Immunization with Formaldehyde Inactivated Whole Cell Vaccine against Multidrug Resistant Pseudomonas aeruginosa Produces Protective IgG Antibodies in Mice Model
Article Information
Modina Ansary Nabonee*, Asma Rahman, Nafisa Jabin Mishu, Nigha Zannat Dola, and SM Shamsuzzaman
Department of Microbiology, Dhaka Medical College and Hospital, Dhaka-1000, Bangladesh
*Corresponding author: Modina Ansary Nabonee, Department of Microbiology, Dhaka Medical College and Hospital, Dhaka-1000, Bangladesh
Received: 24 September 2023 Accepted: 04 October 2023 Published: 16 October 2023
Citation: Modina Ansary Nabonee, Asma Rahman, Nafisa Jabin Mishu, Nigha Zannat Dola, and SM Shamsuzzaman. Immunization with Formaldehyde Inactivated Whole Cell Vaccine against Multidrug Resistant Pseudomonas aeruginosa Produces Protective IgG Antibodies in Mice Model. Archives of Microbiology and Immunology. 7 (2023): 230-235.
View / Download Pdf Share at FacebookAbstract
The emergence of multidrug resistant (MDR) Pseudomonas aeruginosa (P. aeruginosa) are particularly challenging for clinician. Development of an effective vaccine may overcome the problems associated with treatment failure and antibiotic resistance. This study was designed to determine immune response against MDR P. aeruginosa using formaldehyde inactivated P. aeruginosa in mice model. In this study, twelve Swiss albino mice were used. Intramuscular inoculation was implemented three times at 14 days interval with formaldehyde inactivated MDR P. aeruginosa. The mice were given intraperitoneal challenge with live P. aeruginosa and observed for 14 days. Tail blood was collected to assess antigen binding capacity and bactericidal capacity of the serum antibody of immunized mice by ELISA and serum bactericidal antibody (SBA) assay, respectively. The survival rate was 100% among the mice of immunized group at 14 days of post challenge. In ELISA, OD values of serum immunoglobulin G of pre and post challenge immunized mice were significantly higher (p < .001). The SBA assay of the immunized mice sera revealed 100% bactericidal activity after 3h of incubation with 50% guinea pig complement. It was observed that formaldehyde inactivated MDR P. aeruginosa induced protective antibodies in mice model.
Keywords
ELISA, Immune response, MDR P. aeruginosa, Serum bactericidal antibody assay, Whole cell vaccine
Article Details
1. Introduction
The versatility of Pseudomonas aeruginosa (P. aeruginosa) allows it to flourish in different environmental situations (soil, aquatic environments or plants) and clinical settings (nosocomial infections and medical equipment, such as inhalers, respirators, and vaporizers etc.) [1]. This opportunistic pathogen is one of the most common causes of nosocomial infections in the intensive care unit (ICU), commonly in mechanically ventilated patients [2,3]. It also causes chronic respiratory infections in people with cystic fibrosis (CF), bronchiectasis or chronic obstructive pulmonary disease (COPD), and acute infections in immunocompromised individuals [4]. Infections with MDR P. aeruginosa are associated with high rates of mortality, currently estimated at about 18–61% [5]. Aside from its intrinsic resistance to several antibiotics, its propensity to acquire resistance is responsible for its multidrug resistance profile, rendering the pathogen a therapeutic challenge. Therefore, it is essential to develop an alternative strategy to tackle this highly antibiotic-resistant pathogen (e g vaccine, antibody therapy etc.) [6,7]. The development of novel immunotherapies and vaccines against P. aeruginosa could help to prevent infections caused by this highly antibiotic-resistant microorganism [4]. In order to develop an effective vaccine against P. aeruginosa, detailed knowledge of the host immune responses and the bacterial defense mechanisms is important. Both innate and adaptive immune responses work in synergy to act against P. aeruginosa infection. As P. aeruginosa is an extracellular pathogen, humoral, mucosal or systemic opsonizing immunity is most effective to prevent bacterial colonization and infection. However, T-cell responses can also mediate protective immunity in individuals with P. aeruginosa infections [8].
Numerous experimental vaccine candidates have been evaluated such as LPS, alginate, pili, flagella, whole cell etc. In order to improve the presentation of multiple antigens to the immune system, whole-cell and live-attenuated P. aeruginosa mucosal vaccines were shown to be protective in animal models [4]. As an innovative strategy for development of bacterial vaccine, inactivation method could be simultaneously applied and for which formaldehyde is an effective inactivating agent [9]. The intramuscular (IM) route optimizes the immunogenicity of the vaccine and minimizes adverse reactions at the injection site as because muscles have larger and more numerous blood vessels and absorption rate are usually faster than other routes [10].
However, still no study regarding protective vaccine efficacy for P. aeruginosa has been carried out in Bangladesh. Therefore, this study was designed to evaluate the immune response of whole cell vaccine after intramuscular immunization with formaldehyde inactivated multi drug resistant P. aeruginosa in murine model.
2. Materials and Methods
2.1 Animals
Twelve 6 to 8 weeks old female Swiss albino mice weighing 12-15 gram and two guinea pigs were obtained from ICDDR’B, Breeding house, Dhaka, Bangladesh. All mice were maintained at the animal house of the microbiology department of the affiliated institution providing particular pathogen-free facility. All experimental Protocols and animal care were reviewed and approved in accordance with the regulations of the Ethical Review Committee of Dhaka Medical College.
2.2 Preparation of Bacterial Strains
A mixed solution of MDR P. aeruginosa (in PBS) obtained from different samples were chosen to use as a candidate for formalin inactivated whole-cell vaccine preparation. At first culture of bacteria were done in Blood agar and MacConkey agar media. Then subculture was done in Tryptic soy broth for 24 hours at 370 C and washed twice with sterile phosphate-buffered saline (PBS). After that formalin was added to the suspension (final concentration 3%, v/v) and incubated at 370 C. The inactivated P. aeruginosa then washed twice. Then re-suspended with sterile PBS to achieve a concentration of 3×107 CFU/ml and used for the immunization. Viability of bacteria was checked by observing no growth after overnight incubation at 37° C following streaking on Mueller Hinton agar plate [9, 11].
2.3 Immunization Schedule
The mice were randomly divided into three groups such as Group 1 (experimental group), Group 2 & Group 3 (control group). The Group 1 mice were immunized with formalin-inactivated whole-cell P. aeruginosa on day 0, 14 and 28 intramuscularly. The control group received sterile PBS simultaneously [9,12].
2.4 Challenge
Fourteen days after last immunization, Group 1 and Group 2 mice were challenged intraperitoneally with 3×108 CFU/ml live MDR P. aeruginosa suspended in 500 µl of PBS. All mice were monitored for 14 days post challenge for any clinical features like reluctant to feed, lack of movement, weight loss, etc. [9, 13].
2.5 Collection of Blood for SBA and ELISA
Blood was collected from tail end 10 days after 1st inoculation and then 7 days after each booster. Firstly, the tail end was cut about 2-3 mm close to its blunt tip. Then blood was collected into a micro centrifuge tube containing PBS (1:5 dilution). After 2 hours, the blood was centrifuged at 3,000 g for 10 minutes. Clear sera from the top of the tube were taken into another sterile micro centrifuge tube and used for ELISA and SBA assay [12].
2.6 Antibody Detection by ELISA
Indirect ELISA was performed for detection of IgG antibody from mice sera which is specific for P. aeruginosa antigen. Antigen was separated by sonication of P. aeruginosa [13].
2.6.1 Procedure of Sonication
Briefly, 100 µL of distilled water was mixed with bacterial pellets and set aside on ice for 30 min. Then sonication was done at 20 kHz for 2 × 10 s (subjected to viscidness of the samples) and centrifugation was done for 20 min at 10,000 g. Then, supernatant was kept at -200 C and used as antigen later on. Antigen concentration was optimized at 10µg/ml by checkerboard titration method [13].
2.6.2 Optimized ELISA
Antigen coating was done in a 96 well immunoabsorbent plates with 100 µl/well of antigen (10µg/ml) in sodium bicarbonate buffer (pH=9.6) by overnight incubation at 40 C. The wells were washed with PBS three times. Then the wells were blocked with 5% skimmed milk (w/v) in PBS for 30 min at 370C (200 μl/well). The plate was washed three times with 0.05% PBST (0.05% Tween20 in PBS) and once with PBS. Then sera (100 μl/well) were added at a 100-fold dilution and incubated for 90 min at 370 C followed by whole night at 40 C. Horseradish peroxidase (HRP) conjugated anti-mouse IgG (Thermo Fisher Scientific, USA) in PBST (1:5000) were added (100 μl/well) and incubation was done for 90 min at 37 °C. After washing, tetramethyl benzidine (TMB) and urea peroxide (substrate) were added and kept for 10 min under dark condition. Then stop solution (1M H2SO4) was added. ELISA plate reader (BioTek Inc., USA) was used for measurement of optical density value at 450 nm [9]. Following formula was applied for calculation of cut-off value of OD:
Cut-off value of OD = M (mean) + 2 × Standard deviation
2.7 SBA Assay
2.7.1 Preparation of Bacterial Strains
A MDR P. aeruginosa strain was secluded and cultured in Mueller-Hinton agar media for 18–20 h at 37°C. Then a bacterial suspension was prepared (1 × 108 CFU/ml) and serially diluted in PBS at different concentrations (upto 1 × 103 CFU/ml) [13].
2.7.2 Complement
Sera was collected from guinea pig and used for complement. Following blood collection, centrifugation was done for 10 min at 3000 g. Then serum was transferred to sterilized cryotubes and preserved at −20°C. To determine the effectiveness of functional antibody of mouse serum, complement was diluted with PBS in a various concentration (i.e. 50%, 25%, and 12.5%) and added along with bacterial suspension. Viability of P. aeruginosa was also checked using immunized and unimmunized mice sera and bacterial suspension in absence of complement in a different well of microtiter plate [13].
2.7.3 Optimization of SBA Assay
Serum samples of immunized and unimmunized group of mice were inactivated at 56°C for 30 min and serially diluted in PBS (1:10, 1:20, 1:40, and 1:80). Then 10 µl of each of the diluted serum were taken in a microtiter plate. Pooled guinea pig sera of above mentioned dilutions were placed to each well. Then, 10 µl of bacterial suspension of previously prepared concentrations was mixed with the identical wells. Thus final reaction volume of 50 µl was obtained and incubated for 1 h, 2 h, 3 h, and 4 h at 37° C [14]. From every well, 1 µl of suspension was placed in Mueller-Hinton agar plate followed by incubation at 37°C for overnight. Presence of viable bacteria was checked by detecting their growth. All the measures were done in triplicate [15].
2.7.4 Optimized Serum Bactericidal Antibody Assay
Optimum SBA finding was attained by 30 µl suspension of 50% guinea pig sera, 10 µl serum of immune mice (1:10 & 1:20 dilution) and 10 µl of bacterial inoculum (1.5 × 104 CFU/ml) followed by incubation at 37°C for 3 h. Viable CFU counts were noted. The negative control well was inoculated with bacteria and complement [16, 17].
2.8.1 Data Analysis
The data were documented methodically. All statistical analysis was done by SPSS version 25. Statistical significance between groups was compared by unpaired T test. p- value < .05 was considered as a minimum level of significance.
3. Results
3.1. Bacterial Inactivation by Formaldehyde
Following inactivation, the bacterial suspension was cultured overnight in Mueller Hinton agar plate. No colonies were detected on the plate. Un-inactivated bacteria were used as a control.
3.2 Survival of Mice
After lethal challenge, 100% survival was observed at 14 days post challenge among the experimental group of mice. All mice from Group 2 died within 24 hour of challenge.
3.3 Immune Response against P. aeruginosa
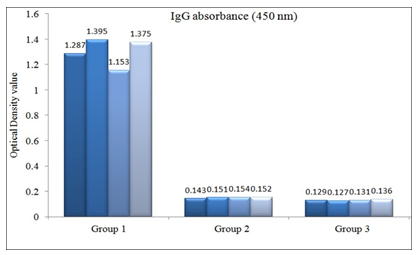
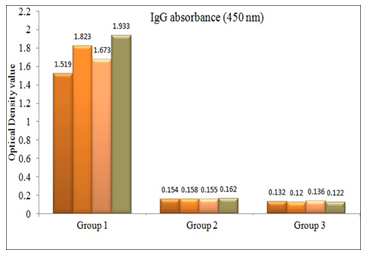
OD value of IgG antibodies in sera of experimental and control group of mice were measured following 10 days after 1st immunization [data not shown] and 7 days after 1st booster [Figure 1] and 2nd booster [Figure 2]. OD value of all the immunized mice sera was above the cutoff value (0.139 and 0.144 respectively). After each immunization, significant statistical difference was observed between the OD values of immunized and control mice sera (p < .001).
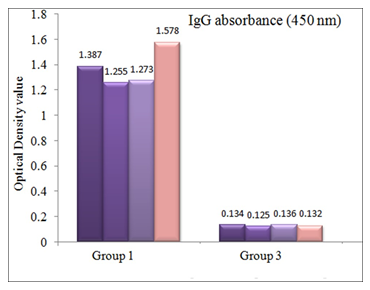
OD value of serum anti-P. aeruginosa IgG antibodies following lethal challenge is shown in Figure 3 which was also above the cutoff value (0.142) and statistically significant (p value <.001).
There were significant differences of serum IgG antibody response of the experimental group of mice within subsequent doses of immunization schedule (Table 1).
Table 1: The optical density value of IgG absorbance (450 nm) within the subsequent booster doses of immunization schedule of experimental group of mice were interpreted by ANOVA: single factor
|
Source of Variation |
SS |
Df |
MS |
F |
p-value |
F crit |
|
Between Groups |
4.113928 |
3 |
1.371309 |
78.8597 |
3.63E-08 |
3.490295 |
|
Within Groups |
0.208671 |
12 |
0.017389 |
|||
|
Total |
4.322599 |
15 |
3.4 SBA Assay
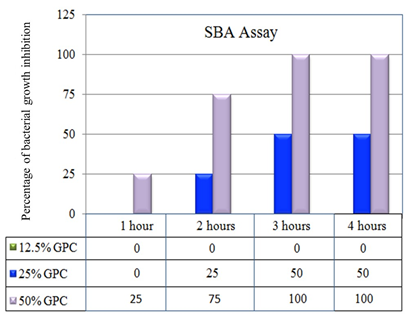
SBA assay of pre challenge immunized mice sera (1:10 dilution) with bacterial inoculum (1.5 × 104 CFU/ml) inhibited bacterial growth using 50% GPC which has been shown [Figure 4]. Serum samples were collected seven days before lethal challenge.
Sera at 1:20 dilution also caused bacterial growth inhibition by using with 50% complement after 3 hours of incubation. SBA assay was also done with post challenge sera which also exerted growth inhibition with 1:10 diluted sera.
4. Discussion
The complexity in the pathogenesis of P. aeruginosa and the emergence of multidrug-resistant strains are the leading cause of the increased susceptibility of infections by this notorious pathogen to the vulnerable groups. Hence the development of a vaccine for this challenging pathogen might be a hopeful alternative to antibiotics [4].In the current study, the survival rate of the immunized group of mice was 100% after 14 days of lethal challenge. A study observed that following intramuscular immunization with PcrVNH (PcrVNH is an artificial PcrV derivative where PcrV is a structural protein of the type 3 secretion system of P. aeruginosa), the percentage survival was 100% to 50% from 36 hours to 7 days of post challenge [18]. No data regarding survival rates of mice after lethal challenge with live MDR P. aeruginosa is available in Bangladesh. Whole cell vaccine was used for enhancing multiple antigen presentation to the immune system resulting a more complete and effective immune response. Thus may ensure broad coverage and clinical efficacy. Formaldehyde inactivation was chosen because integrity of surface antigens is well maintained on the bacteria while inactivated with it [13, 19]. In the present study, immunization with formaldehyde inactivated whole cell vaccine stimulated production of IgG polyclonal antibody against P. aeruginosa after each immunization as well as following lethal challenge which was measured by ELISA. A study conducted in China reported that formaldehyde inactivated bacteria induced both IgG1 and IgG2a immune responses. The same study also reported that the IgG titer was highest at 56th days after first immunization [9]. A different study showed that immunization with heat-inactivated whole cell P. aeruginosa stimulates significant serum IgM, IgG and lung supernatant IgA antibody titers against P. aeruginosa that induced both mucosal as well as systemic humoral immune responses [20]. In this study, the highest OD values of the IgG antibody were noticed following the second booster. This might be due to the fact that memory cells produced more IgG antibody following second booster than first booster. After lethal challenge, IgG antibodies were slightly decreased in mice sera due to removal of the offending pathogen from the body [12].
An assay was performed in this study to determine the serum bactericidal activity of antibody produced against P. aeruginosa in immunized mice. In the present study, immunized mice sera were able to kill MDR P. aeruginosa using 50% guinea pig complement. A study found that SBA for P. aeruginosa revealed bacterial killing by activation of classical pathway of complement indicating presence of anti-pseudomonas functional antibody in human serum [15]. The serum antibodies might be essential for the initiation of the classical pathway of complement system in the early period of infection and confers killing of bacteria. Besides this, antibodies also can cause opsonization of infectious microorganisms followed by stimulating effector functions on neutrophils [21]. A study reported the presence of sufficient IgG2b and IgG3 isotypes against P. aeruginosa in CD4+ T cell deficient whole cell vaccinated mice sera which may be enough to enhance bacterial clearance [20]. The whole-cell vaccine-mediated humoral immune response can play the vital role in immunity for Gram negative bacteria [21].
5. Conclusion
Development of an effective vaccine against this pathogen is a burning issue that can reduce the rate of infection as well as the sufferings of the vulnerable groups and decreases the selective pressure on antibiotics. Although other measures of immunological responses are needed to be evaluated in a larger group of animal model, the present study delivers a hope that formaldehyde inactivated whole cell vaccine against MDR P. aeruginosa induces protective antibodies in Swiss albino mice.
Acknowledgements
Animal house facility and laboratory support were provided by the Department of Microbiology, Dhaka Medical College, Dhaka.
Statement of Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
List of Abbreviations
ELISA, Enzyme Linked Immunosorbent Assay; GPC, Guinea pig complement; MDR, multidrug resistant; OD, optical density; SBA, Serum bactericidal antibody
Statements & Declarations
Funding:
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests:
The authors have no relevant financial or non-financial interests to disclose.
Author Contributions:
Modina Ansary Nabonee was the main contributor of this research. Asma Rahman, Nafisa Jabin Mishu, and Nigha Zannat Dola were the co-investigator during the laboratory works, SM Shamsuzzaman was the supervisor of this research work.
Data Availability:
All the raw data and specific materials are presented in the manuscript.
Ethics Approval:
Ethical clearance was obtained by the Ethical Review Committee (ERC) of Dhaka Medical College.
Consent to Participate:
Not applicable
Consent to Publish:
Not applicable
References
- Cao H, Xia T, Li Y, Xu Z, Bougouffa S, Lo YK, et al, “A multidrug resistant clinical P. aeruginosa isolate in the MLST550 clonal complex: uncoupled quorum sensing modulates the interplay of virulence and resistance”, Antimicrobial Agents and Chemotherapy 22 (2019): AAC-01944.
- Tumbarello M, De PG, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R. et al. “Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients”, Intensive Care Medicine 2013 (39): 682-692.
- Kollef MH, Chastre J, Fagon JY, François B, Niederman MS, Rello J. et al. “Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa”, Critical Care Medicine 2014 (42): 2178-2187.
- Mejías MS, Jurado-Martín I, and McClean S. “Understanding Pseudomonas aeruginosa–host Interactions: The ongoing quest for an efficacious vaccine”, Cells 9 (2020): 2617.
- Ramphal R, Infectious Due to Psudomonas, Burkholderia, and Stenotrophomonas Species. In Harison‘s Prinsiples of Internal Medicine, 20th ed Jameson JL, Kasper DL, Longo DL, Fauci AS, Hauser SL, Loscalzo J, Eds McGraw-Hill Education: New York, NY, USA 1 (2018): 1167-1173
- Kocsis B. “The investigation of colistin resistance mechanism in clinical isolate of Enterobacter asburiae”. in 25th European Cong Clin Microbiol Infect Dis 1007 (2015).
- Juan C, Peña C, Oliver A. “Host and pathogen biomarkers for severe- Pseudomonas aeruginosa infections”. Journal of Infectious Disease 215 (2017): S44-S51.
- Sharma A, Krause A, Worgall S. “Recent developments for Pseudomonas vaccines”. Human Vaccines 7 (2011): 999-1011.
- Fan Y, Mu Y, Lu L, Tian Y, Yuan F, Zhou B, et al. “Hydrogen peroxide- inactivated bacteria induces potent humoral and cellular immune responses and releases nucleic acids”, International Immunopharmacology 69 (2019): 389-397.
- Taylor CR, Lamone P, Lillis C, Lynn P. In: Fundamentals of Nursing Care: The Art and Science of Nursing Care. Walters Kluwer (2011).
- Takahashi K, Hanamura Y, Tokunoh N, Kassai K, Matsunishi M, Watanabe S, et al. “Protective effects of oral immunization with formalin-inactivated whole-cell Citrobacter rodentium on Citrobacter rodentium infection in mice”, Journal of Microbiological Methods 159 (2019): 62-68.
- Kawser Z, Shamsuzzaman SM. “Intradermal immunization with heat-killed Klebsiella pneumoniae Leading to the production of protective immunoglobulin G in BALB/c mice”, International Journal of Applied & Basic Medical Research 11 (2021): 160-165.
- Rahman A, Shamsuzzaman SM, Dola NZ, and Nabonee MA. “Protective Effects of Immunization with Formalin Inactivated Oral Whole Cell Vaccine against Multidrug Resistant Citrobacter freundii in Murine Model”, American Journal of Infectious Diseases and Microbiology 10 (2022): 92-97.
- Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G. et al. “Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines”, Clinical and Vaccine Immunology 21 (2014): 712-721.
- Anandan R, Nandhini D. “Development of serum bactericidal assay for P. aeruginosa”, International Journal of Science and Research 6 (2017): 261-268.
- Lindow JC, Fimlaid KA, Bunn J, Kirkpatrick BD.“Antibodies in action: role of human opsonins in killing salmonella enteric serovar typhi”, Infection and Immunity 79 (2011): 3188-3194.
- Pulickal AS, Gautam S, Clutterbuck EA. et al. “Kinetics of the natural, humoral immune response to Salmonella enterica serovar Typhi in Kathmandu, Nepal”, Clinical and Vaccine Immunology 16 (2009): 1413-1419.
- Wan C, Zhang J, Zhao L, Cheng X, Gao C, Wang, Y, et al. “Rational design of a chimeric derivative of PcrV as a subunit vaccine against Pseudomonas aeruginosa”, Frontiers in Immunology 10 (2019).
- Arshadi N, Mousavi SL, Amani J, and Nazarian, S. “Immunogenic potency of formalin and heat inactivated E. coli O157: H7 in mouse model administered by different routes”, Avicenna Journal of Medical Biotechnology 12 (2020): 194.
- Sen-Kilic E, Blackwood CB, Huckaby AB, Horspool AM. et al. “Defining the mechanistic correlates of protection conferred by whole-cell vaccination against Pseudomonas aeruginosa acute murine pneumonia”, Infection and Immunity 89 (2021).
- Mayadas TN, Tsokos GC, Tsuboi N. “Basic science for clinicians mechanisms of immune complex – mediated neutrophil recruitment and tissue injury”, Circulation 120 (2009): 2012-2024.