Hysteroscopic Imaging of Health Endometrium in Premenopausal Breast Cancer Patients Taking Anti-estrogen Therapies
Article Information
Giancarlo Garuti1*, Paola Francesca Sagrada2, Maurizio Mirra3, Serena Migliaccio1, Andrea Finco1, Marco Soligo1
1Gynecology and Obstetrics Department, Public Hospital of Lodi, via Savoia 1, 26900-Lodi, Italy
2Medical Oncology Department, Public Hospital of Lodi, via Savoia 1, 26900-Lodi, Italy
3Pathology Department, Public Hospital of Lodi, via Savoia 1, 26900-Lodi, Italy
*Corresponding Author: Giancarlo Garuti, Gynecology and Obstetrics Department, Public Hospital of Lodi, via Savoia 1, 26900-Lodi, Italy.
Received: 06 July 2023; Accepted: 13 July 2023; Published: 21 July 2023
Citation: Giancarlo Garuti, Paola Francesca Sagrada, Maurizio Mirra, Serena Migliaccio, Andrea Finco, Marco Soligo. Hysteroscopic Imaging of Health Endometrium in Premenopausal Breast Cancer Patients Taking Anti-estrogen Therapies. Obstetrics and Gynecology Research. 6 (2023): 197-203
View / Download Pdf Share at FacebookAbstract
Purpose:
To assess the physiological response of endometrium to different anti-estrogenic treatments in premenopausal breast-cancer patients, based on hysteroscopy imaging and endometrial biopsy.
Methods:
We reviewed the hysteroscopic findings of 26 patients suffering from breast cancer currently taking anti-estrogenic treatments and in whom an endometrial biopsy yielded a diagnosis of functional conditions. Fourteen patients were treated by Tamoxifen (Tam), 8 with Tam and Gonadotropin-Releasing Hormone (Gn-RH) analogues, 1 with Tam and Gn-RH analogue plus Megestrole acetate and 3 with Exemestane and Gn-RH analogue. Hysteroscopic findings were related to each scheduled treatment.
Results:
Proliferative, secretory and hyperplastic endometrial features have been found in all patients taking Tam. The addition of Gn-RH analogue to Tam led invariably to atrophic endometrial imaging, exhibiting gland cyst differentiation in half of the cases. A progestogen agent addition, such as Megestrole acetate to this latter schedule induced endometrial hyperplastic-like appearances sustained by stromal decidualization. In patients undergoing Exemestane and Gn-RH analogue association we found inactive endometrium and proliferative features in two and one case, respectively.
Conclusion:
In premenopausal patients, the anti-estrogenic drug schedule to treat estrogen-receptor positive breast cancer leads to different but quite predictable molding effects on endometrium. Due to the frequent need for gynecological consultation and endometrial assessment in this group of women, hysteroscopy practitioners should gain awareness about the expected iatrogenic effects of anti-estrogens on endometrial lining.
Keywords
Anti-estrogen therapy; Breast cancer; Endometrium; Exemestane; Gonadotropin Realising-Hormone analogues; Hysteroscopy; Tamoxifen
Anti-estrogen therapy articles Anti-estrogen therapy Research articles Anti-estrogen therapy review articles Anti-estrogen therapy PubMed articles Anti-estrogen therapy PubMed Central articles Anti-estrogen therapy 2023 articles Anti-estrogen therapy 2024 articles Anti-estrogen therapy Scopus articles Anti-estrogen therapy impact factor journals Anti-estrogen therapy Scopus journals Anti-estrogen therapy PubMed journals Anti-estrogen therapy medical journals Anti-estrogen therapy free journals Anti-estrogen therapy best journals Anti-estrogen therapy top journals Anti-estrogen therapy free medical journals Anti-estrogen therapy famous journals Anti-estrogen therapy Google Scholar indexed journals Breast cancer articles Breast cancer Research articles Breast cancer review articles Breast cancer PubMed articles Breast cancer PubMed Central articles Breast cancer 2023 articles Breast cancer 2024 articles Breast cancer Scopus articles Breast cancer impact factor journals Breast cancer Scopus journals Breast cancer PubMed journals Breast cancer medical journals Breast cancer free journals Breast cancer best journals Breast cancer top journals Breast cancer free medical journals Breast cancer famous journals Breast cancer Google Scholar indexed journals Endometrium articles Endometrium Research articles Endometrium review articles Endometrium PubMed articles Endometrium PubMed Central articles Endometrium 2023 articles Endometrium 2024 articles Endometrium Scopus articles Endometrium impact factor journals Endometrium Scopus journals Endometrium PubMed journals Endometrium medical journals Endometrium free journals Endometrium best journals Endometrium top journals Endometrium free medical journals Endometrium famous journals Endometrium Google Scholar indexed journals Exemestane articles Exemestane Research articles Exemestane review articles Exemestane PubMed articles Exemestane PubMed Central articles Exemestane 2023 articles Exemestane 2024 articles Exemestane Scopus articles Exemestane impact factor journals Exemestane Scopus journals Exemestane PubMed journals Exemestane medical journals Exemestane free journals Exemestane best journals Exemestane top journals Exemestane free medical journals Exemestane famous journals Exemestane Google Scholar indexed journals Gonadotropin Realising-Hormone analogues articles Gonadotropin Realising-Hormone analogues Research articles Gonadotropin Realising-Hormone analogues review articles Gonadotropin Realising-Hormone analogues PubMed articles Gonadotropin Realising-Hormone analogues PubMed Central articles Gonadotropin Realising-Hormone analogues 2023 articles Gonadotropin Realising-Hormone analogues 2024 articles Gonadotropin Realising-Hormone analogues Scopus articles Gonadotropin Realising-Hormone analogues impact factor journals Gonadotropin Realising-Hormone analogues Scopus journals Gonadotropin Realising-Hormone analogues PubMed journals Gonadotropin Realising-Hormone analogues medical journals Gonadotropin Realising-Hormone analogues free journals Gonadotropin Realising-Hormone analogues best journals Gonadotropin Realising-Hormone analogues top journals Gonadotropin Realising-Hormone analogues free medical journals Gonadotropin Realising-Hormone analogues famous journals Gonadotropin Realising-Hormone analogues Google Scholar indexed journals Hysteroscopy articles Hysteroscopy Research articles Hysteroscopy review articles Hysteroscopy PubMed articles Hysteroscopy PubMed Central articles Hysteroscopy 2023 articles Hysteroscopy 2024 articles Hysteroscopy Scopus articles Hysteroscopy impact factor journals Hysteroscopy Scopus journals Hysteroscopy PubMed journals Hysteroscopy medical journals Hysteroscopy free journals Hysteroscopy best journals Hysteroscopy top journals Hysteroscopy free medical journals Hysteroscopy famous journals Hysteroscopy Google Scholar indexed journals Tamoxifen articles Tamoxifen Research articles Tamoxifen review articles Tamoxifen PubMed articles Tamoxifen PubMed Central articles Tamoxifen 2023 articles Tamoxifen 2024 articles Tamoxifen Scopus articles Tamoxifen impact factor journals Tamoxifen Scopus journals Tamoxifen PubMed journals Tamoxifen medical journals Tamoxifen free journals Tamoxifen best journals Tamoxifen top journals Tamoxifen free medical journals Tamoxifen famous journals Tamoxifen Google Scholar indexed journals progesterone-receptor articles progesterone-receptor Research articles progesterone-receptor review articles progesterone-receptor PubMed articles progesterone-receptor PubMed Central articles progesterone-receptor 2023 articles progesterone-receptor 2024 articles progesterone-receptor Scopus articles progesterone-receptor impact factor journals progesterone-receptor Scopus journals progesterone-receptor PubMed journals progesterone-receptor medical journals progesterone-receptor free journals progesterone-receptor best journals progesterone-receptor top journals progesterone-receptor free medical journals progesterone-receptor famous journals progesterone-receptor Google Scholar indexed journals estrogen-receptor articles estrogen-receptor Research articles estrogen-receptor review articles estrogen-receptor PubMed articles estrogen-receptor PubMed Central articles estrogen-receptor 2023 articles estrogen-receptor 2024 articles estrogen-receptor Scopus articles estrogen-receptor impact factor journals estrogen-receptor Scopus journals estrogen-receptor PubMed journals estrogen-receptor medical journals estrogen-receptor free journals estrogen-receptor best journals estrogen-receptor top journals estrogen-receptor free medical journals estrogen-receptor famous journals estrogen-receptor Google Scholar indexed journals premenopausal articles premenopausal Research articles premenopausal review articles premenopausal PubMed articles premenopausal PubMed Central articles premenopausal 2023 articles premenopausal 2024 articles premenopausal Scopus articles premenopausal impact factor journals premenopausal Scopus journals premenopausal PubMed journals premenopausal medical journals premenopausal free journals premenopausal best journals premenopausal top journals premenopausal free medical journals premenopausal famous journals premenopausal Google Scholar indexed journals
Article Details
Introduction
The administration of anti-estrogen drugs improves the clinical outcome of estrogen-receptor (ER) and/or progesterone-receptor (PgR) positive breast cancer patients, both in premenopausal and postmenopausal settings. The selective estrogen receptor modulator Tamoxifen, the third generation Aromatase inhibitors (Letrozole, Anastrozole and Exemestane) and the Gonadotropin Releasing Hormone (Gn-RH) analogues are widely used drugs, either alone or in combination, to antagonize the activation of ER and to inhibit estrogen synthesis in peripheral and ovarian tissues[1-3]. The ER (α and β) and PgR (A and B) families are highly represented in stromal and epithelial cells of endometrium, accounting for its moulding caused by exposure to drugs impairing the biochemical pathways of ER activation[4]. The classic paradigm of this assumption in breast cancer patients is represented by the effect of Tamoxifen on endometrium of menopausal women, based on its tissue-specific agonistic function on the ER metabolic pathway and leading to the promotion of either physiological or pathological estrogen-related mucosal growth[5]. Conversely, Aromatase Inhibitors (AI) administration in postmenopausal people, as expected due to the different biochemistry pathways with respect to Tamoxifen, did not show a significant stimulatory effect on endometrium[6]. In premenopausal women with breast cancer, the endometrial response to anti-estrogen drugs is less understood and no guideline for endometrial surveillance is currently traced[7]. Nevertheless, due to the genetic and acquired risk factors shared by breast and endometrial cancer, a planned assessment of endometrial lining, based mainly on transvaginal ultrasonography, is warranted[8]. Moreover, gynecological complaints such as abnormal uterine bleeding or oligomenorrhea, more frequently observed in women taking only Tamoxifen, often leads the oncologist to recommend gynecological consultations[9]. The current reference diagnostic of endometrial diseases is represented by hysteroscopy with or without endometrial biopsy, overcoming some limits of procedures based on blind endometrial sampling[10]. The proposal of the present report is to assess the hysteroscopic imaging in premenopausal patients currently treated with antiestrogen therapies for breast cancer in whom endometrial histology yielded functional findings.
Patients and Methods
Study design and Patient Selection
This is a single-centre retrospective observational study carried-out in the public Hospital of Lodi (Italy). It is based on the review of a hysteroscopic video-clips collection of premenopausal breast cancer patients undergoing various anti-estrogen schedules of hormonal therapy throughout a 5 years period, starting in January 2017 through December 2022. All video-clips were obtained from the personal data base of one author (G.G.). The clinical and pathological findings were retrieved from our Institutional data base. We selected patients with a video-recorded hysteroscopic examination who underwent an endometrial biopsy resulting in functional histology. Hysteroscopy was performed as a second-line investigation after gynecological physical examination and/or transvaginal ultrasound assessment of the endometrial lining presented abnormal findings. An abnormal uterine bleeding was always considered an indication for hysteroscopic assessment. Moreover, a transvaginal ultrasonography showing a non-homogeneous endometrial echotexture in the proliferative phase of menstrual cycle or an endometrial thickness above 5 mm on the longitudinal scan in amenorrheic and asymptomatic patients undergoing Gn-RH analogues treatment were arbitrarily considered indications for hysteroscopy assessment. The endoscopic imaging was related to four schedules of adjuvant hormonal treatment currently administered to treat ER positive breast cancer. Given the retrospective and observational nature of the study, based on procedures performed as part of routine care, the local Ethical Committee approval was waived. This study was performed in line with the principles of the 1964 Declaration of Helsinki and its amendments.
Hysteroscopy and Imaging Classification
Both clinical-outpatient and inpatient hysteroscopic procedures were carried-out. All examinations were performed by means of video-assistance using 16-18Fr operative hysteroscopes and conducted by vaginoscopic uterine entering using saline as the distending medium. In all cases endometrial biopsy was accomplished by mechanical instrumentation using 5Fr scissors and grasping forceps for fashioning and tissue retrieval, respectively. Based on previously described patterns of hysteroscopic morphology, we classified hysteroscopic imaging as follows[10-12]:
i. Atrophic Endometrium: Describing a thin, smooth mucosa evenly shaping the endometrial cavity showing few gland openings and cases in which no gland openings were found while a very thin endometrium mirrored the trabecular pattern of underlying myometrium.
ii. Cystic Atrophy: Focal mucosal cyst-gland prominences sometimes with button-like features (psammoma bodies) in an atrophic environment as described above.
iii. Functional Endometrium: Displaying both proliferative and secretory pathways. Proliferative features were characterised by a smooth endometrial surface or minimal roughness in an evenly-lined uterine cavity, showing a regular arrangement of gland openings. Secretory endometrium identified a thicker evenly-lined mucosa, with a smooth and velvety surface maintaining regularly-spaced gland openings.
iv. Hyperplastic Endometrium: Describing a focal or extended thickened endometrium with a rough, uneven surface showing polypoid projections with gland-cysts and irregularly-spaced or crowded gland openings.
Results
During the study period, we found and reviewed 35 hysteroscopic video-clips of premenopausal breast cancer patients undergoing anti-oestrogen therapies. Five women were excluded from analysis due to pathological reports of non-atypical (2 cases) or atypical (1 case) hyperplasia and endometrial polyp (2 cases), while 4 patients were excluded due to incomplete medical records. Overall, we evaluated the hysteroscopy imaging of 26 patients with normal findings at pathological assessment (Figure 1). The clinical data of these women are resumed in Table 1. Among these, only Tamoxifen was administered to 14 patients, Tamoxifen and Gn-RH analogue to 8, Tamoxifen and Gn-RH analogue plus Megestrole acetate to 1 and Exemestane and Gn-RH analogue to 3 patients. Abnormal uterine bleeding, consisting of inter-menstrual bleeding, periodic heavy menstrual bleeding and poly-menorrhea was reported on 20 patients (76.9%). In Table 2 we show the administered hormonal schedules related to both hysteroscopic imaging and histologic reports. Out of the 14 women taking Tamoxifen monotherapy, hysteroscopic-view displayed proliferative or secretory functional features in 10 cases and all the imaging findings were confirmed at histologic assessment. In 4 cases (28.5%) hysteroscopic-view suggested an endometrial hyperplasia whereas the biopsy pathology reported a diagnosis of functional proliferative endometrium (Figure 2). The combination of Tamoxifen with a Gn-RH analogue was investigated in 8 patients. Hysteroscopic inspection showed atrophic endometrial features in all cases, with gland-cyst differentiation found in half of them (Figure 3). Atrophic endometrium was confirmed through biopsy pathology assessments in all cases. In one patient Megestrole acetate was added to Tamoxifen and Gn-RH analogue schedule for control of severe hot-flash symptoms. In that unique case, endometrial hyperplasia was suspected on hysteroscopic-view whereas a functional finding of stromal pseudo-decidualization was reported by histologic assessment (Figure 4). In the 3 patients undergoing therapy with Exemestane and Gn-RH analogue, hysteroscopic-view showed proliferative and atrophic patterns in 1 and 2 patients, respectively (Figure 5). An agreement with histologic findings on biopsy specimens has been obtained in all three cases.
|
Mean age, years (range) |
43.4 (32-53) |
|
Mean Body Mass Index (range) |
25.9 (18-34) |
|
Previous Chemotherapy |
10 patients |
|
Mean time of antiestrogenic drugs administration, months (range) |
24.5 (6-56) |
|
Bleeding symptoms |
5 patients |
|
Abnormal Transvaginal Sonography |
6 patients |
|
Bleeding symptoms and Abnormal Transvaginal Sonography |
15 patients |
Table 1: Clinical parameters of 26 premenopausal breast cancer patients currently treated by anti-estrogen hormonal therapy who underwent hysteroscopic assessment due to gynecological complaints
|
Hormone schedule |
Patients |
Hysteroscopic-view |
Histology |
|
Tamoxifen (20 mg/day) |
14 |
Proliferative 6 |
Proliferative 10 |
|
Tamoxifen (20mg/day) / Gn-RH analogue (Leuprolide, three monthly 11.25 mg) |
8 |
Atrophic 4 |
Atrophic 8 |
|
Tamoxifen / Gn-RH analogue / Megestrole acetate (20 mg / day) |
1 |
Hyperplasia 1 |
Stromal decidualization 1 |
|
Exemestane (25 mg/day) / Gn-RH analogue |
3 |
Proliferative 1 |
Proliferative 1 |
Table 2: Hysteroscopic-view and histologic findings following an endometrial biopsy are related to four different schedules of anti-estrogen therapy in 26 premenopausal patients with breast cancer
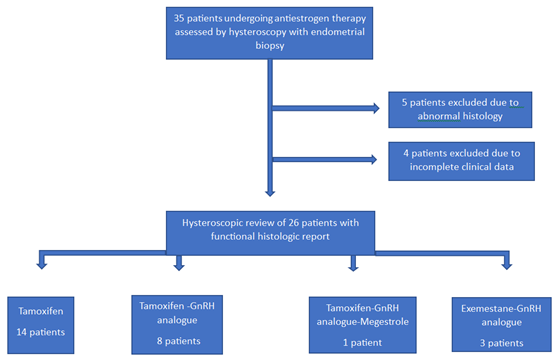
Figure 1: Flow-chart diagram of premenopausal breast-cancer patients included in the study
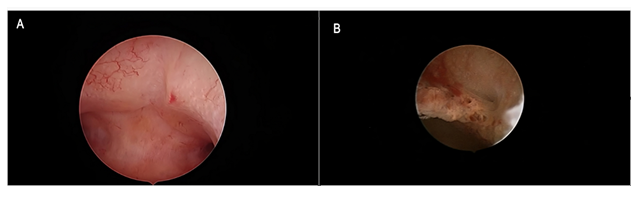
Figure 2: Tamoxifen monotherapy. Figure 2a: Hysteroscopic view of anterior uterine wall showing regular arrangement of gland openings in a background of evenly and lightly thickened mucosa. The hysteroscopic diagnosis of proliferative endometrium was confirmed through biopsy pathology. Figure 2b: Focal polypoid rough growth showing enhanced vascular network and crowding of gland openings suggested endometrial hyperplasia during hysteroscopic inspection. Functional proliferative endometrium was found at histologic assessment.
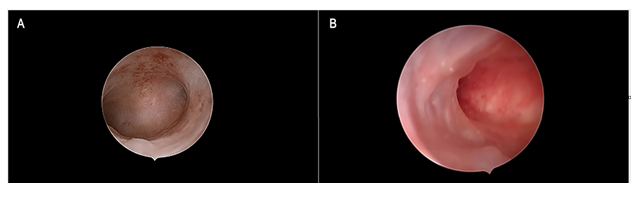
Figure 3: Tamoxifen and Gn-RH analogue Figure 3a: Panoramic view of the endometrial cavity showing an evenly thin atrophic endometrium. Figure 3b: Focal, smooth endometrial thickenings due to under-epithelial gland cysts formation are shown. Such as in Figure 3a, an atrophic picture was reported by histologic assessment.
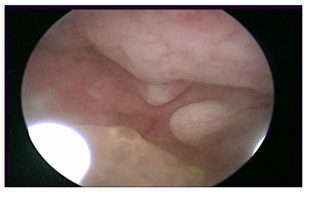
Figure 4: Tamoxifen, Gn-RH analogue and Megestrole acetate
An evenly-extended appearance of endometrial polypoid overgrowth suggested endometrial hyperplasia on hysteroscopic-view whereas functional stromal decidualization was recorded on biopsy pathology report.
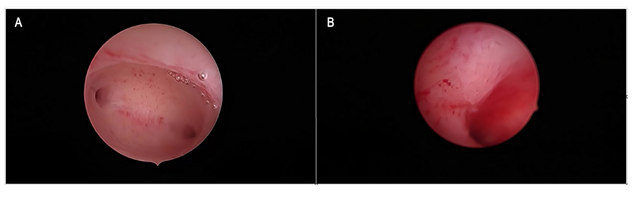
Figure 5: Exemestane and Gn-RH analogue Figure 5a: Panoramic hysteroscopic-view showing an evenly atrophic endometrial shape, confirmed by histologic assessment. Figure 5b: A slightly thickened endometrial lining showing the maintenance of a gland-openings network was found on hysteroscopic imaging and described as proliferative endometrium; the diagnosis was confirmed on biopsy specimen histology.
Discussion
The presented results, based on hysteroscopic imaging findings supported by histology reports of health endometrium, suggest that the iatrogenic response of endometrial morphology to anti-estrogenic drugs administered to treat ER-positive breast cancer in premenopausal women is quite predictable. The biochemical pathway activated by the Selective Oestrogen Receptor Modulator, Tamoxifen, on the hypothalamic-pituitary axis is not well understood. Nevertheless, clinical studies showed that the drug did not impair ovarian function and its single use in breast cancer patients can frequently up-regulate the synthesis and secretion of gonadotropins, often leading to ovarian hyperstimulation[9,13]. Accordingly, in all patients undergoing Tamoxifen monotherapy we found hysteroscopic imaging and biopsy pathology findings to be consistent with functional proliferative or secretory features frequently characterised by a hyperplastic-like growth. These pictures are representative of maintained ovarian endocrine function, possibly sometimes enhanced by hyperstimulation leading to a hyper-estrogenic condition responsible for functional endometrial overgrowth. The Gn-RH analogues down-regulate the hypothalamic synthesis of Gn-RH resulting in the reversible block of pituitary Gonadotropins release leading to the suppression of ovarian steroidogenesis. We found that the concurrent administration of Tamoxifen, although known to act as an ER-partial agonist on endometrial tissue, does not overcome the inhibition of endometrial growth promoted by the Gn-RH analogue suppression of ovarian steroidogenesis[5,7]. In all 8 women undergoing combined Gn-RH analogue and Tamoxifen treatment, both hysteroscopic imaging and histopathology were consistent with atrophic endometrium. Nevertheless, based on hysteroscopic imaging and histology findings, some biochemical pathways possibly induced by Tamoxifen-related ER activation on endometrium appear maintained. The frequent ultrasound and hysteroscopic findings of endometrial cyst-gland differentiation in postmenopausal Tamoxifen users is related to exuberant focal stromal cells proliferation leading to the obstruction of gland ducts resulting in mucous trapping and cyst formation. Such endometrial inspective appearance was found in half of the premenopausal patients here examined and we can suppose that a similar physiopathology may be shared with respect to menopausal women. Secondly, the addition of a progestogen agent such as Megestrole acetate to a Gn-RH analogue/Tamoxifen schedule led to a hyperplastic-like imaging caused by stromal decidualisation, as observed in one patient. This endometrial differentiation following progestin administration cannot be expected in absence of oestrogen-induced synthesis of PgR, in this case likely promoted and maintained by the ER activation due to the chronic intake of Tamoxifen. The endometrial response of combined Exemestane and Gn-RH analogue therapy was assessed in 3 women and in one of them hysteroscopic imaging and histology were consistent with proliferative features. Exemestane is a steroid drug acting as an irreversible inhibitor of aromatase enzyme with the potential binding and activation of endometrial ERs. Although in vitro studies suggest that Exemestane does not bind to ERα, clinical reports on postmenopausal patients indicate that a weak endometrial stimulation induced by its intake may be expected[14,15]. Therefore, we can assume that also in premenopausal women undergoing a concurrent ovarian suppression, Exemestane may maintain some stimulatory effects on endometrium by its direct binding to ER. The main weakness of this study is represented by the low number of patients recruited for analysis. However, to our knowledge no study aimed to assess the physiological response of endometrium to anti-estrogenic drugs administered in premenopausal women has been produced in current literature. Due to the wider indications of endocrine treatments in ER-positive premenopausal breast cancer patients, in whom frequent gynaecological consultations are required, hysteroscopy practitioners should gain awareness about the iatrogenic impact on endometrial morphology caused by antioestrogen drug administration. Indeed, further studies on this topic on a larger number of patients are warranted.
Acknowledgments and Funding Information
We thank Caroline Calnan Sagrada, a native English speaker experienced in reviewing medical papers, for the support in English grammar and style revision of the manuscript. All authors have no conflict of interest or financial conflicts to disclose. All authors declare that no funds were received during the preparation of the manuscript.
Statement & Declarations
Funding:
The authors declare that no funds, grants or other support were received during the preparation of the manuscript
Competing Interests:
The authors have no financial or non-financial interests to disclose.
Author Contributions:
All authors contributed to the study conception and design. Analysis, investigation and data collection were performed by Giancarlo Garuti. Analysis and material preparation were performed by Paola Francesca Sagrada. Investigation and data collection were performed by Maurizio Mirra. Material preparation and data curation were performed by Serena Migliaccio. Material preparation and data curation was performed by Andrea Finco. Analysis and writing-review was performed by Marco Soligo. The first draft of the manuscript was written by Giancarlo Garuti and all authors commented on previous versions of manuscript. All authors read and approved the final manuscript.
Data Availability:
The datasets generated and/or analysed during the current study are not publicly available due to the rules of patient’ privacy but are available from the corresponding author upon reasonable request.
Ethics Approval:
This study was performed in-line with the principle of the Declaration of Helsinky. This is an observational and retrospective study based on routinely delivered care. The Research Ethics Committee of the Public Sanitary Utility of Lodi (Italy) has confirmed that no ethical approval is required.
References
- Francis PA, Regan MM, Fleming GF et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372 (2015): 436-446.
- Pagani O, Regan MM, Walley BA et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371 (2014): 107-118.
- Burstein HJ, Lacchetti C, Anderson H et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 37 (2019): 423-438.
- Hewitt SC, Winuthayanon W, Korach KS. What’s new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol 56 (2016): 55-71.
- Perez-Medina T, Salazar FJ, San-Frutos L, et al. Hysteroscopic dynamic assessment of the endometrium in patients treated with long-term tamoxifen. J Minim Invasive Gynecology 18 (2011): 349-354.
- Garuti G, Cellani F, Centinaio G, et al. Prospective endometrial assessment of breast cancer patients treated with third generation aromatase inhibitors. Gynecol Oncol 103 (2006): 599-603.
- Lee M, Piao J, Jae Jeon M. Risk factors associated with endometrial pathology in premenopausal breast cancer patients treated with tamoxifen. Yousei Med J 61 (2020): 317-322.
- Chlebowski RT, Schottinger JE, Shi J, et al. Aromatase inhibitor, tamoxifen and endometrial cancer in breast cancer survivors. Cancer 121 (2015): 2147-2155.
- Yamazaki R, Inokuchi M, Ishikawa S, et al. Ovarian hyperstimulation closely associated with resumption of follicular growth after chemotherapy during tamoxifen treatment in premenopausal women with breast cancer: a multicenter retrospective cohort study. BMC Cancer 20 (2020): 67.
- Garuti G, Sambruni I, Colonnelli M, et al. Accuracy of hysteroscopy in predicting histopathology of endometrium in 1500 women. J Am Assoc Gynecol Laparosc 8 (2001): 207-213.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Inv Gynecol 22 (2015): 1215-1224.
- Garuti G, Colonnelli M, Soliani A, et al. Estro-progestin and progestogen intake: what’s the impact on hysteroscopic imaging? Fact View Obgyn 15 (2023): 61-67.
- Berliere M, Duhoux FP, Dalenc F, et al. Tamoxifen and ovarian function. PloS one 8 (2013): e66616.
- Ingle JN, Cairns J, Suman VJ et al. Anastrozole has an association between degree of estrogen suppression and outcomes in early breast cancer and is a ligand for estrogen receptor α. Clin Cancer Res 26 (2020): 2986-2996.
- Kieback DG, Harbeck N, Bauer W, et al. Endometrial effects of exemestane compared to tamoxifen within the Tamoxifen Exemestane Adjuvant Multicenter (TEAM) trial: results of a prospective gynecological ultrasound sub-study. Gynecol Oncol 119 (2010): 500-505.
