Imaging Manifestations of Soft Tissue Lymphoma
Article Information
Uma Debi MD*, Maoulik Kumar MD, Lokesh Singh MD, Sathya Sagar MD, Muniraju Maralakunte MD, Vikas Bhatia MD, Anindita Sinha MD, Gita Devi MD, M S Sandhu MD
Department of Radio diagnosis, PGIMER Chandigarh, India
*Corresponding Author: Uma Debi MD, Department of Radio diagnosis, PGIMER Chandigarh, India
Received: 16 June 2020; Accepted: 17 July 2020; Published: 09 September 2020
Citation: Uma Debi, Maoulik Kumar, Lokesh Singh, Sathya Sagar, Muniraju Maralakunte, Vikas Bhatia, Anindita Sinha, Gita Devi, M S Sandhu. Imaging Manifestations of Soft Tissue Lymphoma. Archives of Clinical and Medical Case Reports 4 (2020): 797-812.
View / Download Pdf Share at FacebookAbstract
Malignant bone lymphomas are a rare disease. According to an initial extent, bone lymphoma can be primary or secondary. Primary lymphoma of bone (PLB) has an overall good prognosis, commonly involves meta-diaphysis of long bones. Secondary osseous lymphoma preferentially involves an axial skeleton. Musculoskeletal lymphoma requires multimodality imaging evaluation by radiograph, CT, MRI, bone scintigraphy, and 18F-FDG PET-CT. Medullary bone lesion without cortical involvement is an important feature of lymphoma and it must be considered as a differential of a permeative bone lesion with soft tissue involvement. Muscular lymphoma does not have characteristic imaging findings. The final diagnosis of musculoskeletal lymphoma requires histopathology analysis.
Keywords
Malignant bone lymphomas; Muscular lymphoma; Imaging
Malignant bone lymphomas articles, Muscular lymphoma articles, Imaging articles
Malignant bone lymphomas articles Malignant bone lymphomas Research articles Malignant bone lymphomas review articles Malignant bone lymphomas PubMed articles Malignant bone lymphomas PubMed Central articles Malignant bone lymphomas 2023 articles Malignant bone lymphomas 2024 articles Malignant bone lymphomas Scopus articles Malignant bone lymphomas impact factor journals Malignant bone lymphomas Scopus journals Malignant bone lymphomas PubMed journals Malignant bone lymphomas medical journals Malignant bone lymphomas free journals Malignant bone lymphomas best journals Malignant bone lymphomas top journals Malignant bone lymphomas free medical journals Malignant bone lymphomas famous journals Malignant bone lymphomas Google Scholar indexed journals lymphomas articles lymphomas Research articles lymphomas review articles lymphomas PubMed articles lymphomas PubMed Central articles lymphomas 2023 articles lymphomas 2024 articles lymphomas Scopus articles lymphomas impact factor journals lymphomas Scopus journals lymphomas PubMed journals lymphomas medical journals lymphomas free journals lymphomas best journals lymphomas top journals lymphomas free medical journals lymphomas famous journals lymphomas Google Scholar indexed journals Muscular lymphoma articles Muscular lymphoma Research articles Muscular lymphoma review articles Muscular lymphoma PubMed articles Muscular lymphoma PubMed Central articles Muscular lymphoma 2023 articles Muscular lymphoma 2024 articles Muscular lymphoma Scopus articles Muscular lymphoma impact factor journals Muscular lymphoma Scopus journals Muscular lymphoma PubMed journals Muscular lymphoma medical journals Muscular lymphoma free journals Muscular lymphoma best journals Muscular lymphoma top journals Muscular lymphoma free medical journals Muscular lymphoma famous journals Muscular lymphoma Google Scholar indexed journals Imaging articles Imaging Research articles Imaging review articles Imaging PubMed articles Imaging PubMed Central articles Imaging 2023 articles Imaging 2024 articles Imaging Scopus articles Imaging impact factor journals Imaging Scopus journals Imaging PubMed journals Imaging medical journals Imaging free journals Imaging best journals Imaging top journals Imaging free medical journals Imaging famous journals Imaging Google Scholar indexed journals PET-CT articles PET-CT Research articles PET-CT review articles PET-CT PubMed articles PET-CT PubMed Central articles PET-CT 2023 articles PET-CT 2024 articles PET-CT Scopus articles PET-CT impact factor journals PET-CT Scopus journals PET-CT PubMed journals PET-CT medical journals PET-CT free journals PET-CT best journals PET-CT top journals PET-CT free medical journals PET-CT famous journals PET-CT Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals radiograph articles radiograph Research articles radiograph review articles radiograph PubMed articles radiograph PubMed Central articles radiograph 2023 articles radiograph 2024 articles radiograph Scopus articles radiograph impact factor journals radiograph Scopus journals radiograph PubMed journals radiograph medical journals radiograph free journals radiograph best journals radiograph top journals radiograph free medical journals radiograph famous journals radiograph Google Scholar indexed journals Electrocardiography articles Electrocardiography Research articles Electrocardiography review articles Electrocardiography PubMed articles Electrocardiography PubMed Central articles Electrocardiography 2023 articles Electrocardiography 2024 articles Electrocardiography Scopus articles Electrocardiography impact factor journals Electrocardiography Scopus journals Electrocardiography PubMed journals Electrocardiography medical journals Electrocardiography free journals Electrocardiography best journals Electrocardiography top journals Electrocardiography free medical journals Electrocardiography famous journals Electrocardiography Google Scholar indexed journals
Article Details
1. Introduction
Musculoskeletal lymphoma is an infrequent but important differential of primary bone/ soft tissue tumor and must be considered as a differential of a permeative bone lesion with soft tissue involvement. In 1928, Oberling suggested that the reticuloendothelial cells of bone are primary tumor cells for primary reticulum cell sarcoma of bone. In 1939, Parker and Jackson presented 17 cases of primary reticulum cell sarcoma of bone and described their clinical, radiographic, and histopathological characteristic. They reported an overall good prognosis and survival rate in these cases. It was included as a separate type of bone tumor by Ewing in 1939 in the Registry of Bone Sarcoma of American College of Surgeons [1-4]. Studies from Mayo clinic demonstrated that though reticulum cells were predominant cells, the majority of these tumors had cells of lymphoid origin and few having Reed Sternberg cells and the term malignant lymphoma of bone was coined in these studies [3, 5-7].
Malignant bone lymphomas are a rare disease [8]. Bone involvement in malignant lymphoma can be primary or there may be secondary involvement of skeleton as part of disseminated lymphoma, differentiation between these two may at times be difficult in clinical practice [9, 10]. Secondary involvement of bone marrow(BM) is reported in 16-20% of lymphoma and is more common than primary lymphoma of bone(PLB). PLB is uncommon constituting approximately 3- 7% of malignant neoplasms of bone, 3% of primary bone malignancy, 3-5% of extra-nodal site of lymphoma, and < 1% of Non-Hodgkin's lymphoma(NHL) cases [11-16]. Secondary lymphomatous bone involvement has a poor survival rate, on the other hand, PLB has good clinical outcome especially if treatment initiated early in the disease course [17-19].
Coley et al. [20] defined criteria for reticulum cell sarcoma of bone: (a) Primary focus in a solitary bone, (b) Positive histological diagnosis from the bony lesion, (c) only regional metastasis on a presentation or metastatic lesion appearing after 6 months of symptom onset of the primary tumor. As per classification by Ostrowski [21] in 1986, malignant bone lymphoma can be classified into 4 groups. Group 1-PLB without disease elsewhere, Group 2- Two or more osseous site involvement. In groups 1 and 2, nodal or soft tissue involvement is absent at presentation or for a minimum of 6 months. Group 3- Bone involvement with nodal or/and soft tissue involvement at presentation or within 6 months, Group 4- Secondary bone involvement at least 6 months after diagnosis of malignant lymphoma in nodal and/or soft tissue.
There is controversy in works of literature regarding the definition of primary lymphoma of bone. It has been described that commonly used criteria for defining primary bone lymphoma include:Tumor arising from bone marrow without disease elsewhere, no distant site of disease for at least 6 months after diagnosis of bone lymphoma, Histological confirmation [11]. As per WHO classification (2002, updated in 2006) bone involvement in malignant lymphoma can be subdivided into four categories- (a) those affecting single bone with or without regional lymph nodes, (b) affecting multiple skeletal sites without involvement of lymph node or viscera, (c) present as a primary tumor but reveal visceral or nodal lesions (d) Bone involvement in a known case of lymphoma elsewhere. Primary bone lymphoma comprised of the group a and b. As described in literature it is worth mentioning that 2013 WHO classification does not provide such definitions or criteria [9, 14, 22, 23].
2. Pathological Subtypes
NHL constitutes the majority of cases with the most common subtype being diffuse large B cell lymphoma. Uncommonly peripheral T cell lymphoma, Anaplastic large cell lymphoma, and Hodgkin type may be seen. Also, other subtypes like marginal zone lymphoma, Burkitt lymphoma, primary follicular lymphoma, small B cell lymphocytic lymphoma, precursor B cell lymphoblastic lymphoma, lymphoplasmacytic lymphomas may uncommonly manifest as PLB [17, 24, 25].
3. Risk Factors for Lymphoma
In general, some risk factors for lymphoma (not limited to primary bone lymphoma) include immunosuppressant medications, severe autoimmune conditions, Epstein-Barr virus, chronic infections like H.pylori, and HIV, family history of lymphoma, certain genetic variations eg in HLA system, excess obesity, certain chemicals exposures [26]. Though certain conditions like Paget's disease, Gaucher disease, sarcoidosis, osteochondromas, infections eg osteomyelitis, HIV, and immunosuppression have been reported in association with PLB exact relationship of these association remain undetermined and etiology is still not known [9].
4. Clinical Features
There is a slightly male predominance (M: F- 1.5:1) [8, 21]. It is more common in middle age. In a study by Mankin et al. of 140 patients of bone lymphoma, they found a wide variation in age group affected with a mean age of 45 years [27]. There are no characteristic clinical features [8]. Most commonly patient presents with pain, often there may be swelling or mass involving soft tissue. A patient may also present with pathological fracture, systemic findings (like fever, lethargy, weight loss), or neurologic symptoms [8, 14, 21]. The commonly affected site in PLB is the femur, tibia, humerus, pelvis [14, 27]. Though long bones are more commonly involved sites, flat bones can also be affected. It is usually localized to shaft involving meta-diaphysis [28-30]. Secondary osseous lymphoma, on the contrary, preferentially involves the axial skeleton such as the vertebra, ribs, pelvis, skull including the facial skeleton [11, 18, 31].
5. Imaging Modalities
Imaging modalities commonly used for evaluation of bone lymphoma include radiographs, computed tomography (CT), magnetic resonance imaging (MRI). F-18 FDG PETCT is an important part of imaging workup especially to detect BM involvement and as a workup for systemic lymphoma. Though bone scintigraphy is more sensitive (as compared to conventional radiograph), it is a non-specific imaging modality whereas PETCT is far superior to scintigraphy for detecting skeletal involvement in lymphoma. [32,33].
6. Radiograph
The most common finding in PLB on conventional radiographs is the lytic destruction of bone. In a retrospective study of 237 cases by Mulligan et al, lytic destruction of bone was seen in 70 % cases, 28% had a mixed lytic-blastic pattern, 2% were blastic, and 5% initial radiographs were normal [30]. Lytic lesions in the lymphoma of bone may show a permeative or moth-eaten pattern of bone destruction (Figure 1). As a tumor arises from a medullary portion of the bone, permeative lytic lesion involving medullary bone is the initial radiographic appearance. Medullary bone lesion without obvious cortical involvement is a pointer towards lymphoma, however, cortical destruction is encountered in the advanced course of the disease. Later as tumor extends through cortex and periosteum, soft tissue mass can be seen. A similar appearance may be seen in another round cell tumor especially Ewing sarcoma but the patient of Ewing sarcoma is younger [33-35].
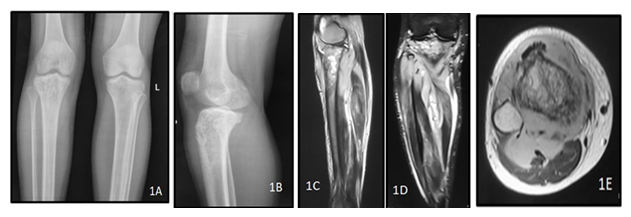
Figure 1: A 40y old male presented with complaints of weight loss with pain in the right knee joint. Plain radiograph of the right knee joint AP (A) and lateral (B) views show permeative lytic lesion of the metadiaphyseal region of the right proximal tibia. MRI of right leg T2 weighted (C,D) and post contrast T1 weighted (E) images show hyperintensity involving the right proximal tibia with associated enhancing extra-osseous soft tissue involving the proximal thigh muscles. The histopathology report came out to be non hodgkin lymphoma of the right tibia.
The purely blastic pattern is rare in PLB when compared with the number of metastatic lymphomas showing this pattern [29, 36]. Similarly mixed lytic sclerotic lesions(Figure 2) are relatively more commonly seen in secondary involvement of bone in lymphoma [29, 36, 37]. Though pure sclerotic or mixed type lesions are more common in Hodgkin disease(HD) of bone than in NHL, lytic lesions are overall more common than sclerotic lesions in HD [38]. Post radiotherapy and chemotherapy, lytic lesions may show changes of sclerosis [36, 37]. A vertebral body showing diffuse sclerosis, or "ivory vertebra", is a radiological sign which can be seen in osseous lymphoma, particularly Hodgkin lymphoma. Other important causes of the ivory vertebra are metastasis, Paget's disease, osteosarcoma, carcinoid. In lymphoma associated paraspinal soft-tissue mass may be seen and there may be vertebral body scalloping which may be due to enlarged lymph node or adjacent soft tissue [39, 40]. Plain radiographs in PLB may be subtle or normal-appearing. Symptomatic patients with normal radiographs should be evaluated further with other imaging modalities like skeletal scintigraphy and especially MRI which are more sensitive for detecting pathologic lesions [36, 41].
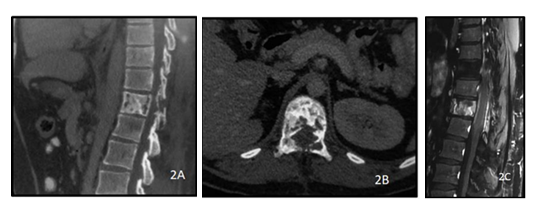
Figure 2: A 28y old male presented with complaints of backache. CT of the dorsolumbar spine sagittal (A) and axial (B) views show lytic sclerotic lesion of the D11 vertebral body including bilateral pedicles. PC T1 weighted sagittal image of the dorsolumbar spine(C) shows heterogeneous post contrast enhancement of the D11 vertebra with ill defined soft tissue extending into the epidural space. The histopathology report came out to be non Hodgkin lymphoma of the spine.
7. Computed Tomography
Findings on CT are similar to radiographic features (Figure 3, 4), but with better visualization and more sensitivity in demonstrating lytic destructive lesions, periosteal new bone formation, extraosseous masses, and bony sequestrum. CT chest and abdomen alone or as part of PET- CT/PET- CECT is done routinely to detect enlarged lymph nodes and lymphomatous infiltration of other extranodal sites which is important for initial staging workup and also for follow up [9, 41, 42].
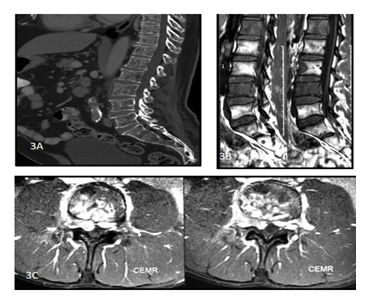
Figure 3: A 45y old male presented with complaints of backache. CT of the dorsolumbar spine sagittal (A) view shows permeative lytic lesions of the dorsolumbar vertebral bodies. T1 weighted sagittal images. (B) and Post contrastT1 weighted axial images(C) of the lumbosacral spine show altered vertebral marrow signal intensity with heterogeneously enhancing soft tissue encasing the spinal cord with extracanalicular extension. The histopathology report came out to be non Hodgkin lymphoma of the spine.
Previously it was considered that periosteal reaction is rare in lymphoma and was considered as one of the important differentiating features from Ewing's sarcoma. Mulligan et al. in a study of 237 cases reported periosteal new bone formation in 58% of cases. Periosteal reactions may be interrupted, multilamellar (onion skin type), or sunburst type. The single-layered solid type of periosteal reaction may also be seen [28, 35, 36]. Aggressive types of periosteal reaction are more common than solid single-layer periosteal reaction [30]. The prominent endosteal reaction can also be seen. Manester described prominent endosteal thickening in the aggressive appearing lesion as the characteristic feature of bone lymphoma. Endosteal thickening may also be seen in Ewing's sarcoma, osteomyelitis, and lytic chondrosarcoma [43]. Less common presentations of PLB include uncommon long bone locations such as in mid diaphysis or cortex, unusual margins including geographic and aneurysmal, and spread across joints to involve contiguous osseous structures [30]. Sequestra though commonly described with chronic osteomyelitis, in a study by Mulligan et al. sequestra were seen in 11% PLB cases [44].
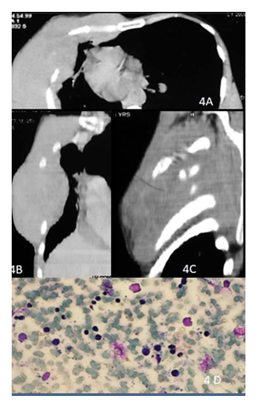
Figure 4: 48y old male presented with right chest wall mass. CECT of the chest axial (A) , coronal (B) and sagittal (C) images show permeative lytic lesions of the ribs on right with large soft tissue component and intrathoracic extension. Smear shows scattered atypical lymphoid cells (2.5-3 x normal lymphocytes) in a hemorrhagic background, have hyperchromatic nuclei and scant cytoplasm consistent with non-Hodgkin’s lymphoma (D).
8. Magnetic Resonance Imaging
8.1 Bone marrow replacement
T1 weighted sequence is very useful for demonstrating marrow changes [42]. BM signal intensity changes are not specific and show variability. On T1WI, signal intensity may be hypointense or isointense [12, 42]., and on T2WI signal intensity are however more variable, and the literature is somewhat contradictory [45]. PLB shows heterogeneous, variable signal changes, and may appear hypo, iso, or hyperintense (relative to signal intensity of fat) (Figure 5) [46].
Hypointense signal intensity may be due to areas of fibrosis in the lesion. Apart from the tumor, perilesional edema and reactive changes in BM can also show T2 hyperintensity [36]. White et al. described that these variable T2-weighted signal characteristics of PLB are not simply due to changes of fibrosis or tumoral neovascularity but are more likely to represent complex pictures of multiple intralesional factors [46]. STIR images also useful in demonstrating abnormal marrow. Post-contrast, enhancing areas may be seen [36]. G Carroll et al. [45] described that T2 heterogeneity was seen in the majority of bone lymphoma. They observed that primary osseous lesions appear as alternating signal intensities on MRI which they described as "mosaic" pattern was seen in 54% of cases.
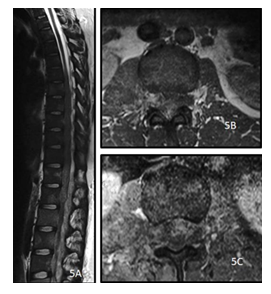
Figure 5: A 28y old female presented with low backache and sensory and motor deficit in both lower limbs. MRI of the dorsolumbar spine sagittal T2 weighted image (A), precontrast axial T1 (B) and post contrast axial T1(C) show diffusely altered vertebral marrow signal intensity with T2 hyperintense, T1 hypointense heterogeneously enhancing soft tissue encasing the spinal cord and cauda equina with extracanalicular extension through the neural foramina and bulky right psoas. The histopathology report came out to be Non Hodgkin Lymphoma.
8.2 Soft-tissue involvement
Soft tissue mass is seen in about 50 % of PLB cases (Figure 6). Limb et al. in their study reported soft tissue mass in about half of their PLB cases, similarly, Mulligan et al. described that out of 237 patients with PLB,48% had soft tissue mass [8, 30]. Usually peri osseous soft tissue masses on MRI show permeative lytic destruction on a conventional radiograph. Intramedullary lesion without obvious cortical disruption and presence of significant surrounding soft tissue mass are important diagnostic clues for bone lymphoma and are characteristic features of round cell tumors like Ewing sarcoma, lymphoma, and multiple myeloma [30, 36, 45]. In general, MRI features are usually not able to differentiate PLB from other small round cell tumors [12]. Soft tissue masses are usually hypointense on T1WI, hyperintense on T2WI, and show predominantly homogenous enhancement(Figure 7) [12]. Manaster suggested that soft tissue mass often aids in diagnosing bone lymphoma. Soft tissue lesion in most cases of bone sarcomas is usually round–oval in shape and it displaces surrounding muscles and neurovascular structures. Whereas extraosseous soft tissue mass in PLB is often disproportionately large, appears infiltrative with multicompartment involvement, encases neurovascular bundle, and does not appear to be pseudoencapsulated [43, 45].
Pretreatment radiographs of PLB showing findings of pathologic fracture, periosteal layering, interrupted or discontinuous periosteal new bone, cortical disruption, soft-tissue mass, soft tissue swelling have a poor prognosis as compared to patients not having these findings. Because they are features indicative of a more aggressive, rapidly growing, infiltrative, and highly destructive tumor compared with lesions that do not have these features. [30,35].
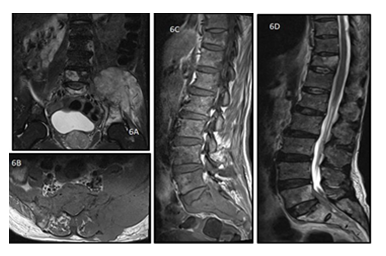
Figure 6: A 50 y old male presented with weight loss and pain in the left hip joint. MRI of pelvis with bilateral hip joints, bilateral coronal T2 weighted (A) and axial T1 weighted (B) images show altered signal intensity involving the left sacrum and iliac bone with associated extra-osseous soft tissue along the left sacroiliac joint. The histopathology report came out to be non hodgkin lymphoma of the skeletal muscle. Sagittal T1 (C) and T2 weighted (D) images of the spine of the same patient shows T1 hypo and T2 hyperintense lesions involving the D11, L5 vertebrae as well as the sacral spine with extra-osseous soft tissue.
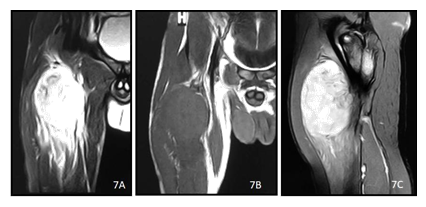
Figure 7: A 21y old male presented with right leg radiculopathy for 15 months. T2 (A)and T1 (B) weighted coronal images and post contrast T1 (C) weighted image show a heterogenously enhancing lobulated soft tissue mass in the anterior aspect of right thigh with surrounding edema.
9. Primary Multifocal Osseous Lymphoma
There is controversy in the literature regarding primary bone lymphoma with multifocal bone involvement. Some authors exclude this entity in their study or description of PLB. Authors supporting this entity usually defines this as lesions in multiple osseous sites without the involvement of distant lymph nodes or viscera for at least 6 months of initial diagnosis [3, 29, 36]. Clinical presentation and imaging features are the same as PLB [29]. Bone scan is useful for demonstrating multifocal skeletal site involvement. Marrow involvement can be assessed by MRI or PETCT. It has been reported that multifocal osseous lymphoma commonly involves the knee region. Abnormalities involving bones around the knee with skull findings support the diagnosis. Conventional radiographs are very useful for treatment response assessment [3, 41]. Overall multiple skeletal site involvements in PLB have a worse prognosis than those with unifocal involvement [29].
10. Muscular Lymphoma
Muscular lymphoma is rare, constituting up to 1.4% of cases of lymphomas. Lymphoma involving muscles can be secondary to disseminated lymphoma, maybe a local extension of bone- adjacent nodal lymphoma, or in a rare case it may be a primary site of involvement [47, 48]. Primary muscular lymphoma (PML) is exceedingly rare, seen in only 8 of 7000 patients with malignant lymphoma in a 10-year study [49]. Muscular lymphoma is commonly due to NHL [48]. PML is seen commonly in the elderly age group usually after the sixties. The most common site of involvement is thigh, arms, and chest [45]. Lymphoma involving muscles has no characteristic imaging findings with varied imaging features including focal mass, focal or diffuse muscle enlargement, and infiltration with latter appearance being more common than former. [42, 50]. Ultrasonography may be utilized as an initial imaging modality to assess accessible sites of the body which reveals hypoechoic mass lesion, coarsened fibro adipose septa, and enlarged muscles fibers [51]. MRI (Figure 8, 9) reveals mass lesion often demonstrating isointense to mildly hyperintense signal on T1W SE (relative to muscle), the intermediate signal between fat and muscle on T2 weighted FSE, a hyperintense signal on STIR and enhancement may be heterogeneous or homogenous. It often shows multicompartment involvement and extension along with the neurovascular bundle. Associated thickening of subcutaneous tissue – skin and marrow abnormality may be seen [47]. CT has a very limited role in evaluation (Figure 10) [42, 52].
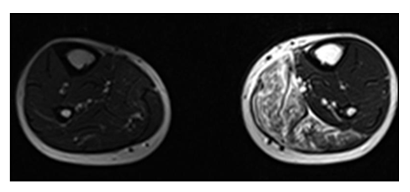
Figure 8: A 38y old male presented with weight loss and pain in the left leg. MRI of bilateral legs axial view shows hyperintensity involving muscles of the medial compartment of the left leg. The histopathology report came out to be non Hodgkin lymphoma of the skeletal muscle.
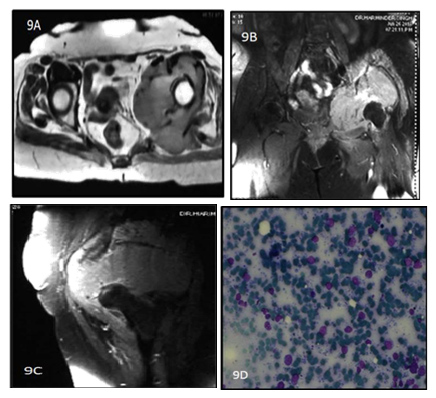
Figure 9: 62y old female presented with left hip pain for 4 months. T1 weighted Precontrast (A)and T1 weighted image postcontrast (B and C)coronal reformat Images show a heterogenously enhancing lobulated soft tissue mass in the anterior aspect of left thigh with Diffuse enhancement.9(D) shows atypical, large lymphocytes with hyperchromatic nuclei and scant cytoplasm.
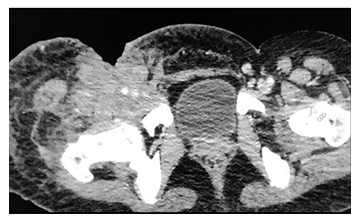
Figure 10: A 38y old male presented with weight loss and pain in the right thigh. CECT of the pelvis axial view shows ill defined hyperdensity in the right thigh involving the skin, subcutaneous tissue and muscles of the anterior compartment with encasement of the right common femoral vessels and associated skin defect. The histopathology report came out to be non Hodgkin lymphoma of the skeletal muscle.
11. Lymphoma in Aids
Immunosuppression is considered a risk factor for NHL with the prevalence of NHL being 60 times more common in patients with AIDS as compared to that in the general population [53]. Disease course of lymphoma in a patient with AIDS is very aggressive with widespread dissemination and involvement of the musculoskeletal system with almost one-third of patients having the involvement of BM, though radiological appearance per se remains the same as seen in a patient without AIDS [42].
12. Staging
Clinical examination, certain blood examination, chest radiograph, CECT of neck, chest, abdomen, MRI of the skeletal lesion, F18-FDG PETCT, histopathological examination of BM, and another organ specific investigation like upper GI endoscopy are part of staging workup [9, 16]. There are four stages in the Ann Arbor staging system. Solitary osseous lesion constitutes stage I. Bone lesion with the involvement of multiple lymph nodes on the ipsilateral side is stage II and on both sides of diaphragm considered stage III. Stage IV includes disseminated lymphoma [11, 27, 54]. As per IELSG staging, bone lymphomas can be divided into 4 stages: stage IE – solitary skeletal lesion, if it is associated with regional lymph nodes involvement it is considered stage IIE. IVE- Multifocal osseous lymphoma. Stage IV- A disseminated form of lymphoma with the involvement of a minimum one skeletal site [9].
As per musculoskeletal tumor society staging system, stage I- low-grade tumor, IA is intraosseous and IB being an extraosseous extension. Stage II- high-grade tumor, IIA comprising intraosseous high-grade tumor, and stage IIB being an extraosseous extension. Local tumor with distant metastases in stage III. Patient with stage IIB has a poor prognosis as compared to those with stage I or IIA [27].
13. Diagnosis and Treatment
Whole-body MRI(WBMRI) and F-18FDG PETCT are useful imaging modality for detecting lymphomatous BM infiltration. These imaging modalities can demonstrate multifocality and help in guiding biopsy. The final diagnosis requires histopathology and immunohistochemistry examination of bone marrow (BM) biopsy specimen. The sensitivity of WB MRI is low for indolent lymphoma and is equal to PETCT for aggressive lymphoma. PETCT has high sensitivity but false positive and false negative findings do occur. There are limited data that suggest lymphomatous infiltration of BM not detected by PET- CT but diagnosed on BM biopsy does not alter staging and subsequent treatment [22, 42, 55-57].
Combined treatment with chemotherapy and radiotherapy (usually chemotherapy followed by radiotherapy) has shown higher clinical remission –response rate and better survival rate. If a patient presents with pathological fracture initial surgical stabilization is done before initiating definitive treatment [54, 58- 61].
14. Response Monitoring
18-F FDG PETCT and MRI are commonly used for imaging assessment of treatment. Post-treatment tumors may show reduced T2 signal intensity and tumoral enhancement. The international working group emphasized the change in tumor size, 18-F FDG uptake, and immunohistochemistry as response criteria and encourages the use of flow cytometry and genetic analysis to be incorporated in clinical trials as a future directive. After initiating treatment, FDG activity is reduced well before the tumor becomes small in size. With the combined advantage of evaluating post-treatment functional and morphological changes, currently, PETCT plays a very important role in assessing treatment response [33, 62-64].
15 Conclusion
The imaging evaluation of musculoskeletal lymphoma requires multimodality imaging including radiography, CT, and MRI. Since it is a heterogeneous disease, its imaging features are often variable. Musculoskeletal lymphoma is an infrequent but important differential of primary bone/ soft tissue tumor and must be considered as a differential of a permeative bone lesion with soft tissue involvement.
References
- Rosendal T. On Reticulum Cell Sarcoma in the Bones. Acta Radiologica 26 (1945): 210-221.
- Potdar GG. Primary Reticulum-Cell Sarcoma of Bone in Western India. Br J Cancer 24 (1970): 48-55.
- Melamed JW, Martinez S, Hoffman CJ. Imaging of primary multifocal osseous lymphoma. Skeletal Radiol 26 (1997): 35-41.
- Schobinger Von Schowingen R. Primary reticulum cell sarcoma of bone. Am J Surg. 93 (1957): 41-9.
- Ivins JC, Dahlin DC. Malignant lymphoma (reticulum cell sarcoma) of bone. Proc Staff Meet Mayo Clin 38 (1963): 375-385.
- Boston HC Jr, Dahlin DC, Ivins JC, Cupps RE. Malignant lymphoma (so-called reticulum cell sarcoma) of bone. Cancer 34 (1974): 1131-1137.
- Aye M, Cabot J, Roslly Z, et al. Primary Lymphoma of Bone: A case report and brief review of literature. Clin Case Rep Rev 3 (2017): 1-3.
- Limb D, Dreghorn C, Murphy J, et al. Primary lymphoma of bone. Int Orthop 18 (1994): 180-3.
- Messina C, Christie D, Zucca E, Gospodarowicz M, Ferreri AJ. Primary and secondary bone lymphomas. Cancer Treat Rev. 41 (2015): 235-246.
- Gianelli U, Patriarca C, Moro A, et al. Lymphomas of the Bone: A Pathological and Clinical Study of 54 Cases. Int J Surg Pathol 10 (2002): 257-266.
- Zhou H-Y, Gao F, Bu B, et al. Primary bone lymphoma: A case report and review of the literature. Oncology Letters 8 (2014): 1551-1556.
- Heyning FH, Kroon HMJA, Hogendoorn PCW, et al. MR imaging characteristics in primary lymphoma of bone with emphasis on non-aggressive appearance. Skeletal Radiol 36 (2007): 937-944.
- Kitsoulis P, Vlychou M, Papoudou-Bai A, et al. Primary Lymphomas of Bone. Anticancer Res 26 (2006): 325-338.
- Demircay E, Hornicek FJ, Mankin HJ, et al. Malignant Lymphoma of Bone: A Review of 119 Patients: Clin Orthop Relat Res 471 (2013): 2684-2690.
- Zinzani PL, Carrillo G, Ascani S, et al. Primarybone lymphoma: experience with 52 patients. Haematologica. 88 (2003): 280-5.
- Maruyama D, Watanabe T, Beppu Y, et al. Primary bone lymphoma: a new and detailed characterization of 28 patients in a single-institution study. Jpn J Clin Oncol. 37 (2007): 216-223.
- Wu H, Bui MM, Leston DG, et al. Clinical characteristics and prognostic factors of bone lymphomas: focus on the clinical significance of multifocal bone involvement by primary bone large B-cell lymphomas. BMC Cancer 14 (2014): 900.
- Braunstein EM, White SJ. Non-Hodgkin Lymphoma of Bone. Radiology 135 (1980): 59-63.
- Hicks DG, Gokan T, O’Keefe RJ, et al. Primary lymphoma of bone: Correlation of magnetic resonance imaging features with cytokine production by tumor cells. Cancer 75 (1995): 973-980.
- Coley BL, Higinbotham NL, Groesbeck HP. Primary reticulum-cell sarcoma of bone: summary of 37 cases. Radiology 55 (1950): 641-658.
- Ostrowski ML, Unni KK, Banks PM, et al. Malignant lymphoma of bone. Cancer 58 (1986): 2646-2655.
- Greenspan A, Beltran J. Orthopedic imaging a practical approach.6th Philadelphia: Lippincott Williams & Wilkins (2015).
- Wang J, Liu J, Fan S, et al. A rare case report of primary bone lymphoma and a brief review of the literature. OncoTargets and Therapy 9 (2016): 4923-4928.
- Jain A, Alam K, Maheshwari V et al. Primary bone lymphomas-Clinical cases and review of literature. J Bone Oncol 2(2013): 132-136.
- Bhagavathi S, Fu K. Primary bone lymphoma. Arch Pathol Lab Med. 133 (2009): 1868-1871.
- American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society (2015).
- Mankin HJ, Hornicek FJ, Harmon DC, et al. Lymphoma of bone: a review of 140 patients. Therapy 3 (2006): 499-507.
- Greenspan A, Jundt G, Remagen W Beltran. Differential diagnosis in Orthopaedic oncology .2nd Philadelphia: Lippincott Williams & Wilkins (2007).
- O’Neill J, Finlay K, Jurriaans E, Friedman L. Radiological Manifestations of Skeletal Lymphoma. Curr Probl Diagn Radiol 38 (2009): 228-236.
- Mulligan ME, McRae GA, Murphey MD. Imaging features of primary lymphoma of bone. AJR Am J Roentgenol 173 (1999): 1691-1697.
- Ruzek KA, Wenger DE. The multiple faces of lymphoma of the musculoskeletal system. Skeletal Radiol 33 (2004): 1-8.
- Moog F, Kotzerke J, Reske SN. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. J Nucl Med. 40 (1999): 1407-1413.
- Keraliya AR, Krajewski KM, Jagannathan JP, et al. Multimodality imaging of osseous involvement In haematological malignancies. Br J Radiol 89 (2016): 980-986.
- Yochum TR, Rowe LJ. Yochum and Rowe’s Essentials Of Skeletal Radiology. 3rd Philadelphia: Lippincott Williams & Wilkins (2005).
- Phillips WC, Kattapuram SV, Doseretz DE, et al. Primary lymphoma of bone: relationship of radiographic appearance and prognosis. Radiology 144 (1982): 285-290.
- Krishnan A, Shirkhoda A, Tehranzadeh J, Armin AR, Irwin R, Les K. Primary Bone Lymphoma: Radiographic-MR Imaging Correlation. RadioGraphics 23 (2003): 1371-1383.
- Navarro SM, Matcuk GR, Patel DB, et al. Musculoskeletal Imaging Findings of Hematologic Malignancies. RadioGraphics 37 (2017): 881-900.
- Ozdemirli M, Mankin HJ, Aisenberg AC, et al. Hodgkin's disease presenting as a solitary bone tumor: A report of four cases and review of the literature. Cancer 77 (1996): 79-88.
- Graham TS. The Ivory Vertebra Sign. Radiology 235 (2005): 614-615.
- Braun RA, Milito CF do RB, Goldman SM, et al. Ivory vertebra: imaging findings in different diagnoses. Radiol Bras 49 (2016): 117-121.
- Suleman FE, Bellew N. Primary bone lymphoma: Imaging findings of a rare primary bone tumour. SA Orthopaedic Journal 10 (2011): 68-70.
- Hwang S. Imaging of Lymphoma of the Musculoskeletal System. Radiol Clin North Am 46 (2008): 379-396.
- Manaster BJ. Primary bone lymphoma: radiographic-MR imaging correlation. Invited commentary. Radiographics 23 (2003): 1384-1386.
- Mulligan ME, Kransdorf MJ. Sequestra in primary lymphoma of bone: prevalence and radiologic features. AJR Am J Roentgenol 160 (1993): 1245-1248.
- Carroll G, Breidahl W, Robbins P. Musculoskeletal lymphoma: MRI of bone or soft tissue presentations. J Med Imaging Radiat Oncol 57 (2013): 663-673.
- White LM, Schweitzer ME, Khalili K, et al. MR imaging of primary lymphoma of bone: variability of T2-weighted signal intensity. AJR Am J Roentgenol 170 (1998): 1243-1247.
- Lee VS, Martinez S, Coleman RE. Primary muscle lymphoma:clinical and imaging findings. Radiology 203 (1997): 237-244.
- Chun CW, Jee W-H, Park HJ, et al. MRI Features of Skeletal Muscle Lymphoma. AJR Am J Roentgenol 195 (2010): 1355-1360.
- Travis WD, Banks PM, Reiman HM. Primary extranodal soft tissue lymphoma of the extremities. Am J Surg Pathol 11 (1987): 359-366.
- Malloy PC, Fishman EK, Magid D. Lymphoma of bone, muscle, and skin: CT findings. AJR Am J Roentgenol. 159 (1992): 805-809.
- Hongsakul K, Laohawiriyakamol T, Kayasut K. A rare case of primary muscular non-Hodgkin’s lymphoma and a review of how imaging can assist in its diagnosis. Singapore Med J 54 (2013): 179-182.
- Lim CY, Ong KO. Imaging of musculoskeletal lymphoma. Cancer Imaging 13 (2013): 448-457.
- Beral V, Peterman T, Berkelman R, et al. AIDS-associated non-Hodgkin lymphoma. Lancet 337 (1991): 805-809.
- Power DG, McVey GP, Korpanty G, et al. Primary bone lymphoma: single institution case series. Ir J Med Sci. 177 (2008): 247-251.
- Johnson SA, Kumar A, Matasar MJ, Schöder H, Rademaker J. Imaging for Staging and Response Assessment in Lymphoma. Radiology 276 (2015): 323-338.
- Adams HJ, Kwee TC, Vermoolen MA, de Keizer B, de Klerk JM, Adam JA, et al. Whole-body MRI for the detection of bone marrow involvement in lymphoma: prospective study in 116 patients and comparison with FDG-PET. Eur Radiol 23 (2013): 2271-2278.
- Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JMH, Nievelstein RA. FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 41 (2014): 565-574.
- Beal K, Allen L, Yahalom J. Primary bone lymphoma: Treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer 106 (2006): 2652-2656.
- Singh T, Satheesh CT, Lakshmaiah KC, et al. Primary bone lymphoma: A report of two cases and review of the literature. J Cancer Res Ther 6 (2010): 296-298.
- Jawad MU, Schneiderbauer MM, Min ES, et al. Primary lymphoma of bone in adult patients. Cancer 116 (2010): 871-879.
- Rahmat K, Wastie M, Abdullah B. Primary bone lymphoma: report of a case with multifocal skeletal involvement. Biomed Imaging Interv J. 3 (2007): 52-54.
- Rahmouni A,Luciani A, Itti E. MRI and PET in monitoring response in lymphoma. Cancer Imaging 5 (2005): 106-112.
- Hutchings M, Barrington SF. PET/CT for Therapy Response Assessment in Lymphoma. J Nucl Med 50 (2009): 21-30.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised Response Criteria for Malignant Lymphoma. J Clin Oncol 25 (2007): 579-586.
