Identification of a Novel Aggressive CD52+ Tumor Cell Subpopulation in Metastatic Prostate Cancer
Article Information
Marina G. Ferrari1, Paari Murugan2, Timothy M. Kuzel3, Eunsil Hahm4, Jindan Yu5, Sergio A. Gradilone6, Adrian P. Mansini*, 1
1Department of Urology, Rush University Medical Center, Rush University, Chicago, IL, USA
2Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, Minnesota.
3Department of Internal Medicine, Division of Hematology, Oncology and Cell Therapy, Rush Medical College, Rush University Medical Center, Chicago, IL, USA
4Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois, USA
5Department of Urology and Department of Human Genetics, Emory University School of Medicine. Winship Cancer Institute of Emory University, Atlanta, GA, USA.
6The Hormel Institute, University of Minnesota, Austin, MN, USA
*Corresponding author: Adrian P. Mansini, Department of Urology, Rush University Medical Center, 1735 W. Harrison St. 60612, Chicago, IL.
Received: 25 September 2023; Accepted: 05 October 2023; Published: 05 December 2023
Citation: Marina G. Ferrari, Paari Murugan, Timothy M. Kuzel, Eunsil Hahm, Jindan Yu, Sergio A. Gradilone, Adrian P. Mansini. Identification of a Novel Aggressive CD52+ Tumor Cell Subpopulation in Metastatic Prostate Cancer. Fortune Journal of Health Sciences. 6 (2023): 494-507.
View / Download Pdf Share at FacebookAbstract
Metastasis is the primary cause of cancer-related deaths in prostate cancer (PCa) patients. The poor prognosis in metastatic PCa (mPCa) is associated with heterogeneous cell populations contributing to the disease’s progression. Unfortunately, there are currently no curative options for patients at this stage. Therefore, identifying subpopulations of PCa cells prone to metastasis is critical for understanding the mechanism of metastasis and identifying novel therapeutic targets. CD52 is expressed in lymphocytes and the male genital tract. The immunological function of CD52 has not been elucidated. Whereas its importance in liquid tumors is well-studied, its relevance in solid tumors remains unknown. This study investigates the relevance of CD52 in PCa. We analyzed clinical data to investigate the relevance of CD52 in the patient’s prognosis. We found that amplification of CD52 and high expression are the most common alterations in patients and are associated with lower disease-free. Flow analysis showed an increased CD52+ subpopulation in human mPCa models DU145 and PC3 cell lines. CD52+ cells show higher metastatic behavior than parental cells. The metastatic potential was associated with the activation of the NF-KB signaling. CD52+ cells release soluble-CD52 in vitro, and validation in patients showed increased serum soluble-CD52 levels in metastasis. We analyzed the immune cell infiltration and showed that CD52 in prostate tumors is associated with poor anti-tumor immune cell infiltration. Additionally, we show that the depletion of CD52 in this subpopulation decreases the metastatic potential. These data suggest therapies targeted at CD52 could be developed for treating aggressive mPCa phenotypes.
Keywords
CD52, prostate cancer, metastasis, tumor microenvironment, immunosuppression
CD52 articles, prostate cancer articles, metastasis articles, tumor microenvironment articles, immunosuppression articles
Article Details
1. Introduction
Approximately 40% of patients diagnosed with PCa will experience a disease recurrence after primary treatment [1, 2]. The presence of aggressive and metastatic cells characterizes the disease's recurrence. These aggressive metastatic cells colonize outside the prostatic region or remain in residual tumors, thus evading the immune system. No current treatment is curative once cancer has reached this stage, and the 5-year overall survival is about 21 months [3]. The poor prognosis in metastatic prostate cancer (mPCa) is associated with heterogeneous cell populations contributing to the disease progression. The progression of metastatic tumors is driven by acquiring genetic and epigenetic traits, providing cells with advantages in proliferation, migration, and survival [4]. The acquisition of these traits generally occurs in a cell that eventually will generate clones and subpopulations of cells that will coexist with the original cells. Indeed, the subpopulations interact and cooperate to enhance metastatic properties with the original tumor cells. Additionally, subpopulations of tumor cells may modify the tumor microenvironment, facilitating the original cells' survival and inducing a suppressive tumor microenvironment evading the anti-tumor immune response. Therefore, identifying aggressive subpopulations prone to metastasize will help to develop more efficacy and personalized therapy.
The tumor microenvironment (TME) is critical in prostate tumor development and metastasis [5]. Prostate tumors are characterized by an immunosuppressive TME associated with nurturing tumor cells that harbor a propensity to become metastatic, suggesting an interplay between molecules regulating metastasis and immunosuppression [6, 7]. During the metastatic process, the formation of new blood vessels and the interaction between the resident cells of the TME is orchestrated by tumor cells. Prostate tumor cells produce VEGF to induce angiogenesis and several cytokines and chemokines contributing to inflammation, immune cell infiltration, and remodeling enzymes such as MMP9, which result in the spread of the tumor. CD52 antigen (CAMPATH-1) is a surface receptor glycoprotein. Although its biological function is unclear, this protein is suggested to regulate T and B cell function [8]. CD52 is abundant in the male reproductive organs, such as the epididymis and seminal vesicles [9, 10]. CD52 is highly expressed on the surface of T-lymphocytes and B-lymphocytes. In contrast, it is scantly found in NK cells, monocytes, and dendritic cells and nearly absent in macrophages, eosinophils, and bone marrow stem cells [9]. The role of CD52 in hematopoietic cancers is well-studied [11, 12]. Indeed, the serum level of CD52 is associated with poor prognosis and disease progression in chronic lymphocytic leukemia [12]. However, its role in solid tumors is unknown.
This study aimed to analyze the significance of CD52 in PCa using clinical data of patients with PCa and cell lines. We investigated the significance of CD52 in PCa by analyzing clinical and genomic data of patients from the TCGA-PRAD cohorts and determined the expression of CD52 in cell-based models representing different phenotypes of human PCa progression. We found that metastatic patients exhibit the highest level of CD52 expression. We also found that metastatic PCa cell lines expressing CD52 are prone to metastasize, and these characteristics were associated with the activation of the ERK and NF-KB signaling pathways. Additionally, we showed the targeting of CD52 in CD52+ cells resulted in a decrease in the metastatic potential. These data suggest therapies targeted at CD52 could be developed for treating aggressive mPCa phenotypes.
2. Materials and Methods
Anti-CD52 antibody was obtained From Novus Biologicals (Centennial, CO). Anti-VEGF and anti-MMP9 antibodies were obtained from Abcam (Cambridge, MA). Anti-phospho-NFκB-p65, NFκB-p65, anti-phospho-ERK, ERK, and GAPDH antibodies were purchased from Cell Signaling (Danvers, MA). Anti-CD52-PE was purchased from Abnova (Taipei, Taiwan). The IgG2b-PE control isotype was purchased from Invitrogen (Carlsbad, CA). Cell culture. RWPE1, NB26, 22Rv1, LNCaP, PC-3 cells, and DU145 cell models were purchased from ATCC and grown under standard cell culture conditions at 37oC and 5% CO2 environment. Conditional media (CM) was prepared and collected; the cells were first grown to confluence in complete culture media. Next, such cells were grown under serum-free media for 48h. The serum-free media was collected at 48h and centrifuged. The supernatant condition media was stored at -20 C until its use. ELISA kit for CD52 was purchased from Lifespan Biosciences (Seattle,WA) and performed following the kit instructions.
2.1 CD52+ cell isolation
Cells were detached using 0.025% trypsin, resuspended in labeling buffer, and labeled with anti-CD52PE-conjugated antibody as per vendor`s protocol (Abnova, Walnut, CA). Then, CD52+ cells were isolated using a cell sorter (BD FACSAria II P07800142 (BSL2). The quantification of isolated cells was performed by using Cell-Quest software. Next, isolated CD52+ cells were expanded, allowed to grow, and maintained as a pure colony. At every passage, the expression of CD52 was confirmed by immunoblotting assay and RT-qPCR.
2.2 Patient survival analysis
The association of CD52 expression analysis to patient survival was determined by analyzing the TCGA-PRAD clinical dataset for PCa patients using the cBioPortal web platform. Prostate-DECIPHER Classifier test: This study profiled RNA from primary prostate tumor specimens from patients treated with radical prostatectomy (RP) at the Department of Urology, University of Minnesota. After exclusion for tissue unavailability and quality control, the study consisted of 228 patients. The tumor material was shared with GENOME-DX for generating DECIPHER-TEST under a collaborative agreement. The genome and RNA-sequencing data were developed at the GENOME-DX facility (Genome DX biosciences, San Diego, CA) and provided to the University of Minnesota investigators for further analysis. Dr. Jinhua Wang performed the bioinformatic analysis at the Institute of Informatics, Masonic Cancer Center of the University of Minnesota.
2.3 Expression of CD52 in PRAD patient cohort
The transcription expression levels of CD52 in PRAD cohort patients was determined by analyzing data from UALCAN database [13].
2.4 Quantitative real-time PCR
qRT-PCR assays were performed in cell lines as previously described [14]. The primer sequences for each gene are provided in Supplementary Table 1. GAPDH was used to normalize the gene expression (N. to GAPDH).
Luciferase reporter assays, immunoblot, IF, MTT, wound healing, chemoinvasion, and transendothelial migration assays were performed as previously published methods [15-17]. For proliferation, transendothelial migration, and chemoinvasion assays, 24h after transfection, the cells were detached using 0.025% trypsin and seed in 24 well-plates, then the assays were performed. For the western blotting assay, the cells were lysed with RIPA buffer (Bio-Rad, Hercules, CA) 30h after transfections. In transendothelial migration assays, CD52+ PCa cells were labeled with a fluorescence-tracker blue dye after 24h of transfection and loaded the dye-labeled cells in free-serum media in the upper insert of the co-culture plate and added complete media (with serum) in the bottom. The transmigration was assessed as previously described [15].
Transfections: CD52 was suppressed using a specific pool of small interfering RNA (siRNA) (Integrated DNA Technology, Coralville, IA) using RNAi MAX per the vendor`s instructions (Thermo Fisher, Waltham, MA).
Immune cell infiltration analysis: Tumor Immune Estimation Resource 2.0 (TIMER2.0) provides compressive analysis of tumor-infiltrating immune cells with data from TCGA-PRAD [17]. We used TIMER2.0 to analyze the association between CD52 expression and types of infiltrating immune cells into the tumor zone. The association analysis was performed using the partial Spearman’s correlation.
Statistical analysis: The statistical analysis and the graphical summaries were generated using GraphPad Prism statistical software (GraphPad Software, San Diego, CA). The student t-test for independent analysis was applied to evaluate differences between the treated and untreated cells or parental and derived cells. All statistical measures were summarized as mean ± SE. A p-value of <0.05 was considered statistically significant. All the experiments were performed at least three times.
3. Results
3.1 Genomic data analysis of prostate cancer patient
We performed a comprehensive analysis of the tumor genome data of PCa patients using cBioPortal, a public web platform hosted by Memorial Sloan Kettering Cancer Center (MSK). We analyzed genomic data of 21 clinical studies comprised of 8,060 patients. We found that 13 studies (n=3,377 patients) out of 21 have patients showing alterations in the CD52 gene and/or in the level of the CD52 mRNA expression in prostatic tumors (Table 1). We identified that amplification in the copy number of the CD52 gene was the most frequent genomic alteration in prostate tumors, while 12 out of 13 studies found that prostate tumors exhibited high expression levels of CD52 mRNA (Table 1). Only one study showed low levels of CD52 mRNA expression as a unique alteration (MCTP, Nature 2012). Notably, the MSK, Cancer 2010 clinical study showed that PCa patients with CD52 gene alterations exhibit a lower disease-free survival than patients with unaltered CD52 gene with a median survival of 27.86 months (Fig. 1A). Complementary, we used the ULCAN platform and the PRAD-TCGA clinical data to study if there is any association between the expression of CD52 in prostate tumors and metastasis in patients. The data obtained from 424 patients with prostate tumors (N0: no regional lymph node metastasis n=345, N1: metastases in 1 to 3 axillary lymph nodes n=79) compared with 52 normal tissues showed significantly high expression ofCD52in tumors with metastasis in axillary lymph nodes (Fig. 1B). All these data show that some groups of patients with PCa exhibit alteration in CD52 expression, which is associated with metastasis and poor disease-free survival.
3.2 Expression of CD52 in prostate cancer patients
We asked if CD52 independently could predict the metastasis occurrence in PCa disease. For this purpose, we employed a Decipher-genomic test of tumors/biopsies of patients who undergo radical prostatectomy (RP) treatment. The prostate Decipher genomic test provides multiple algorithms that can predict the clinical outcomes, including the risk of invasiveness or metastasis in patients at the diagnosis, based on RNA-sequencing/whole transcriptome data of tumor biopsies [18, 19]. The first signs of local metastasis in PCa patients are when tumor cells invade extra-prostatic spaces or prostate adjoining tissues such as seminal vesicles. Seminal vesicle invasion (SVI) is considered the hallmark of disease aggressiveness. Decipher genomic-test classified the patient cohort (n=228) into high-risk, low-risk, and No-risk groups. We observed that a significantly high CD52 transcript level in biopsies/tumors (p=0.0015) predicts the increased risk of SVI in PCa patients (Fig. 1C).
Table1:AlterationsintheCD52geneinclinicalstudies
3.3 CD52 is highly expressed in metastatic prostate cancer cell lines
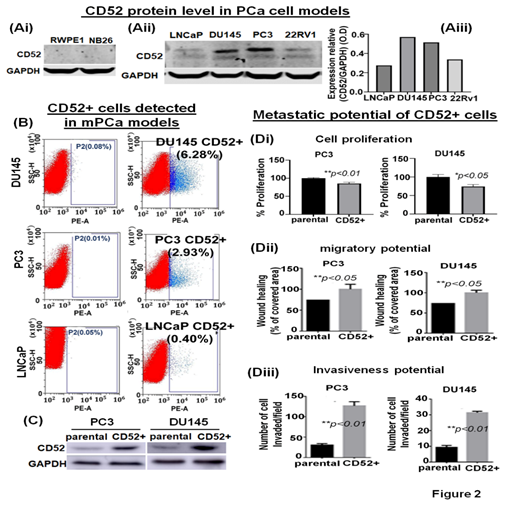
The expression of CD52 in prostate tumors is not reported. Therefore, we employed cell-based models representing different phenotypes of human PCa to determine the expression levels of CD52 by immunoblotting. The immunoblotting results showed that CD52 is extremely low to be detected in normal prostate epithelial (RWPE1) and premalignant prostate epithelial neoplastic (NB26) cell models (Fig. 2Ai). CD52 protein was observed to be scantly detectable in LNCaP cells, which exhibit poor metastatic potential and weak in 22Rv1 cells (Fig. 2Aii, iii). Conversely, CD52 protein was markedly detectable in metastatic models, PC3 and DU145 (Fig. 2Aii, iii). The cell line data validates the results found in patients where the CD52 high expression is associated with PCa and metastasis.
3.4 CD52+ subpopulation of prostate cancer cells exhibits an aggressive metastatic behavior
PCa aggressiveness and poor prognosis are associated with heterogeneous cancerous cells in the tumors [20]. We posit that tumor cells, during progression, acquire differential phenotypic and genotypic traits. It is noted that the evolution of subpopulations of tumor cells is required for seedlings and settling in the microenvironments at distant metastasis. Based on our data, we posit that the prostate tumor cells expressing CD52 are highly prone to metastasis, resulting in the disease's poor outcome. We asked if all mPCa cells express CD52 or is expressed in a subpopulation distinct and present in mPCa cells. CD52 protein exists in a cell-membrane glycoprotein and a secretory form found in the TME [12]. This characteristic provides an advantage for isolating the CD52+ cells from mPCa cell lines using the fluorescence-activated cell sorting (FACS) technique. The FACS-based cell isolation data showed the presence of CD52+ subpopulation in PC3 (2.93%) and DU145 (6.28%) cells (Fig. 2B). Complementary, since we found a very weak expression of CD52 in LNCaP cells in the immunoblot, we use LNCaP cells as a negative control in FACS. The results showed that in LNCaP cells (low metastatic potential), the percentage of CD52+ subpopulation is very low (0.40%) (Fig. 2B). To study the metastatic behavior of the CD52+ subpopulation, isolated cells were allowed to grow under standard culture conditions. After reaching full confluence and serial propagation, we compared the levels of CD52 between parental and isolated cells, confirming the isolation's success (Fig. 2C). Then, we compared the growth, migration, and invasive potential of the CD52+ subpopulation. We evaluated the cell proliferation by MTT assay, finding that CD52+ cells proliferate more slowly than the parental cells (Fig. 2Di). The migratory potential was measured in the distance covered by tumor cells to fill the artificially generated wound/gap in the culture dish. The 24h post-wound healing analysis showed that CD52+ cells covered the wound 26% more than parental PCa cells (Fig. 2Dii). Finally, the invasiveness of CD52+ cells was measured by chemoinvasion assay. The analysis showed that CD52+ cells exhibit more invasion than parental cells (Fig. 2Diii). These data indicate that the CD52+ subpopulation in prostate tumors could be the basis of the aggressive mPCa subtype in humans.
Figure 2: Identification of the CD52+ subpopulations of metastatic cells in prostate cancer cell models. (Ai, ii) immunoblot image shows the protein level of CD52 in PCa cell models representing normal immortalized (RWPE1), premalignant/indolent PCa (NB26), and metastatic cells (LNCaP, DU145, PC3 and 22Rv1) assessed by immunoblot. The GAPDH protein levels in cell lysates were used as a loading control. (Aiii) Bar graph shows the densitometry analysis of the CD52 expression in metastatic PCa cell lines. (B) The scatter/cell distribution images show the enrichment of the CD52+ subpopulation of tumor cells from parental PC3, DU145 and LNCaP cells by the FACS-based cell-sorter technique. (C) image compares the level of CD52 in parental and CD52+ subpopulations assessed by immunoblot. GAPDH was used as a control. (Di-iii) Histograms compare the cell proliferation, the migratory and invasive potential of the CD52+ subpopulation, and parental PCa cells assessed by MTT, scratch-wound, and chemoinvasion assays, respectively.
3.5 Molecular characterization of CD52 prostate cancer cells
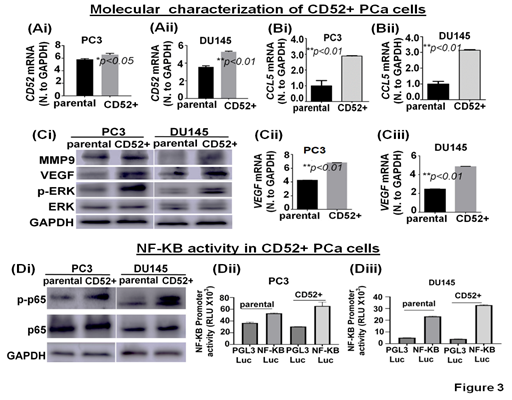
To investigate potential signaling pathways and metastatic-related proteins associated with the metastatic behavior observed, we molecularly characterized the CD52+ cell population. First, we determined the CD52 transcript levels in parental and isolated CD52+ cells. We found that CD52+ cells exhibit higher transcript levels than parental cells (Fig 3Ai, ii). Then, because the expression of CCL5 is positively correlated with metastasis and poor prognosis PCa patients [21] and we observed that patients with alteration in the CD52 gene exhibit poor disease-free survival (Fig. 1B), we speculate that CD52 cells would express high levels of CCL5. Therefore, we evaluated the levels of CCL5 transcript in parental and CD52+ cells. The results showed that CD52+ cells exhibit higher transcript levels than the parental cells (Fig. 3Bi, ii). Because activation of the ERK signaling pathways is critical for metastasis of PCa [22], we evaluated the phosphorylation of ERK and the downstream targets MMP9 and VEGF by immunoblot. The comparative analysis of cells showed that phospho-ERK, VEGF, and MMP9 levels are increased in CD52+ compared to the parental cells (Fig. 3Ci). Additionally, CD52+ PCa subpopulations exhibited an increased level of VEGF transcript (Fig. 3Cii, iii). Multiple molecular pathways associated with metastasis, proliferation, inflammation, and immune-cell activity converge on NF-kB [23]. Inflammatory pathways such as NF-kB have been reported to play an essential role in metastasis and PCa progression [24]. Therefore, we compared the status of NF-kB signaling in CD52+ cells versus parental cells. The immunoblot analysis showed that phosphorylated-p65 subunit levels are elevated in the CD52+ cells compared to parental (Fig. 3Di). It is to be noted that the NF-kB's activation in tumor cells depends on the phosphorylation and subsequent nuclear translocation of its p65 subunit. We next measured the nuclear activity of NF-kB transcriptional factor in CD52+ cells by a luciferase-based reporter assay. This reporter assay measures the binding of NF-kB transcriptional factor to its responsive element (NRE) located at target genes. We found that the CD52+ cells exhibit an increased NF-kB transcriptional activity than the parental PCa cells (Fig. 3Dii, iii). These data show that the metastatic potential of the CD52+ subpopulation is associated with the constitutive activation of the ERK and NF-KB signaling pathways.
Figure 3: Molecular characterization of CD52+ cells. (Ai-Bii) Bar graphs show the expression of CD52 and CCL5 mRNA in parental and CD52+ cells assessed by RT-qPCR. (Ci) image compares the level of MMP9, VEGF, phospho-ERK, and total-ERK in parental and CD52+ subpopulations assessed by immunoblot. GAPDH was used as a control. (Cii, iii) Bar graphs show the expression of VEGF mRNA in parental and CD52+ cells assessed by RT-qPCR. (Di) Immunoblot images compare phospho-p65 (p-p65) and p65 expression levels in parental and CD52+ subpopulations assessed by immunoblot. GAPDH was used as control. (Dii, iii) Histograms compare NFκB transcriptional activity in CD52+ subpopulation and parental PCa cells assessed by dual-luciferase reporter assays. Each bar of histograms represents the average of three independent experiments. Renilla luciferase activity served as the internal control for each group.
3.6 Soluble CD52 protein is released by metastatic prostate cancer cells
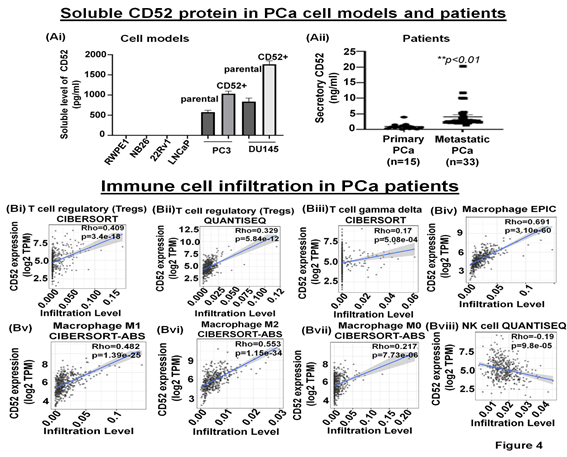
The immune-suppressive TME comprises fibroblasts, MDSC, Tregs, and secretory proteins/factors inhibiting NK cell function [25]. The cleavage of the transmembrane CD52 protein is reported to result in the release of a soluble form of CD52 (sCD52) protein in the extracellular space [26, 27]. However, whether prostate tumor cells release sCD52 in the TME remains unknown. Therefore, we asked if sCD52 is detectable in the extracellular space or TME of the prostate tumor cells. For this purpose, we evaluated the FBS-free condition culture media of parental and CD52+ subpopulation of PCa cells and measured the sCD52 by ELISA. Whereas the culture media of metastatic cells (PC3, DU145 cells) exhibited sCD52 protein, no protein was detected in the conditioned media of primary prostate cells (Fig. 4Ai). This data suggests that the CD52+ subpopulation which is prone to metastasis could be an important source of sCD52 protein in prostate tumors. These data prompted us to determine the serum levels of sCD52 protein in PCa patients. We measured sCD52 levels in serum of patients with primary PCa (n=15) and mPCa (n=33). We found that serum-sCD52 levels were significantly (p<0.05) higher in mPCa (mean value =4 ng/ml) than primary PCa (mean value = 0.94 ng/ml) (Fig. 4Aii). These data suggest the relevance of soluble CD52 as a potential indicator of mPCa in patients.
Figure 4: Soluble CD52 in prostate cancer cell lines and the association between CD52 and immune cell infiltration in patients
(A) Bar graphs show the secretory level of CD52 in the FBS-free conditioned culture media of PCa cell models assessed by the ELISA method. (B) The graph shows the serum-CD52 levels in PCa patients (primary PCa and mPCa) assessed by ELISA. TIMER analysis results. (Bi-viii) The CD52 expression levels have a significant positive correlation with infiltration levels of Tregs, gamma delta T cells, and macrophages but negatively correlated with the infiltration of NK cells.
3.7 Expression of CD52 in Prostate tumors is associated with an immunosuppressive Tumor Microenvironment
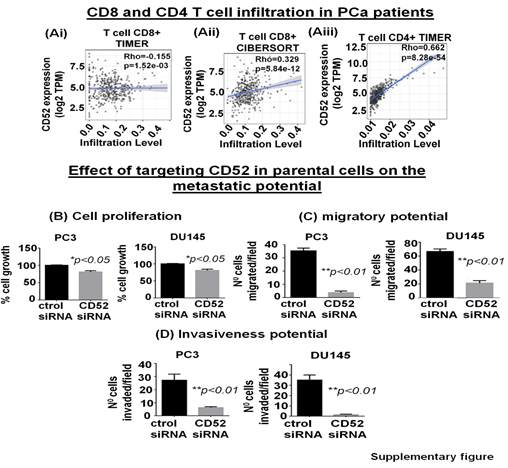
The tumor immune microenvironment (TME) critically affects tumor behavior and plays an essential role in developing metastasis in PCa [28]. PCa is a cold tumor characterized by low infiltration of anti-tumor immune cells, and most of infiltrating cells are immunosuppressive or dysfunctional. Tumor infiltrating CD8+ and NK cells have been related with a good outcome [29, 30]. Soluble CD52 protein is released from immune cells and by interacting with Siglec-10 on T cells results in an inhibitory effect on the cell function [31]. The immunosuppressive TME facilitates the growth of aggressive PCa cell subpopulations, distinct from each other in their oncogenic signatures and responses to therapies. Therefore, we asked if CD52+ prostate tumor cells have any bearing or association with the level and type of immune cells within the prostate tumor zone. For this purpose, we used the TIMER 2.0 web platform that integrates multiple state-of-the-art algorithms (TIMER, xCell, MCP-counter, CIBERSORT, EPIC, Quantiseq) to analyze tumor infiltration present in patient tumors. These algorithms are applied to the expression profiles of the Cancer Genome Atlas (TCGA) tumors, allowing users to explore various associations between immune infiltrates and genetic features in the TCGA cohorts, such as TCGA-PRAD (TCGA-prostate adenocarcinoma) clinical data. We first determined a correlation between CD52 and Treg infiltrate levels in prostate tumors of patients studied under the TCGA-PRAD cohort (n =498). As per CIBERSORT analysis that deconvolves detailed subsets of T-cell signatures, we found a positive correlation (rho= 0.409; p=3.4 e-18) between CD52 and Treg cell infiltrates in prostate tumors of the PRAD cohort (n=498) and is presented in terms of a linear regression graph (Fig. 4Bi). As per Quantiseq analysis that directly generates scores interpreted as cell fractions, a positive correlation (rho= 0.329; p=5.84 e-12) between CD52 and Treg population in prostatic tumors was observed in the PRAD cohort (n=498) (Fig 4Bii). We next measured the level of CD8+ and CD4+ T cells in the PRAD cohort's immune infiltrate of prostate tumors. The TIMER algorithm-generated data shows a strong negative correlation between CD52 and the presence of CD8+ T cells in the prostate tumor zone. In contrast, CIBERSORT-generated data shows a positive correlation between CD52 and CD8+ T cell levels (Supplementary Fig. Ai, ii). Next, we evaluated CD4+ T cell levels in CD52+ prostate tumors and found a positive correlation between CD52 and CD4+ T cell infiltration (Rho 0.662) in CaP patients (Supplementary Fig. Aiii). Gamma-delta (γδ) T cells are a subset of T cells that promote the inflammatory responses of lymphoid and myeloid lineages in the tissues, and prostate TME is laden with lymphoid and myeloid cells. We analyzed the infiltration of the γδ T cells associated with the presence of CD52. The PRAD clinical data analysis shows a positive correlation between CD52 and γδ T cell levels in the immune infiltrate in the prostate TME (Fig. 4Biii).
Macrophages are the most abundant component of tumor-infiltrating immune cells that promote CaP initiation, progression, and metastasis. Tumor-associated macrophages (TAMs) play an essential role in tumor progression. Therefore, we investigated the association between CD52 and TAM infiltration levels in prostate tumors of the patient cohort of the TCGA-PRAD clinical study. The analysis shows a strong and positive correlation between the CD52 and the presence of macrophages (Rho 0.691; p=3.10 e-60) in TME (Fig. 4Biv). Furthermore, the analysis showed the ratio of macrophages anti-tumor (M1)/pro-tumor (M2) population favoring a shift towards tumor growth and positively correlated with the CD52 (Rho 0.482, p=1.39 e-25 and Rho 0.553, p=1.15 e-34 respectively) (Fig. 4Bv, vi). Notably, no correlation was observed between CD52 and the non-activated macrophage (M0) population (Rho 0.217, p=7.73 e-6) in prostate tissues (Fig. 4Bvii). These data show that CD52 is significantly associated with the recruitment of tumor-promoting TAMs in PCa patients. Natural killer (NK) cells belong to the innate immune system and exhibit anti-tumor function by directly killing tumor cells and influencing other immune cells and factors (IFN-γ, TNF-α) [32]. Therefore, we investigated the association between CD52 and NK cell infiltration levels in prostate tumors of the TCGA-PRAD cohort. We found a significant negative correlation (Rho -0.19, p=9.80 e-05) between the presence of CD52 and NK cell infiltration (Fig. 4Bviii). All these data suggest that the expression of CD52 in CaP tumors is associated with a deficient anti-tumor cellular immunity.
3.8 CD52 as a therapeutic target for metastatic prostate cancer
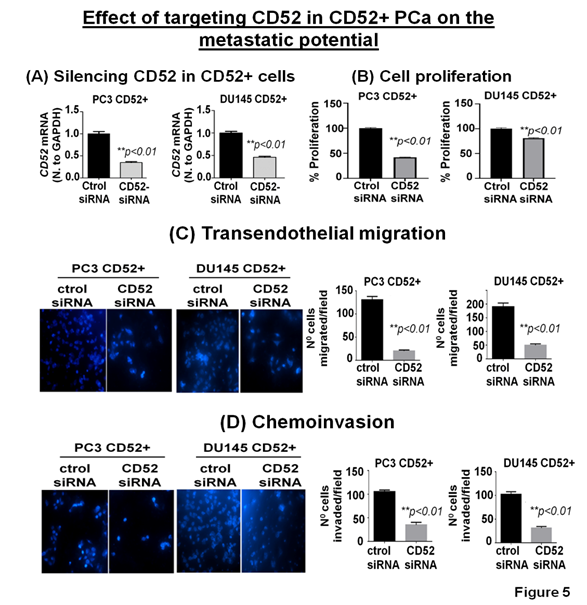
The clinical and preclinical data show the relevance of CD52 in the metastasis of the PCa; therefore, we posit that targeting CD52 could improve the therapeutic outcome. For this purpose, we conducted genetic targeting of CD52 in the CD52+ subpopulation of PCa cells and, 24h post-transfection, validated the suppression of CD52 assessed by RT-qPCR (Fig. 5A). We next determined the effect of the CD52 silencing on proliferation, transmigration of prostate tumor cells across the layer of endothelial cells (HUVEC), and invasiveness of CD52+ cells. First, we evaluated the proliferation of the cells at 72h. We found that silencing of CD52 caused a significant inhibition in the proliferation (p<0.01) compared to the control (Fig 5B). We also found that suppression of CD52 significantly (p<0.05) reduced the growth of parental PC3 cells and moderately decreased the growth of parental DU145 cells assessed by MTT assays (Supplementary Fig. B). A critical step of the metastatic process is the invasion of cancer cells and their entry into the circulatory system through endothelial transmigration. Therefore, we performed a transendothelial migration assay through a thin monolayer of HUVEC spread over a membrane. The suppression of CD52 significantly reduced the transmigratory potential of CD52+ PCa cells (Fig. 5C). Complementary, we performed a chemoinvasion assay using CD52-suppressed cells. The results show that silencing of CD52 causes a significant decrease in the invasive potential of CD52+ PCa cells (Fig 5D). In addition, the silencing of CD52 in parental PC3 and DU145 cells significantly decreases their transmigration and chemoinvasion properties (Supplementary Fig. C and D). These data suggest that the invasiveness and metastatic behavior may be attributed mainly to the presence of CD52 protein in PCa cells. To summarize, our data identified (i) a novel subtype of PCa in humans, (ii) CD52+ is a potential indicator of metastasis, and (iii) CD52 as a promising therapeutic target for metastatic disease.
Figure 5: Relevance of CD52 as a therapeutic target for metastatic prostate cancer. (A) Bar graphs show the CD52 silencing in the CD52+ subpopulation assessed by RT-qPCR. (B) Bar graphs compare the effect of CD52 silencing (CD52 siRNA) and control on the proliferation of the CD52+ cells determined by MTT assay. (C, D) imagens and histograms show the effect of CD52 silencing in the CD52+ cell on the transendothelial migration through a layer of endothelial cells (HUVEC) and chemoinvasion.
4. Discussion
The poor prognosis in mPCa is associated with heterogeneous cell populations contributing to the disease progression. These populations can respond or not to therapies and exhibit different metastatic potential. Therefore, identifying subpopulations of PCa cells prone to metastasis is critical for understanding the mechanism of metastasis and identifying novel therapeutic targets for treating metastatic patients. Furthermore, it is also essential to investigate the mechanisms involved in metastasis, such as migration and invasion, and activation of signaling pathways that confer metastatic characteristics to cells. Thus, understanding the mechanisms will lead to exploring novel therapeutic targets.
In this study, we explored the expression of CD52 in patients using the TCGA-PRAD database and PCa cell lines. We found a population of patients exhibiting genomic alterations in the CD52 gene or alterations in the mRNA expression. In a clinical study comprising 126 patients, we found that patients with alteration in CD52 exhibit lower disease-free survival than those with unaltered. However, the study has some limitations, such as not discriminating between patient’s tumor alterations and low number of patients. Even though we note that the study included 9 patients with low expression of CD52 mRNA and 15 with high expression. Additionally, we show that mPCa cell lines express and release CD52, and functional studies show the expression of CD52 provides cells with increased metastatic behavior.
CD52 is a membrane glycoprotein commonly found on the surface of mature immune cells and the male genital tract. CD52's function is still unknown and poorly studied. However, some experiments using anti-CD52 antibody show that CD52 is critical for lymphocyte transendothelial migration [33]. Based on our data, it could be inferred that CD52 confers transmigratory property and imparts the metastatic ability to PCa cells. Indeed, we found that CD52+ cancer cells exhibit a high level of transendothelial migration. Because CD52 is a highly negatively charged molecule, we speculate that tumor cells expressing this molecule harbor anti-adhesion properties, thus allowing prostate tumor cells to migrate and metastasize. Additionally, we found that CD52+ cells exhibited constitutive activation of the ERK and NF-KB signaling pathways and increased VEGF and MMP9 expression levels, which explains the aggressive nature and metastatic capability of CD52+ cells. CD52 glycoprotein is cleaved from the membrane of immune cells and is subsequently released as sCD52 to exert immunosuppressive function [34]. CD52 is also shed from epithelial cells of distal epididymis and ductus deferens into the seminal plasma, which is detectable in the seminal ejaculate of men [35]. An important finding of our study is the significance of increased levels of sCD52 protein detected in the blood of PCa patients diagnosed with metastasis. Our data inference is strong because of the validation experiment that showed the secretion of sCD52 by invasive/metastatic PCa cells in vitro cultures, which matched the scenario of increased sCD52 in the blood of patients with mPCa (Fig. 4Aii). Because sCD52 is detectable in the body fluids, our study opens a critical opportunity to study sCD52 protein as a potential fluid biopsy-biomarker predictive of progression in PCa patients. Furthermore, based on the Decipher genomic test data, we suggest that biopsy-CD52 expression could indicate the risk of poor outcome in patients with primary PCa, whereas serum-CD52 proteins could predict the risk of metastasis in PCa patients who remain under post-treatment surveillance. Prostate tumors are considered immune-cold tissues with high infiltration of immunosuppressive cells that, rather than eliminating, often promote tumor growth and metastasis [28, 36]. Our data clearly show a positive correlation between the presence of CD52 and immunosuppressive cell infiltration in PCa patients (TCGA-PRAD). Furthermore, we found that the presence of CD52 was negatively correlated with the infiltration of NK cells, which are critical for the anti-tumor immune function. In line with this finding, it is reported that a high infiltration of NK cells in prostate tumors is associated with a good prognosis and inversely correlated with SVI [29, 37]. Indeed, our Decipher genomic data shows that the expression of CD52 in prostate tumors predicts SVI in patients. These data encourage us to continue studying the potential neutralization of CD52 in metastatic cells to modify immune cell infiltration in vivo. The outcome of this study suggests that prostate tumor cells CD52+ are at risk of metastasis. Therefore, CD52 could be used as a target for eliminating such tumor cells predestined for metastasis in PCa. CD52 as a therapeutic target has been studied in autoimmune diseases [38]. Recent studies have shown that targeting CD52 by the anti-CD52 antibody (alemtuzumab) could help treat graft versus host disease for organ transplantation [39, 40]. In addition, alemtuzumab has been approved to treat lymphocytic leukemia [41]. However, the systemic administration of alemtuzumab depletes T and B cells, leading to profound immunosuppression and risk of severe infections in patients [40]. Therefore, alemtuzumab for treating metastatic cancer expressing CD52 protein would bring potential clinical issues. However, our data showing that genetic targeting of CD52 reduces the invasive potential of CD52+ mPCa cells suggests that CD52 is a targetable molecule that possibly could be targeted in vivo using a small molecule inhibitor and thus overcome the limitation of alemtuzumab.
5. Conclusion
This is the first study showing the significance of CD52 in PCa disease. We identified a subpopulation of PCa cells that express high levels of CD52, which is associated with metastasis. The CD52+ cells exhibit overactivation of the NF-KB and ERK signaling pathways that may explain its metastatic behavior due to an increase in the expression of MMP9 and VEGF. We identified that alterations in the CD52 gene in PCa patients are associated with poor survival in PCa patients. Our data indicated that genetic suppression of CD52 significantly reduces metastatic cell growth, migration, and invasive potential. Therefore, therapies targeting CD52 may bring new opportunities to develop therapeutic approaches for treating aggressive metastatic PCa.
Acknowledgement
We thank Ashraf Shabenah and Jinhua Wang from the Institute for Health Informatics at the University of Minnesota for their help with the Decipher test analysis. We thank Dr. Saleem for his suggestions and help with the data analysis. We thank Dr. Elai Davicioni (Chief Scientific Officer, Decipher Biosciences, Inc, San Diego, CA) for the Minnesota cohort's Decipher-genome testing (RP-treated patient specimens). Finally, we thank Ashraf Shabenah and Jinhua Wang from the Institute for Health Informatics, University of Minnesota, for their help with the Decipher test analysis.
No Conflict Statement
All authors have participated in the planning, execution, and analysis of the study and have approved this version. The authors declare no potential conflicts of interest.
Financial Declaration
This study was supported by the US PHS grant (R01-CA193739). Rush University Medical Center Research Recruitment supported the authors Ferrari and Mansini.
References
- Kinsella N, et al, Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Androl Urol 7 (2018): 83-97.
- Kolodziej M, Management of biochemically recurrent prostate cancer following local therapy. Am J Manag Care 20 (2014): S273-81.
- Antonov PG, Raycheva and Popov V. Unexpected long-term survival in an adult patient with metastatic prostate cancer. Urol Case Rep 37 (2021): 101634.
- Valastyan S and Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 147 (2011): 275-92.
- Messex JK, and Liou GY. Impact of Immune Cells in the Tumor Microenvironment of Prostate Cancer Metastasis. Life (Basel) 13 (2023).
- Bellmunt JKS, Fizazi, and Srikrishna G. Immunosuppressed Microenvironment An Emerging Target in Prostate Cancer Management (2014).
- Wu SQ, et al, Role of tumor-associated immune cells in prostate cancer: angel or devil? Asian J Androl 21 (2019): 433-437.
- Rowan W, et al. Cross-linking of the CAMPATH-1 antigen (CD52) mediates growth inhibition in human B- and T-lymphoma cell lines, and subsequent emergence of CD52-deficient cells. Immunology 95 (1998): 427-36.
- Kirchhoff C, et al. A major mRNA of the human epididymal principal cells, HE5, encodes the leucocyte differentiation CDw52 antigen peptide backbone. Mol Reprod Dev 34 (1993): 8-15.
- Hale G, et al. The glycosylphosphatidylinositol-anchored lymphocyte antigen CDw52 is associated with the epididymal maturation of human spermatozoa. J Reprod Immunol 23 (1993): 189-205.
- Jiang L, et al. Variable CD52 expression in mature T cell and NK cell malignancies: implications for alemtuzumab therapy. Br J Haematol 145 (2009): 173-9.
- Vojdeman FJ, et al. Soluble CD52 is an indicator of disease activity in chronic lymphocytic leukemia. Leuk Lymphoma 58 (2017): 2356-2362.
- Chandrashekar DS, et al. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 19 (2017): 649-658.
- Umbreen S, et al. COMMD3:BMI1 Fusion and COMMD3 Protein Regulate C-MYC Transcription: Novel Therapeutic Target for Metastatic Prostate Cancer. Mol Cancer Ther 18 (2019): 2111-2123.
- Ganaie AA, et al. Anti-S100A4 antibody therapy is efficient in treating aggressive prostate cancer and reversing immunosuppression: Serum and biopsy S100A4 as clinical predictor. Mol Cancer Ther (2020).
- Parray A, et al. ROBO1, a tumor suppressor and critical molecular barrier for localized tumor cells to acquire invasive phenotype: study in African-American and Caucasian prostate cancer models. Int J Cancer 135 (2014): 2493-506.
- Ferrari MG, et al. Identifying and treating ROBO1(-ve) /DOCK1(+ve) prostate cancer: An aggressive cancer subtype prevalent in African American patients. Prostate 80 (2020): 1045-1057.
- Marrone M, et al. A 22 Gene-expression Assay, Decipher (R) (GenomeDx Biosciences) to Predict Five-year Risk of Metastatic Prostate Cancer in Men Treated with Radical Prostatectomy. PLoS Curr 7 (2015).
- Lee HJ, et al. Evaluation of a genomic classifier in radical prostatectomy patients with lymph node metastasis. Res Rep Urol 8 (2016): 77-84.
- Skvortsov S, et al, Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells 36 (2018): 1457-1474.
- Huang R, et al, Research Trends and Regulation of CCL5 in Prostate Cancer. Onco Targets Ther 14 (2021): 1417-1427.
- Nickols NG, et al. MEK-ERK signaling is a therapeutic target in metastatic castration resistant prostate cancer. Prostate Cancer Prostatic Dis 22 (2019): 531-538.
- Xia YS, Shen and Verma IM. NF-κB, an active player in human cancers. Cancer immunology research 2 (2014): 823-830.
- Jin RJ, et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res 68 (2008): 6762-9.
- Tormoen GW, Crittenden MR, and Gough MJ. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol 3 (2018): 520-526.
- Toh B-H, et al. Immune regulation by CD52-expressing CD4 T cells. Cellular & Molecular Immunology 10 (2013): 379-382.
- Samten B. CD52 as both a marker and an effector molecule of T cells with regulatory action: Identification of novel regulatory T cells. Cellular & Molecular Immunology 10 (2013): 456-458.
- Palano MT, et al The tumor innate immune microenvironment in prostate cancer: an overview of soluble actors and cellular effectors. Explor Target Antitumor Ther 3 (2022): 694-718.
- Pasero C, et al. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget 6 (2015): 14360-73.
- Yang Y, et al. High intratumoral CD8(+) T-cell infiltration is associated with improved survival in prostate cancer patients undergoing radical prostatectomy. Prostate 81 (2021): 20-28.
- Toh BH, et al. Immune regulation by CD52-expressing CD4 T cells. Cell Mol Immunol 10 (2013): 379-82.
- Paul S and Lal G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Frontiers in Immunology 8 (2017).
- Kasarello K and Mirowska-Guzel D. Anti-CD52 Therapy for Multiple Sclerosis: An Update in the COVID Era. Immunotargets Ther 10 (2021): 237-246.
- Zhao Y, et al. The immunological function of CD52 and its targeting in organ transplantation. Inflamm Res 66 (2017): 571-578.
- Yeung CH, et al Epididymal secretion of CD52 as measured in human seminal plasma by a fluorescence immunoassay. Mol Hum Reprod 4 (1998): 447-51.
- Bruni D, Angell HK and Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer 20 (2020): 662-680.
- Gannon PO, et al. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods 348 (2009): 9-17.
- Ruck T, et al. Alemtuzumab in Multiple Sclerosis: Mechanism of Action and Beyond. Int J Mol Sci 16 (2015): 16414-39.
- Bouvy AP, et al. Alemtuzumab as Antirejection Therapy: T Cell Repopulation and Cytokine Responsiveness. Transplant Direct 2 (2016): e83.
- Guthoff M, et al. Low-dose alemtuzumab induction in a tailored immunosuppression protocol for sensitized kidney transplant recipients. BMC Nephrology 21 (2020): 178.
- Keating MJ, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 99 (2002): 3554-61.