -20°C until further analysis. CD40L-expressing fibroblasts were replaced with soluble CD40L for DC stimulation when it became available from Miltenyi Biotech (Bergisch Gladbach, Germany). For stimulation with soluble CD40L, a final concentration of 25 μg/mL CD40L was used for 2x104 DCs. Control wells contained medium plus CD40L. The plates were incubated for 24 hrs before culture supernatant media were transferred to a V-bottom 96-well plate and stored at -20°C for further analysis. To test for the presence of IL-10 and IL-12 in culture media, ELISA MAX deluxe sets specific for human IL-10 and human IL-12 (p70) were used from BioLegend (San Diego, USA). The assay was performed according to the manufacturer’s directions, with minor adjustments. The absorbance was measured at 450 nm and 570 nm using a Victor plate reader (PerkinElmer Waltham, Massachusetts, USA). To correct for the absorbance of the plate itself, the absorbance at 570 nm was subtracted from the absorbance at 450 nm. A standard curve was used to calculate the levels of IL-10 and IL-12 secreted by the DCs into the supernatant medium.
2.8 T-cell assays
Frozen peripheral blood mononuclear cells (PBMCs) from different time points were thawed and resuspended in TexMACS medium (Miltenyi Biotec) at a density of 1x107 cells/mL. Thereafter, 1 μL of 10 μg/μL stock of Nivolumab (Bristol-Myers Squibb, New York, USA) was added and mixed for 30 min at room temperature using a lab-mixing device. After blocking, the PBMCs were washed twice with 10-15 mL TexMACS medium, centrifuged (400g, 5 min, acceleration 9/break 7), and resuspended at a concentration of 2x106 cells/mL. PBMCs were plated in 24- or 6-well plates 10 IU/mL IL-2 and 6 µL/mL (stock 30 nmol/mL) of hTERT or Survivin peptides (PepTivator; Miltenyi Biotech) were added and incubated at 37°C in 5% CO2. After 3 or 4 days of incubation, 0.4 mL of fresh medium with 10 IU/mL IL-2 was added per millilitre of old medium and the plates cultured further. On day 7, cells were harvested for restimulation and the cell concentration adjusted to 1x107 cells/mL in TexMACS medium. The cell suspension (100 μL) was seeded in flat-bottom 96-well plates (Costar, Washington DC, USA). hTERT or Survivin peptides (2 µL of a stock 30 nmol/mL stock solution) were added to the wells. The plates were incubated overnight at 37°C in 5% CO2. To test for the presence of IFNγ-producing CD4+ and CD8+ T cells, the IFNγ Rapid Cytokine Inspector kit with IFNγ-PE (Miltenyi Biotech) was used as recommended by the manufacturer, with minor adjustments regarding length of stimulation. Stimulation was performed overnight before adding Brefeldin A.
Flow cytometric data were acquired by FACS Canto and analysed using FlowJo software.
3. Results
3.1 Characterization of DCs transfected with mRNA encoding full-length hTERT and Survivin antigens
FACS analysis confirmed that all vaccine formulations consisted of mature DCs. As expected, differentiation of monocytes into mature DCs led to down-regulation of CD14 expression, which is characteristic of monocytes, and up-regulation of the known DC maturation markers CD80, CD83, and CD86, as well as CD274, CCR7, CD40, and HLA-DR (Figure 1). The two formulations of Oslo fast DCs produced intermediate or high levels of IL-10 but no IL-12, whereas Munich fast DCs produced high levels of IL-12 and lower levels of IL-10 (Figures 2a and 2b). When migration capacity of DCs towards CCL19 was investigated no differences could be observed among the three DC formulations used in these patients (data not shown).
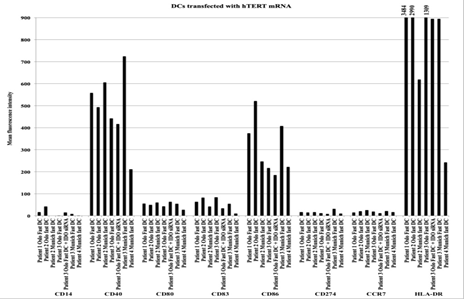
Figure 1: Antigen expression on different DC formulations transfected with hTERT from individual patients. Following thawing of frozen samples DCs were stained with antibodies against the antigens: CD14, CD40, CD80, CD83, CD86, CD274, CCR7 and HLA-DR and analysed by FACS. The results are presented as mean fluorescence intensity of the different antigens tested.
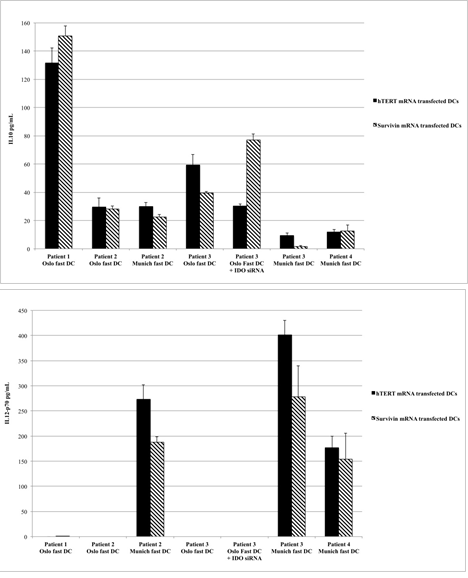
Figure 2: CD40L-induced production of IL-10 (Figure 2a) and IL-12 (Figure 2b) from different DC formulations. Data are presented as mean values and standard deviations from six to eight replicates (stimulation by L-cells) or triplicate measures (stimulation with soluble CD40L).
3.2 Immune responses
To assess immune responses against the two antigens chosen for DC vaccination, patient PBMCs were acquired at different time points throughout treatment and cryopreserved for later assessment. Thawed cells were stimulated with pools of peptides spanning the full-length hTERT and Survivin antigens. Separate aliquots of cells were stimulated with the two different antigens. Intracellular IFNγ was analysed in stimulated CD4+ and CD8+ T-cell subsets, which served as the read-out for specific immune responses. These subsets were differentiated by binding of respective cell surface antibodies for CD4 and CD8. Since the treatment period in Patients 2, 3 and 4 was on-going over several years, investigation of immune responses was done by spot-checks at three to five different time points selected to represent early, middle and late points in the vaccine schedules of the individual patients. In addition, immune response status was investigated before the start of vaccination (baseline) when possible.When PBMCs were stimulated with peptide pools for Survivin, some increases in numbers of CD4+ and CD8+ cells expressing intracellular IFN-γ were observed throughout vaccination, but the values were generally low and further follow-up assays for response to this antigen were not continued (data not shown). The remaining assessments of immune response were thereby limited to responses stimulated by the peptide pools of hTERT antigen. CD4+ responses were much stronger than CD8+ responses. The classification as high, intermediate, and low responses were adapted for each T-cell subset.
Patient 1 had an intermediate level of IFNγ production in CD4+ T cells at time point 1 (TP 1) which was lower but still detectable at later time points. CD8+ responses were also intermediate at TP 1 and persistent during the treatment phase. Analysis of responses at baseline could not be done for this patient (Figures 3a and 3b).
Patient 2 had positive CD4+ responses at baseline that increased during the first 73 months (TP 2) and decreased at the later time points. CD8+ responses were present at base line and remained detectable from TP 1 to TP 3. CD8+ responses were not detectable at the very late time points of TP 4 (106 months) and TP 5 (113 months) (Figures 3a and 3b).
Patient 3 had a low CD4+ response at baseline that persisted to TP 2 (52 months), strongly increased at TP 3 (month 65) and was very low at TP 4 (month 70) and TP 5 (month 83). In contrast, CD8+ responses were very high at baseline, almost vanished at TP 1 and TP 2 and strongly increased again from TP 3 to TP 5 (Figures 3a and 3b).
Patient 4 had very low CD4+ T-cell responses at baseline, which increased during vaccination and intermittent ADT. CD8+ responses could not be detected at baseline, but increased during vaccination (Figures 3a and 3b).
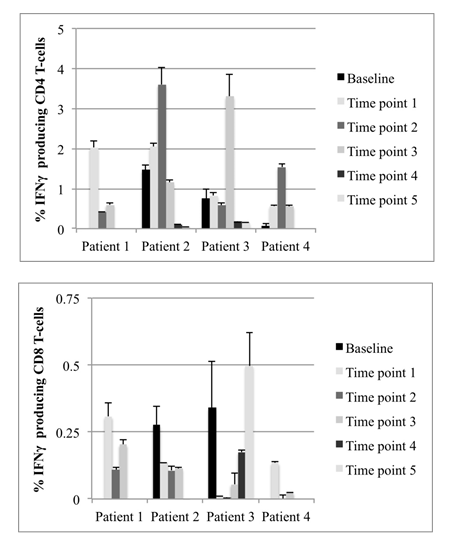
Figure 3: Percent of IFNγ-producing CD4+ (Figure 3a) and CD8+ (Figure 3b) T-cells after stimulation of PBMCs with Peptivator hTERT peptide pool for 6-7 days. Data show results from individual patient samples acquired at different times during DC vaccination. Due to a lack of available peripheral blood samples, baseline was not monitored for Patient 1. For all other time points, the data are presented as mean values and standard deviations of duplicates for baseline patient 3 and at least triplicate measures, depending on sample size for all other time points.
3.3 Clinical outcome during DC vaccination
Patient 1 had aggressive progressive disease on ADT before the initiation of vaccination with Oslo fast DCs. Twelve months after DC vaccination, his PSA levels increased and the patient developed bone metastases; additional RT and chemotherapy combined with DC vaccination had no clinical effect. The overall survival time for this patient from start of DC vaccination was 18 months (Table 2, Supplementary Figure S1a).
Patient 2 started with ADT combined with Oslo fast DC vaccination since he was diagnosed with a minimal residual disease after surgery. After 74 months, vaccination with Oslo fast DCs was changed to use of Munich fast DCs. During the subsequent vaccination period, the patient continued in complete clinical remission but with a recent modest increase in PSA. The observation time since start of vaccination is 113 months (Table 2, Supplementary Figure S1b).
Patient 3 had increasing PSA values on ADT when additional treatment with Oslo fast DCs was added. Initially this combination stabilized the PSA level followed by a slow increase of PSA. Low dose cyclophosphamide was added to the vaccines and ADT. When additional RT against paraaortic LN was given with this combination, an initial drop in PSA occurred but was then followed by an increase in PSA. Based on these observations, low dose cyclophosphamide was stopped and Oslo fast DCs were changed to Oslo fast DCs + IDO siRNA and used for 29 months in combination with ADH. PSA levels continued to show modest increases during this treatment. In an attempt to further enhance vaccine efficacy, Munich fast DCs were exchanged for Oslo fast DCs + IDO siRNA and continued with ADT for a further 28 months (Table 2, Supplementary Figure S1c). The patient maintained a clinical stable disease and, in an attempt to increase the effect of Munich fast DCs and ADT it was decided to give additional RT against persistent abdominal LNs 18 months after start of Munich fast DCs. Thereafter, the patient had stable clinical disease under continuing vaccination until he developed bone metastasis 108 months after start of vaccination.
Patient 4 was given Munich fast DC vaccines in combination with intermittent ADT. Following this therapy, he went into complete biochemical and clinical remission with an observation time of 72 months from start of DC vaccination (Table 2, Supplementary Figure S1d).
4. Discussion
In the setting of hospital exemption, an exploratory group of four patients with minimal disease or progressing prostate cancer was treated with fast DC vaccines. The fast DC vaccines were applied monthly over extended periods of time and were used in combination with ADT in all cases. RT and chemotherapy were included as needed in some patients. The observation that two patients (Patient 2 and 4) showed no BCR and remained in complete clinical remission over observation times of 113 and 72 months, respectively, indicates that persistent DC vaccination may have contributed to prevention of disease progression. A third patient (Patient 3) achieved clinically stable disease with an observation time of 95 months from start of vaccination. Important to note is that all three patients received the personalized tailored DC vaccines at secondary progression. These results indicate that combined treatment of DC vaccines with ADT may prolong time to clinical recurrence in high-risk relapsed PC patients. Patient 1 who was diagnosed with bone metastases immediately after start of DC vaccination had modest or no clinical effect even when the DC vaccines were combined with ADT, RT, and chemotherapy. This is in line with previous studies showing that patients with high tumour burden have little or no benefit from DC vaccines when combined with second-line treatments [14,21].
Immune monitoring of T cell responses in these patients confirmed that DC vaccination supported development or maintenance of specific CD4+ and CD8+ immune responses, potentially related to positive clinical effects, although clear conclusions regarding Patient 1 cannot be made due to missing background values. The evidence of immune responses of T cells to stimulation with hTERT peptide pools in vitro was further substantiated by detection of DTH responses as evidence of T-cell activity in vivo (Table 2), already appearing at the first assessment at week six and continuing throughout the vaccine treatment periods for Patients 2, 3 and 4. In contrast, Patient 1 who showed no clinical benefit from DC vaccination, failed to demonstrate DTH responses at multiple times of challenge. CD4+ and CD8+ responses were detected in vitro in this patient but seemed to have no clinical effect. He was the only patient whose DCs showed high IL-10 production upon DC-stimulated T-cell interactions in vitro. This might have resulted in a predominant activation of regulatory T cells in vivo.
Sipuleucel-t, is the first immunotherapy approved for PC and targets the prostate tumour-associated antigen, prostatic acid phosphatase (PAP), fused with GM-CSF. In a phase III study, Sipuleucel-t resulted in moderate improvement in overall survival compared to placebo (25.8 months versus 21.7 months) [22]. This immunotherapy consisted of three intravenous infusions of apheresis products containing some autologous DCs, spanning a period of eight weeks. In previous studies of highly enriched populations of DCs as generated according to our formulations and applied by an intradermal route, clinical effects were apparent only after 3-6 months of vaccination, and monthly booster vaccination was required [10,14]. As the four PC patients studied here had progressive disease before start of DC therapy and scientific data is lacking on how long vaccine boosting is desirable for patients with persisting residual disease, we elected to continue with DC boosters and combination therapy until tumour progression. Therefore, this approach is substantially different from the phase III Sipuleucel-t study. In addition, we applied highly enriched mature DCs directly transfected with the selected target antigens, hTERT and Survivin, whereby protein expression was strictly controlled in the DCs following electroporation of ivt-RNA encoding the target molecules. Thereby, our approach represents the direct impact of DCs themselves in the immunotherapy designed for these patients. Vaccination was also combined with ADT in these case examples. Recently, Gulley et al. [23] reported the clinical results of a phase III trial, PROSTVAC, in metastatic PC using a viral vector-based immunotherapy targeting PSA. ADT was stopped 6 weeks before start of vaccination. The patients were given seven subcutaneous vaccinations over a period of 21 weeks. The PROSTVAC study was safe but without benefit in overall survival. While the viral vectors applied as vaccines would be expected to reach some DCs enabling them to present antigen to T-cells in vivo, the efficiency of this process and the types of DCs that present antigen in the end cannot be controlled, leaving open any conclusions about the role of DCs in this approach.
The fast DC vaccines used in these four patients were generated with some differences in production protocols but they all shared the properties of being fully mature in phenotype, expressing high levels of co-stimulatory molecules and demonstrating good migration capacities. Furthermore, full-length antigen introduced by electroporation of ivt-RNA led to detectable protein expression in the majority of DCs. The two selected antigens, hTERT and Survivin, were found to be expressed by PC cells and thereby deemed to be suitable target antigens for specific T-cell responses against PC. The maturation cocktails used to develop the Oslo and Munich DC vaccines led to differences in IL-10 and IL-12 cytokine secretion patterns, with the Munich fast DCs having the capacity to produce bioactive IL-12p70 [18]. However, an impact of this difference was not clear with respect to development of CD4+ T-cell responses. Importantly, CD4+ immune responses were detected in patients over time and these occurred in parallel with positive clinical benefits, although a direct correlation cannot be drawn given that the patients received additional treatments, including ADT and RT.
In this study, we used as targets for immune responses the universal tumour antigens hTERT and Survivin. hTERT is broadly overexpressed in different types of tumours and has a 10-fold higher expression in tumour cells compared with normal cells [24-26]. The safety profile of this antigen was demonstrated by the fact that patients here were given booster vaccinations for more than 100 months without any signs of toxicity.
Survivin is widely expressed in many cancers and has been shown to be present in fetal cells, but not in terminally differentiated adult cells, except for cells in the thymus, placenta and CD34+ stem cells [26]. In addition to its high expression on PC cells, Survivin plays a role in development of resistance to anti-androgen treatment [27]. Recently, Survivin was also reported to be involved in the development of radio-resistance [28]. As both castration and radio-resistance are major factors causing treatment failure in PC, DCs transfected with Survivin mRNA were included in this study. Like hTERT, DC vaccines with Survivin mRNA were used over extended time periods with no toxicity.
All patients in this study continued with ADT during DC vaccination. In a recent study using hTERT peptide vaccines in men with metastatic hormone-naive PC, all patients were treated with concomitant ADT during vaccination: 17 of 21 patients had a good clinical response [29]. Recent data suggest that ADT may indirectly lead to priming of tumour-specific adaptive immune responses [30]. In line with this observation, Morse et al. [31] showed that ADT promotes adaptive anti-tumour T- and B-cell responses, but Th1 and Th17 effector memory subsets were reduced after 2 years of therapy. Continuing DC vaccination with boosters, as we have done here, may stimulate the effector memory cells and prevent this from occurring. ADT-induced anti-tumour T-cell responses were accompanied by a concomitant increase in CD4+CD25+FoxP3+ T-regulatory cells [32]. Similar mechanisms can apply when DC vaccines are used [33]. As high numbers of T-regulatory cells act as negative immune regulators, we added low dose cyclophosphamide in one patient progressing during DC vaccination and ADT [34]. The patient was given additional RT against LN while on low dose cyclophosphamide. This treatment combination led to an initial decrease in PSA which thereafter was followed by a PSA increase while still on low dose cyclophosphamide.
Both ADT and RT have synergistic immune modulatory properties [35,36]. The three long-term responders in this study had ADT and RT as primary treatments, and one of them (Patient 3) had additional RT before and after start of vaccination. The limited number of cases prevents conclusions being drawn as to whether ADT and RT combined with DC vaccination contributed to response, but this merits further assessment.
5. Conclusion
In this personalized medicine approach, three high-risk patients with PC progressing after primary treatment and secondary multimodal therapy achieved durable clinical responses using ADT combined with long-term continuing DC vaccination. These patients had low tumour burdens before vaccination, whereas one patient with a high tumour burden developing bone metastases shortly after start of vaccination showed little or no clinical benefit from vaccination, ADT, RT and chemotherapy. The lack of toxicity seen in responding patients suggests that this DC vaccination approach combined with ADT could be offered to patients at high-risk of recurrence before they develop castration-resistant disease.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68 (2018): 394-424.
- van den Bergh RCN, Briers E, Bourke L, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer. Europena Association of Urology (2018): 1-145.
- Chandrasekar T, Yang JC, Gao AC, et al. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol 4 (2015): 365-380.
- Saraon P, Drabovich AP, Jarvi KA, et al. Mechanisms of Androgen-Independent Prostate Cancer. EJIFCC 25 (2014): 42-54.
- Jaworska D, Król W, Szliszka E. Prostate Cancer Stem Cells: Research Advances. IJMS 16 (2015): 27433-27449.
- Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 373 (2015): 737-746.
- Kyriakopoulos CE, Chen Y-H, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 36 (2018): 1080-1087.
- Evans CP, Higano CS, Keane T, et al. The PREVAIL Study: Primary Outcomes by Site and Extent of Baseline Disease for Enzalutamide-treated Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer. European Urology 70 (2016): 675-683.
- SIOUD M, Sæbøe-Larssen S, HETLAND TE, et al. Silencing of indoleamine 2,3-dioxygenase enhances dendritic cell immunogenicity and antitumour immunity in cancer patients. Int J Oncol 43 (2013): 280-288.
- Suso EMI, Dueland S, Rasmussen A-M, et al. hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol Immunother 60 (2011): 809-818.
- Kyte JA, Kvalheim G, Aamdal S, et al. Preclinical full-scale evaluation of dendritic cells transfected with autologous tumor-mRNA for melanoma vaccination. Cancer Gene Ther 12 (2005): 579-591.
- Sæbøe-Larssen S, Fossberg E, Gaudernack G. mRNA-based electrotransfection of human dendritic cells and induction of cytotoxic T lymphocyte responses against the telomerase catalytic subunit (hTERT). Journal of Immunological Methods 259 (2002): 191-203.
- Vik-Mo EO, Nyakas M, Mikkelsen BV, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 62 (2013): 1499-1509.
- Mu LJ, Kyte JA, Kvalheim G, et al. Immunotherapy with allotumour mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. Br J Cancer 93 (2005): 749-756.
- Zhang M, Coen JJ, Suzuki Y, et al. Survivin Is a Potential Mediator of Prostate Cancer Metastasis. International Journal of Radiation Oncology Biology Physics 78 (2010): 1095-1103.
- Jarnjak-Jankovic S, Hammerstad H, Sæbøe-Larssen S, et al. A full scale comparative study of methods for generation of functional Dendritic cells for use as cancer vaccines. BMC Cancer 2007 7: 575-9.
- Subklewe M, Geiger C, Lichtenegger FS, et al. New generation dendritic cell vaccine for immunotherapy of acute myeloid leukemia. Cancer Immunol Immunother 63 (2014): 1093-1103.
- Zobywalski A, Javorovic M, Frankenberger B, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med 5 (2007): 18.
- Wieckowski E, Chatta GS, Mailliard RM, et al. Type-1 polarized dendritic cells loaded with apoptotic prostate cancer cells are potent inducers of CD8+ T cells against prostate cancer cells and defined prostate cancer-specific epitopes. Prostate 71 (2010): 125-133.
- Kalinski P, Vieira P, Schuitemaker JHN, et al. Generation of Human Type 1- and Type 2-Polarized Dendritic Cells from Peripheral Blood. In: Methods in Molecular Biology. Humana Press: New Jersey (2003): 427-436.
- Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol 18 (2011): e150-e157.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363 (2010): 411-422.
- Gulley JL, Borre M, Vogelzang NJ, et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. Journal of Clinical Oncology 37 (2019): 1051-1061.
- Vonderheide RH, Hahn WC, Schultze JL, et al. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity 10 (1999): 673-679.
- Glybochko PV, Zezerov EG, Glukhov AI, et al. Telomerase as a tumor marker in diagnosis of prostatic intraepithelial neoplasia and prostate cancer. Prostate 74 (2014): 1043-1051.
- Lina Matera. The choice of the antigen in the dendritic cell-based vaccine therapy for prostate cancer. Cancer Treatment Reviews 36 (2010): 131-141.
- Zhang M, Latham DE, Delaney MA, et al. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene 24 (2005): 2474-2482.
- Rödel F, Hoffmann J, Distel L, et al. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Research 65 (2005): 4881-4887.
- Lilleby W, Gaudernack G, Brunsvig PF, et al. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol Immunother 66 (2017): 891-901.
- Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Human Immunology 71 (2010): 496-504.
- Morse MD, McNeel DG. T cells localized to the androgen-deprived prostate are TH1 and TH17 biased. Prostate 72 (2011): 1239-1247.
- Brezar V, Godot V, Cheng L, et al. T-Regulatory Cells and Vaccination ‘Pay Attention and Do Not Neglect Them’: Lessons from HIV and Cancer Vaccine Trials. Vaccines 4 (2016): 30-13.
- Liu Y-H, Yeh I-J, Lai M-D, et al. Cancer Immunotherapy: Silencing Intracellular Negative Immune Regulators of Dendritic Cells. Cancers 11 (2019): 108-12.
- Noordam L, Kaijen MEH, Bezemer K, et al. Low-dose cyclophosphamide depletes circulating naïve and activated regulatory T cells in malignant pleural mesothelioma patients synergistically treated with dendritic cell-based immunotherapy. oncoimmunology 7 (2018): 1-10.
- Kalina J, Neilson D, Comber A, et al. Immune Modulation by Androgen Deprivation and Radiation Therapy: Implications for Prostate Cancer Immunotherapy. Cancers 9 (2017): 13-25.
- Nesslinger NJ, Sahota RA, Stone B, et al. Standard Treatments Induce Antigen-Specific Immune Responses in Prostate Cancer. Clinical Cancer Research 13 (2007): 1493-1502.
