Hypoxic Coma Due to Takotsubo Syndrome after Toxic Carbon Monoxide Poisoning
Article Information
Rita Formisano1*, Cesare Greco2, Marta Aloisi1, Marianna Contrada1, Chiara Falletta Caravasso1, Giacomo Luccichenti1, Maria Gabriella Buzzi1
1IRCCS Santa Lucia Foundation, Rome, Italy
2San Giovanni-Hospital, Rome, Italy
*Corresponding Author: Dr. Rita Formisano, IRCCS Fondazione Santa Lucia, Via Ardeatina 306, 00179 Rome, Italy
Received: 10 March 2020; Accepted: 14 April 2020; Published: 03 July 2020
Citation:
Rita Formisano, Cesare Greco, Marta Aloisi, Marianna Contrada, Chiara Falletta Caravasso, Giacomo Luccichenti, Maria Gabriella Buzzi. Hypoxic Coma Due to Takotsubo Syndrome after Toxic Carbon Monoxide Poisoning. Archives of Clinical and Medical Case Reports 4 (2020): 574-579.
View / Download Pdf Share at FacebookAbstract
Objectives: Takotsubo syndrome (TS) is clinically characterized as an acute coronary syndrome with rapid development and subsequent resolution of severe, reversible left ventricular dysfunction. We describe a case of TS induced by an acute, severe reduction of oxygen supply resulting in a life threatening carbon monoxide poisoning and leading to coma. A 58 year-old woman was referred to Emergency Room (ER) with cardiogenic shock and hypoxic coma.
Materials and Methods: At ER admission, the Glasgow Coma Scale score was 5 and she needed intensive cardio-pulmonary support and mechanical ventilation. A malfunctioning of the heating system in her bedroom was demonstrated, thus the diagnosis of carbon monoxide poisoning was applied. After a first recovery of consciousness, her responsiveness progressively worsened to a new coma occurrence as by a delayed leukoencephalopathy. At admission in rehabilitation, the patient was diagnosed as vegetative state/unresponsive wakefulness syndrome, according to the Coma Recovery Scale-Revised. After intensive multidisciplinary rehabilitation, the patient evolved from Minimally Conscious State Minus (akinetic mutism) to MCS Plus and finally emerged to Exit-MCS. At 9 months follow-up, the patient recovered a complete autonomy in daily living activities. This is the first case report of TS after toxic carbon monoxide poisoning.
Keywords
Takotsubo syndrome; Hypoxic coma; Delayed post-hypoxic leukoencephalopathy; Vegetative State; Unresponsive Wakefulness Syndrome; Carbon monoxide poisoning; Parkinsonism; Disorders of consciousness; Akinetic mutism
Takotsubo syndrome articles, Hypoxic coma articles, Delayed post-hypoxic leukoencephalopathy articles, Vegetative State articles, Unresponsive Wakefulness Syndrome articles, Carbon monoxide poisoning articles, Parkinsonism articles, Disorders of consciousness articles, Akinetic mutism articles
Takotsubo syndrome articles Takotsubo syndrome Research articles Takotsubo syndrome review articles Takotsubo syndrome PubMed articles Takotsubo syndrome PubMed Central articles Takotsubo syndrome 2023 articles Takotsubo syndrome 2024 articles Takotsubo syndrome Scopus articles Takotsubo syndrome impact factor journals Takotsubo syndrome Scopus journals Takotsubo syndrome PubMed journals Takotsubo syndrome medical journals Takotsubo syndrome free journals Takotsubo syndrome best journals Takotsubo syndrome top journals Takotsubo syndrome free medical journals Takotsubo syndrome famous journals Takotsubo syndrome Google Scholar indexed journals syndrome articles syndrome Research articles syndrome review articles syndrome PubMed articles syndrome PubMed Central articles syndrome 2023 articles syndrome 2024 articles syndrome Scopus articles syndrome impact factor journals syndrome Scopus journals syndrome PubMed journals syndrome medical journals syndrome free journals syndrome best journals syndrome top journals syndrome free medical journals syndrome famous journals syndrome Google Scholar indexed journals Hypoxic coma articles Hypoxic coma Research articles Hypoxic coma review articles Hypoxic coma PubMed articles Hypoxic coma PubMed Central articles Hypoxic coma 2023 articles Hypoxic coma 2024 articles Hypoxic coma Scopus articles Hypoxic coma impact factor journals Hypoxic coma Scopus journals Hypoxic coma PubMed journals Hypoxic coma medical journals Hypoxic coma free journals Hypoxic coma best journals Hypoxic coma top journals Hypoxic coma free medical journals Hypoxic coma famous journals Hypoxic coma Google Scholar indexed journals Delayed post-hypoxic leukoencephalopathy articles Delayed post-hypoxic leukoencephalopathy Research articles Delayed post-hypoxic leukoencephalopathy review articles Delayed post-hypoxic leukoencephalopathy PubMed articles Delayed post-hypoxic leukoencephalopathy PubMed Central articles Delayed post-hypoxic leukoencephalopathy 2023 articles Delayed post-hypoxic leukoencephalopathy 2024 articles Delayed post-hypoxic leukoencephalopathy Scopus articles Delayed post-hypoxic leukoencephalopathy impact factor journals Delayed post-hypoxic leukoencephalopathy Scopus journals Delayed post-hypoxic leukoencephalopathy PubMed journals Delayed post-hypoxic leukoencephalopathy medical journals Delayed post-hypoxic leukoencephalopathy free journals Delayed post-hypoxic leukoencephalopathy best journals Delayed post-hypoxic leukoencephalopathy top journals Delayed post-hypoxic leukoencephalopathy free medical journals Delayed post-hypoxic leukoencephalopathy famous journals Delayed post-hypoxic leukoencephalopathy Google Scholar indexed journals Vegetative State articles Vegetative State Research articles Vegetative State review articles Vegetative State PubMed articles Vegetative State PubMed Central articles Vegetative State 2023 articles Vegetative State 2024 articles Vegetative State Scopus articles Vegetative State impact factor journals Vegetative State Scopus journals Vegetative State PubMed journals Vegetative State medical journals Vegetative State free journals Vegetative State best journals Vegetative State top journals Vegetative State free medical journals Vegetative State famous journals Vegetative State Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Unresponsive Wakefulness Syndrome articles Unresponsive Wakefulness Syndrome Research articles Unresponsive Wakefulness Syndrome review articles Unresponsive Wakefulness Syndrome PubMed articles Unresponsive Wakefulness Syndrome PubMed Central articles Unresponsive Wakefulness Syndrome 2023 articles Unresponsive Wakefulness Syndrome 2024 articles Unresponsive Wakefulness Syndrome Scopus articles Unresponsive Wakefulness Syndrome impact factor journals Unresponsive Wakefulness Syndrome Scopus journals Unresponsive Wakefulness Syndrome PubMed journals Unresponsive Wakefulness Syndrome medical journals Unresponsive Wakefulness Syndrome free journals Unresponsive Wakefulness Syndrome best journals Unresponsive Wakefulness Syndrome top journals Unresponsive Wakefulness Syndrome free medical journals Unresponsive Wakefulness Syndrome famous journals Unresponsive Wakefulness Syndrome Google Scholar indexed journals Carbon monoxide poisoning articles Carbon monoxide poisoning Research articles Carbon monoxide poisoning review articles Carbon monoxide poisoning PubMed articles Carbon monoxide poisoning PubMed Central articles Carbon monoxide poisoning 2023 articles Carbon monoxide poisoning 2024 articles Carbon monoxide poisoning Scopus articles Carbon monoxide poisoning impact factor journals Carbon monoxide poisoning Scopus journals Carbon monoxide poisoning PubMed journals Carbon monoxide poisoning medical journals Carbon monoxide poisoning free journals Carbon monoxide poisoning best journals Carbon monoxide poisoning top journals Carbon monoxide poisoning free medical journals Carbon monoxide poisoning famous journals Carbon monoxide poisoning Google Scholar indexed journals kidney articles kidney Research articles kidney review articles kidney PubMed articles kidney PubMed Central articles kidney 2023 articles kidney 2024 articles kidney Scopus articles kidney impact factor journals kidney Scopus journals kidney PubMed journals kidney medical journals kidney free journals kidney best journals kidney top journals kidney free medical journals kidney famous journals kidney Google Scholar indexed journals Parkinsonism articles Parkinsonism Research articles Parkinsonism review articles Parkinsonism PubMed articles Parkinsonism PubMed Central articles Parkinsonism 2023 articles Parkinsonism 2024 articles Parkinsonism Scopus articles Parkinsonism impact factor journals Parkinsonism Scopus journals Parkinsonism PubMed journals Parkinsonism medical journals Parkinsonism free journals Parkinsonism best journals Parkinsonism top journals Parkinsonism free medical journals Parkinsonism famous journals Parkinsonism Google Scholar indexed journals
Article Details
Abbreviations:
TS-Takotsubo Syndrome; LV-Left Ventricular; CAD-Coronary Artery Disease; ER-Emergency Room; SSRI-Serotoninergic Anti-Depressants; E-MCS- Emergence from Minimally Conscious State
1. Introduction
Takotsubo syndrome (TS) is an acute coronary syndrome with rapid development and subsequent resolution of severe, reversible left ventricular dysfunction [1]. The onset of TS is often preceded by emotional (27.7%) or physical (36%) stress [2] and has also been reported as consequence of stimulant drugs abuse [3] and acute poisoning [4]. Among the poisoning agents, up to two-thirds of patients who survive the acute phase of carbon monoxide (CO) toxic inhalation present a delayed leukoencephalopathy, usually in a period ranging from 2 to 40 days. Both leukoencephalopathy and cardiogenic shock may be associated with an overstimulation of the sympathetic nervous system [5]. Some studies-reported that patients with TS had a higher prevalence of neurological or psychiatric disorders than did those with an acute coronary syndrome [2], but the causal relationship between brain damage and heart dysfunction is still to be clarified [6]. Here, we describe the clinical course and outcome of a patient who was diagnosed with TS subsequent to a carbon monoxide poisoning, which caused also a delayed post-hypoxic leukoencephalopathy (DPHL). We selected the present case report with features of both TS and DPHL as hypoxic coma sequelae. The diagnosis of TS was based on the Mayo diagnostic criteria [7]; whereas the diagnosis of DPHL was possible for the presence of the 2 required neurological syndromes: parkinsonism (masked face, rigidity, tremor, dystonic posturing) and akinetic mutism (apathy, visual pursuit and loss of initiation) [8]. A legal representative signed the informed consent.
A 58 year-old woman presented with cardiogenic shock and hypoxic coma. At admission to Emergency Room (ER) the patient needed intensive cardio-pulmonary support and mechanical ventilation for a cardiogenic shock and hypoxic coma, with a Glasgow Coma Scale score of 5. Pre-morbid risk factors consisted of stressing life style and abuse of psychotropic drugs; the diagnosis of carbon monoxide poisoning was applied, due to a malfunctioning of the heating system. Brain CT and MRI were normal at ER entry, coronarography did not show any abnormal findings, whereas left ventricular ejection fraction was 25% at trans-oesophageal echocardiography. Repeated EEG showed diffuse slowing cerebral electrical activity, without any paroxystic discharges. In order to counteract critical haemodynamic condition, an aortic contra-pulsator 1:1 was implanted and the patient was sustained with ECMO V-A, IABP 1:1 and mechanical ventilation until ejection fraction recovered at echocardiography control (after 9 days), according to the diagnosis of TS. Repeated cerebral MRI showed diffuse and confluent hypoxic white matter changes at the level of bilateral fronto-mesial regions, involving also the right temporal lobe as in DPHL. At sedation withdrawal, 10 days after coma onset, the patient recovered an interaction with the environment and presented partially disoriented, confused and sometimes agitated.
Fifteen days after extubation, the patient progressively developed a psychomotor slowing, a diffuse rigidity at upper and lower limbs, like a parkinsonian syndrome, she stopped to communicate, with a worsening of vigilance and consciousness up to a new coma state. At admission to our post-acute rehabilitation hospital, 37 days after- cardiogenic shock, the patient presented with vegetative state (VS)/Unresponsiveness Wakefulness Syndrome (UWS), according to the Coma Recovery Scale-Revised. At this stage, EEG showed a basal activity at 7-8 cycles per second, without any epileptic paroxysms and somatosensory, auditory and flash visual evoked potentials were normal. A diffuse rigido-spasticity was prevalent at the lower limbs with muscular hypertonia at the adductor muscles, reduced range of motion at the left elbow, right knee and bilateral ankles. The low responsiveness, the severe apathy and the complete loss of initiation led the rehabilitation staff to diagnose the patient as in akinetic mutism. Intensive rehabilitation program including physical therapy, with stretching exercises, standing with robot stepping device (ERIGO), gait training, respiratory and, swallowing rehabilitation, speech therapy and cognitive-behavioral training, 5 days per week with a daily duration of at least 3 hours, was performed for 3 months. An educational training of the main caregivers and intensive daily nursing program were also provided.
Forty days after the rehabilitation program, the patient regained the eye-tracking ability, evolving from Minimally Conscious State-Minus to Minimally Conscious State-Plus, with first commands following and sporadic, automatic verbalization and she emerged from minimally conscious state (E-MCS). During rehabilitation hospital stay the patient regained verbal communication, a complete and safe oral feeding ability, improved global motor activity, although a post-hypoxic parkinsonism (hypomimia, parkinsonian posture, diffuse rigidity and bradykinesia) was also diagnosed. Brain MRI has been performed around every 3 months in the post-acute phase and was compatible with the diagnosis of DPHL. Cerebral MR at 9 months from the coma onset showed a reduction of the diffuse alteration of bi-hemispheric white matter fronto-parieto-temporo-occipital regions, with a cortico-subcortical atrophy (Figure 1 and 2).
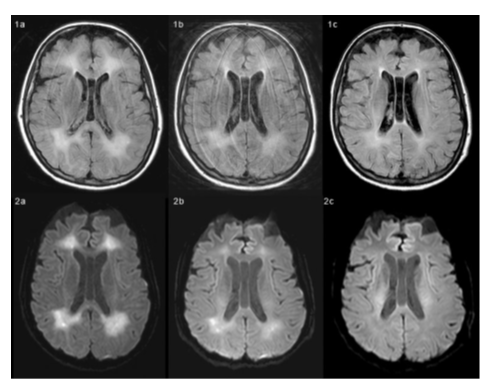
Figure 1-2: MR time-series images obtained with FLAIR (Figure 1 series) and DWI (Figure 2 series) techniques in April (a), June (b) and September (c). Both FLAIR and DWI techniques show abnormal signal intensity areas in deep white matter, suggesting tissue injury and nonspecific slight edema-like condition.
From April to September there is a reduction of the extent and signal intensity of these abnormal hyperintense areas, which can be associated with patient clinical recovery.
Measurements within a region of interest positioned in the abnormal hyperintense areas in ADC maps (not shown) confirm the progressive increase of water apparent diffusion coefficient (ADC=588·10-6 mm2/s in April; 762·10-6 mm2/s in June; 909·10-6 mm2/s in September). The improvement of ADC value is correlated with the decrease, from April to September, of the restriction of water diffusion.
At 9 months follow-up, the patient recovered a complete autonomy in daily living activities. A mild post-hypoxic parkinsonism, characterized by small steps gait, moderate hypomimia and parkinsonian dysarthria still persisted at this time. A neuropsychological evaluation showed a cognitive-behavioral profile characterized by dysexecutive disorders, which impaired problem solving, planning, divided attention and behavioral disinhibition, such as impulsivity and tangential speech; deficit of logical-deductive reasoning for visuo-spatial material, mild explorative asymmetry of the left side, difficulties in short and long-term memory and visuo-spatial working memory. To our knowledge, this is the first case report of TS after toxic carbon monoxide poisoning, whereas a delayed post-hypoxic leukoencephalopathy, as in our case, has been previously reported with a clinical history of carbon monoxide poisoning. The diffuse leukoencephalopathy, demonstrated by cerebral MR in the post-acute phase, may explain the delayed reoccurrence of coma, the prolonged disorder of consciousness and the slow recovery of the neurological and neuropsychological functioning.
Finally, the good recovery of this case, confirmed the benign prognosis of TS due to the reversibility of myocardial and brain dysfunction and of the DPHL, also consequent to the intensive neurorehabilitation treatment. The occurrence of apparently distinct medical disorders (carbon monoxide poisoning, TS-hypoxic coma-DPHL), involving the heart and the brain, seems to confirm the recent theory of the relationship between the two organs, known as “brain-heart connection” [6].
Funding
The acquisition and interpretation of imaging data in the manuscript was supported by grant for the Italian Ministry of Heatlh GR-2013-02359341. This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 778234.
Conflicts of Interests
No conflicts of interests for this manuscript are present.
References
- Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. Journal of cardiology 21(1991): 203-214.
- Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. New England Journal of Medicine 373 (2015): 929-938.
- Butterfield M, Riguzzi C, Frenkel O et al. Stimulant-related Takotsubo cardiomyopathy. The American journal of emergency medicine 3 (2015): 476-e1.
- Jeon U, Park S, Park S, et al. Clinical characteristics of stress cardiomyopathy in patients with acute poisoning. Scientific reports 1 (2018): 1-6.
- Varrassi M, Di Sibio A, Gianneramo C, et al. Advanced neuroimaging of carbon monoxide poisoning. The neuroradiology journal 5 (2017): 461-469.
- Chen Z, Venkat P, Seyfried D, et al. Brain-Heart Interaction: Cardiac Complications After Stroke. Circulation research 4 (2017): 451-468.
- Prasad A, Lerman A, Rihal CS. Apical Ballooning syndrome (Takotsubo or stress cardiomyopathy) a mimic of acute myocardial infarction. American heart journal 3 (2008): 408-417.
- Zamora CA, Nauen D, Hynecek R, et al. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain and behavior 8 (2015): e00364.
