Ibrutinib-Associated Life-Threatening Hemorrhage with Subcapsular Renal Hematoma and Hemothorax
Article Information
Pramod Gaudel1, Autumn Brubaker2, Caitlyn Huang3, Linda Verkruyse4, and Chao Huang5
1Fellow, Department of Hematology/Medical Oncology, University of Kansas Medical Center, Kansas City, KS, USA
2Resident, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, KS, USA
3Student, Pembroke Hill High School, Kansas City, MO, USA
4Attending Physician, Department of Hematology/Oncology, Kansas City VA Medical Center, Kansas City, MO, USA
5Professor, Department of Medical Oncology, University of Kansas Medical Center, Kansas City, KS, USA
*Corresponding Author: Pramod Gaudel, Fellow, Department of Hematology/Medical Oncology, University of Kansas Medical Center, Kansas City, KS, USA
Received: 15 March 2022; Accepted: 19 April 2022; Published: 29 April 2022
Citation: Pramod Gaudel, Autumn Brubaker, Caitlyn Huang, Linda Verkruyse, and Chao Huang. Ibrutinib-Associated Life- Threatening Hemorrhage with Subcapsular Renal Hematoma and Hemothorax. Archives of Clinical and Medical Case Reports 6 (2022): 313-323.
View / Download Pdf Share at FacebookAbstract
Background: Ibrutinib is an irreversible inhibitor of Bruton tyrosine kinase (BTK) which is FDA approved for treatment of chronic lymphocytic leukemia (CLL) and multiple other B-cell lymphoproliferative disorders. Ibrutinib therapy is associated with increased risk of bleeding. We report an unusual but life-threatening case of hemorrhage with subcapsular renal hematoma and large hemothorax secondary to Ibrutinib.
Case Presentation: An 85-year-old male with history of CLL started on a reduced dose of ibrutinib presented with acute onset left sided flank pain and dyspnea. He was found to have bicytopenia with acute anemia, thrombocytopenia, and acute kidney failure. Imaging revealed moderate to large left sided pleural effusion and a renal mass concerning for renal neoplasm. He underwent thoracentesis revealing hemothorax. The patient was managed conservatively with intravenous hydration for his acute renal failure, blood transfusions and pain medications. His pain resolved; blood counts eventually stabilized and he was discharged from the hospital. The renal mass was later diagnosed as a large subcapsular renal hematoma after further review by another radiologist who determined that the Hounsfield unit of the mass was consistent with blood.
Conclusions: This case illustrates a case of life-threatening hemorrhage that required hospitalization after 2 weeks of initiation of ibrutinib. Hemorrhage can occur despite reduced dose, and can result in hemorrhagic pleural effusion and bleeding in solid organs like the kidney. The presence of sudden large masses in a solid organ in a patient on ibrutinib should elicit the differential of intra-organ hemorrhage. Initiation of ibrutinib should be done cautiously, especially in elderly patients.
Keywords
Ibrutinib, CLL, Hemorrhage, Hemothorax, Thrombocytopenia, Renal hematoma
ibrutinib articles, CLL articles, hemorrhage articles, hemothorax articles, thrombocytopenia articles, renal hematoma articles
Ibrutinib articles Ibrutinib Research articles Ibrutinib review articles Ibrutinib PubMed articles Ibrutinib PubMed Central articles Ibrutinib 2023 articles Ibrutinib 2024 articles Ibrutinib Scopus articles Ibrutinib impact factor journals Ibrutinib Scopus journals Ibrutinib PubMed journals Ibrutinib medical journals Ibrutinib free journals Ibrutinib best journals Ibrutinib top journals Ibrutinib free medical journals Ibrutinib famous journals Ibrutinib Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals CLL articles CLL Research articles CLL review articles CLL PubMed articles CLL PubMed Central articles CLL 2023 articles CLL 2024 articles CLL Scopus articles CLL impact factor journals CLL Scopus journals CLL PubMed journals CLL medical journals CLL free journals CLL best journals CLL top journals CLL free medical journals CLL famous journals CLL Google Scholar indexed journals Hemorrhage articles Hemorrhage Research articles Hemorrhage review articles Hemorrhage PubMed articles Hemorrhage PubMed Central articles Hemorrhage 2023 articles Hemorrhage 2024 articles Hemorrhage Scopus articles Hemorrhage impact factor journals Hemorrhage Scopus journals Hemorrhage PubMed journals Hemorrhage medical journals Hemorrhage free journals Hemorrhage best journals Hemorrhage top journals Hemorrhage free medical journals Hemorrhage famous journals Hemorrhage Google Scholar indexed journals Ultra Sound articles Ultra Sound Research articles Ultra Sound review articles Ultra Sound PubMed articles Ultra Sound PubMed Central articles Ultra Sound 2023 articles Ultra Sound 2024 articles Ultra Sound Scopus articles Ultra Sound impact factor journals Ultra Sound Scopus journals Ultra Sound PubMed journals Ultra Sound medical journals Ultra Sound free journals Ultra Sound best journals Ultra Sound top journals Ultra Sound free medical journals Ultra Sound famous journals Ultra Sound Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Radiology articles Radiology Research articles Radiology review articles Radiology PubMed articles Radiology PubMed Central articles Radiology 2023 articles Radiology 2024 articles Radiology Scopus articles Radiology impact factor journals Radiology Scopus journals Radiology PubMed journals Radiology medical journals Radiology free journals Radiology best journals Radiology top journals Radiology free medical journals Radiology famous journals Radiology Google Scholar indexed journals Thrombocytopenia articles Thrombocytopenia Research articles Thrombocytopenia review articles Thrombocytopenia PubMed articles Thrombocytopenia PubMed Central articles Thrombocytopenia 2023 articles Thrombocytopenia 2024 articles Thrombocytopenia Scopus articles Thrombocytopenia impact factor journals Thrombocytopenia Scopus journals Thrombocytopenia PubMed journals Thrombocytopenia medical journals Thrombocytopenia free journals Thrombocytopenia best journals Thrombocytopenia top journals Thrombocytopenia free medical journals Thrombocytopenia famous journals Thrombocytopenia Google Scholar indexed journals Renal hematoma articles Renal hematoma Research articles Renal hematoma review articles Renal hematoma PubMed articles Renal hematoma PubMed Central articles Renal hematoma 2023 articles Renal hematoma 2024 articles Renal hematoma Scopus articles Renal hematoma impact factor journals Renal hematoma Scopus journals Renal hematoma PubMed journals Renal hematoma medical journals Renal hematoma free journals Renal hematoma best journals Renal hematoma top journals Renal hematoma free medical journals Renal hematoma famous journals Renal hematoma Google Scholar indexed journals
Article Details
1. Introduction
Bruton’s tyrosine kinase (BTK) is a downstream component of the B-cell receptor (BCR) involved in normal differentiation, survival and proliferation of peripheral B cells as well as participation in platelet activation signaling pathways [1-3]. BTK is an important factor to the survival of malignant B cells, making it an ideal target in CLL therapy. Ibrutinib is a potent and irreversible BTK inhibitor that covalently binds to the active site of BTK and has received US FDA approval as a first line treatment for chronic lymphocytic leukemia/small lymphocytic lymphoma (based on RESONATE-2 trial) [4] It is also approved for several B cell malignancies like treatment naïve Waldenström’s macroglobulinemia [5, 6], relapsed mantle cell lymphoma [7] and relapsed/refractory marginal zone lymphoma [8]. Multiple studies are currently ongoing in different hematologic malig-nancies and solid tumors, and use of Ibrutinib might further expand in future.
Ibrutinib is associated with increased risk of bleeding compared to other chemotherapy regimens due to inhibition of BTK and TEC tyrosine kinase leading to decreased platelet activation. The hemorrhage associated with ibrutinib varies from mild to severe. We report a case of severe hemorrhage in a patient with CLL who was not on prior anti-platelet therapy or anticoagulation. He presented just two weeks after initiating reduced dose of ibrutinib with grade 3 ibrutinib-associated hemorrhage involving subcapsular renal hematoma and hemothorax.
2. Case Presentation
An 85-year-old Caucasian male with active comorbidities of hypertension, hyperlipidemia, stage 3c chronic kidney disease, had a diagnosis of chronic lymphocytic leukemia (CLL) Rai stage I with symptomatic diffuse adenopathy. FISH for CLL was positive for trisomy 12 and deletion of 13q14.3, negative for deletions of 6q, ATM and TP53. He was initiated on ibrutinib at a reduced dose of 280mg daily (instead of standard 420mg daily) to treat his CLL. The dose reduction was recommended due to the patient's concerns about side effect profile. He reported significant reduction in his cervical lymph nodes size after starting ibrutinib. Two weeks after ibrutinib was initiated, he presented to the emergency department with acute onset, non-traumatic severe 10/10 left sided flank pain, increased dyspnea, nausea, and progressive weakness. He was in acute hypoxic respiratory failure with new oxygen requirement of 2 LPM by nasal cannula. Initial laboratory findings revealed acute anemia with hemoglobin 8.1 g/dL from baseline of 12 g/dL, acute on chronic thrombocytopenia with platelets 55 x 103/µL from baseline of 110 x 103/µL, worsening leukocytosis at 99.5 x 103/µL from baseline of 18 x 103/µL. Urinalysis with 6-10 RBCs/HPF, and 1+ blood. In addition, he developed acute kidney failure with a rise in creatinine up to 1.82 mg/dL (Baseline Creatinine was 1.5mg/dL).
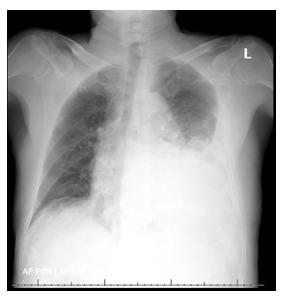
Figure 1: XR chest on admission- large left pleural effusion.
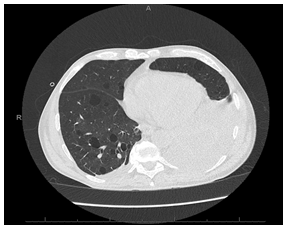
Figure 2: CT chest without IV contrast showing large left pleural effusion with passive atelectasis in the left lower lobe
Chest X-ray revealed moderately large left pleural effusion with volume loss in the left lung secondary to atelectasis associated with the pleural fluid (Figure 1). He was then admitted for further management. CT of the chest without contrast confirmed moderately large left pleural effusion with atelectasis in left lower lobe (Figure 2). CT abdomen and pelvis without contrast obtained on admission revealed a perinephric opacity with proximal obstruction of the left kidney near the ureteropelvic junction with perinephric stranding concerning for infection versus lymphomatous infiltration (Figure 3 and 4). His ibrutinib was held at the time of admission. His anemia worsened on day 1 of his hospitalization, his hemoglobin dropped to 5.7 g/dL which led to transfusion of three units of packed red blood cells. Haptoglobin was normal, indicating a non-hemolytic process. Lactate dehydrogenase (LDH) was mildly elevated to 287 U/L (Ref range: 125-243 U/L). During his hospital stay on day 2 and 3, he had worsening thrombocytopenia with platelets dropping to 41 x 103/µL. Further laboratory testing showed D-dimer elevated at 7403 ng/mL, fibrinogen and prothrombin time were within normal limits. He was initiated on prednisone 1mg/kg daily for possible autoimmune thrombocytopenia on day 3. His workup for thrombocytopenia showed negative platelet antibody and cold agglutinins. Platelet aggregation studies were unable to be performed due to insufficient platelet quantities. Additional anemia work up revealed multifactorial anemia with iron deficiency (Serum Iron: 18 µg/dL; TIBC: 195 µg/dL; Iron saturation: 9%; Ferritin: 102.8 ng/mL). Reticulocyte panel showed low reticulocyte count at 3.54%, corrected at 1.4%, absolute reticulocyte count at 78 x 109/L (Ref range: 22-101 x 109/L) and a reticulocyte index at 1.51 indicating a hyperproliferative state. His upper and lower endoscopy was negative without stigmata of active gastrointestinal bleeding identified, H. pylori immunohistochemical stains were negative.
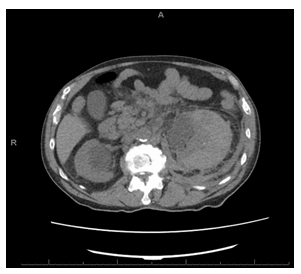
Figure 3: CT abdomen without IV contrast showing markedly enlarged left kidney with stranding in the soft tissues surrounding it.
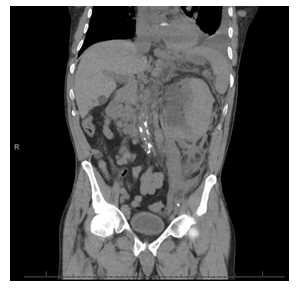
Figure 4: The CT of chest / abdomen and pelvis was done without IV contrast (due renal failure) showing a large left renal mass which was new compared with prior CT. There was considerable stranding in the soft tissues surrounding the left kidney as well, also representing a new finding. Differential diagnosis elicited were urinary obstruction near ureter-pelvis junction, infection or lymphomatous infiltration.
His renal function worsened with creatinine peaking at 2.6 mg/dL, and he developed mild hyperkalemia with potassium 5.1 mEq/L, hyperuricemia with uric acid 9.9 mg/dL, hyperphosphatemia with phosphorus 5.4 mg/dL, hypocalcemia with calcium 7.4 mg/dL concerning for early tumor lysis syndrome (Table 1). He was supported with intravenous fluids and allopurinol. He was also started on empiric broad spectrum antibiotics (IV cefepime 2g every 8 hours) due to concern for left proximal ureteral obstruction and possible pyelonephritis. Renal evaluation with ultrasound showed heterogenous enlargement of the left kidney measuring 13.0 × 7.7 × 8.1 cm without hydronephrosis.
The left renal cortex was markedly thickened measuring 5 cm with loss of corticomedullary differentiation. The left renal mass appeared to be solid, and in the setting of CLL there was concern for Richter transformation with infiltration of the left kidney, however, the clinical picture was not consistent with transformation without significant increase in LDH and lack of B symptoms. Secondary neoplasm of renal parenchyma was also in the differential. Palliative radiation was considered during admission but ultimately withheld due to patient’s condition. The patient reported gradual improvement of his left flank pain with conservative management.
In view of persistent pleural effusion and concern for malignant involvement, he underwent thoracentesis with removal of 1200 cc of bloody fluid. His dyspnea and oxygen requirements then returned to baseline. Pleural fluid analysis showed that effusion was a hemorrhagic exudative effusion with pleural protein 2.9 g/dL, pleural LDH 173 U/L, pleural fluid to serum protein ratio 0.55, RBC 479,000/µL, non-RBC 12,122/µL with 95% lymphocytes. Flow cytometry of the pleural fluid with predominant clonal B cell population matching previously diagnosed CLL.
On further review of the CT of the abdomen and pelvis in the tumor boards, another radiologist indicated that the Hounsfield units for the previously reported perinephric opacity were consistent with subcapsular hematoma in the left kidney (Figure3 and 4). After transfusion and prednisone support, his hemoglobin and platelets gradually improved, and he was discharged on a prednisone taper with close follow up in hematology clinic.
After hospital discharge, the patient remained asymptomatic and his CBC recovered (Table 1). Repeat CT of the abdomen and pelvis without contrast after a month of hospital discharge showed decrease in size and density of left renal subcapsular hematoma and resolution of perinephric soft tissue stranding (Figure 5 and 6). After further discussions and consideration of other treatment options including oral chlorambucil or IV obinutuzumab based regimens, the patient opted for no further treatment and remained on expectant management without significant disease progression.
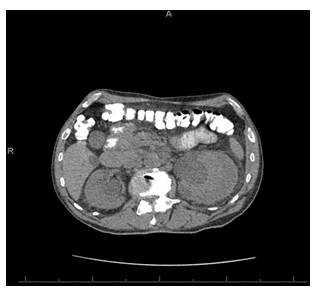
Figure 5: CT abdomen/pelvis without IV contrast (after 1 month) showing decrease in size and density of the left renal subcapsular hematoma.
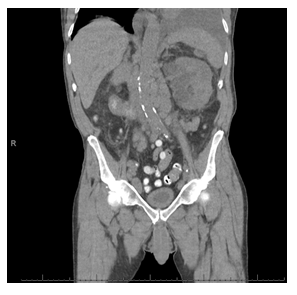
Figure 6: CT abdomen/pelvis without IV contrast (after 1 month) showing decrease in left renal subcapsular hematoma and resolution of soft tissue stranding.
Table 1: Laboratory results.
3. Discussion
Ibrutinib is an irreversible BKT inhibitor which is a highly effective first line oral therapy in CLL. It's use as an initial therapy is associated with thrombocytopenia, anemia and neutropenia in 2%, 6%, and 10% of patients, respectively. It can also cause pneumonia and pleural effusion in 4% and 2% of patients respectively. Atrial fibrillation occurred in about 6% of patients taking ibrutinib [4].
Early clinical trials of ibrutinib revealed increased incidences of major bleeding including gastrointestinal bleeding, intracranial bleeding, subdural hematomas, and hematuria leading to exclusion of patients on Vitamin K antagonists in newer studies [9]. Therefore, patients are instructed to avoid vitamin K antagonists concurrently with Ibrutinib; direct oral anticoagulants (DOACs) should be used if long term anticoagulation is strongly indicated. In the absence of concurrent anticoagulation and antiplatelet therapy, the incidence of ibrutinib associated bleeding is 28% for all grades, with 4% classified as grade III-IV [3].
With increased use of ibrutinib, reports of major hemorrhage associated with this agent has been publicized [10], including intracranial hemorrhage [11], diffuse alveolar hemorrhage [12], and intra-muscular hemorrhage [13]. Although rare cases of spontaneous hemothorax while on Ibrutinib have been reported [14], spontaneous renal subcapsular hematoma secondary to ibrutinib has not been previously reported as per our database search.
The mechanism of hemorrhage due to ibrutinib involves more than inhibition of BTK. Besides irreversible inhibition of BTK, ibrutinib also inhibits several other intracellular molecules involved in platelet signaling including non-receptor tyrosine kinase TEC (tyrosine kinase expressed in hepatocellular carcinoma). BTK and TEC play a role in collagen and collagen-related peptide signaling through the platelet collagen receptor glycoprotein VI (GPVI) (Fig. 7) and C-type lectin-like receptor 2 (CLEC-2) pathways [2, 3]. Inhibition of both BTK and TEC in these pathways results in reduced collagen-mediated platelet aggregation and decreased thrombus stability after platelet adhesion to damaged endothelium [3]. BTK is also involved in the von Willebrand factor (vWF) - Gp1b signaling pathway [15].With BTK inhibition, there is minimal vWF-induced platelet adhesion under high shear stress, particularly in the microvasculature. Fibrinogen binds to Integrin αIIbβ3 on platelets, which activates a signaling cascade that also involves BTK. Inhibition of this pathway leads to decreased clot formation with reduced platelet adhesion, spreading, and clot retraction [3]. This is illustrated and summarized in Figure 7.
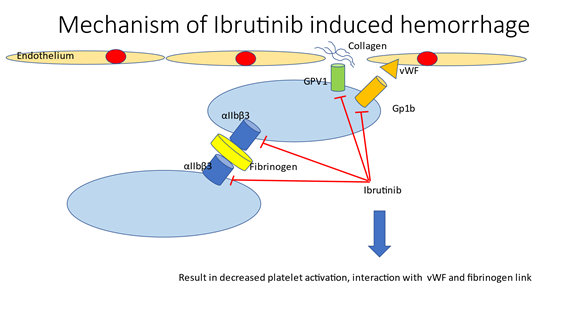
Figure 7: Mechanism of Ibrutinib induced hemorrhage. GPVI, Platelet collagen receptor-Glycoprotein VI; vWF, von Willebrand Factor.
Another reason for increase hemorrhage is drug displacement with increased plasma levels of anticoagulation drugs like dabigatran when concurrently used with ibrutinib [16].
The incidence of major hemorrhage seems to be related with race (nonwhite), gender (male), age (>75 years), history of major hemorrhage, hepatic disease, renal impairment, anemia, thrombocytopenia, coronary artery disease, atrial fibrillation, and alcohol use when compared with the group without major hemorrhage according to Georgantopoulos et al [10]. In this patient, he had at least 3 factors that contributed to hemorrhage, including gender, age, and renal failure which worsened with renal hemorrhage. The patient was treated with a reduced dose of ibrutinib at the onset to try to minimize complications, but despite this reduced dose, the patient unfortunately developed life threatening hemorrhage event. Nevertheless, the use of ibrutinib was effective for his CLL since he had a significant response of his adenopathy with resolution of his neck nodes and other nodes noted in prior CT. Next generation BTK inhibitors are considered to be more selective for BTK with less off target kinase inhibition which may reduce bleeding complications as compared to ibrutinib [17, 18].
4. Conclusions
Ibrutinib is an established and effective therapy for treatment of CLL but can result in increased incidence of bleeding through inhibition of several platelet aggregation pathways. While the bleeding risk is well established with ibrutinib, this case report illustrates the occurrence of a rare case of ibrutinib-associated spontaneous subcapsular renal hematoma and spontaneous hemothorax; even at a lower dose. Initiation of ibrutinib should be done cautiously in elderly patients who have other risk factors for bleeding and should be closely followed for potential severe hemorrhagic complications.
References
- Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton's tyrosine kinase in B cells and malignancies. Mol Cancer 17 (2018): 57.
- Series J, Ribes A, Garcia C, et al. Effects of novel Btk and Syk inhibitors on platelet functions alone and in combination in vitro and in vivo. J Thromb Haemost 18 (2020): 3336-3351.
- Shatzel JJ, Olson SR, Tao DL, et al. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J Thromb Haemost 15 (2017): 835-847.
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 373 (2015): 2425-2437.
- Treon SP, Gustine J, Meid K, et al. Ibrutinib Monotherapy in Symptomatic, Treatment-Naive Patients With Waldenstrom Macroglobulinemia. J Clin Oncol 36 (2018): 2755-2761.
- Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood 136 (2020): 2038-2050.
- Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 387 (2016): 770-778.
- Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 129 (2017): 2224-2232.
- Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol 17 (2016): 200-211.
- Georgantopoulos P, Yang H, Norris LB, et al. Major hemorrhage in chronic lymphocytic leukemia patients in the US Veterans Health Administration system in the pre-ibrutinib era: Incidence and risk factors. Cancer Med 8 (2019): 2233-2240.
- Martin-Moro F, Garcia-Cosio M, et al. Intracranial hemorrhage as presentation of chronic lymphocytic leukemia successfully treated with ibrutinib. Ann Hematol (2021).
- Locke CB, Jr Lansigan F. Diffuse Alveolar Hemorrhage Secondary to Ibrutinib Therapy in a Patient with Refractory Mantle Cell Lymphoma. Cureus 12 (2020): e8743.
- Sarcon A, Botta GP, Patel N, et al. Spontaneous Iliopsoas Muscle Hemorrhage Secondary to Ibrutinib (Imbruvica; Pharmacyclics): Brief Report. J Investig Med High Impact Case Rep 4 (2016): 2324709616648457.
- Lehr A, Condos R. Spontaneous Hemothorax in a Patient on Ibrutinib.American Thoracic Society 2020 International Conference: American Journal of Respiratory and Critical Care Medicine (2020).
- Caron F, Leong DP, Hillis C, et al. Current understanding of bleeding with ibrutinib use: a systematic review and meta-analysis. Blood Adv 1 (2017): 772-778.
- Quintavalla R, Lombardi M, Prandoni P, et al. Increased dabigatran plasma concentration during Ibrutinib treatment: a case of cerebral hemorrhage and successful dabigatran reversal by idarucizumab. Aging Clin Exp Res 30 (2018): 93-95.
- Rhodes JM, Mato AR. Zanubrutinib (BGB-3111), a Second-Generation Selective Covalent Inhibitor of Bruton's Tyrosine Kinase and Its Utility in Treating Chronic Lymphocytic Leukemia. Drug Des Devel Ther 15 (2021): 919-926.
- Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol 9 (2016): 21.
