Hypoglycemia is Associated with Increased In-Hospital Mortality in Patients with Liver Cirrhosis; A Nationwide Study
Article Information
Adham E Obeidat1*, Ratib Mahfouz2, Landon Kozai1, Mahmoud Mansour3, Mohammad Darweesh4, Ahmad Alqam5, Parthav Shah1, Traci Murakami6
1University of Hawaii, Hawaii, United States
2Kent Hospital/Brown University, Rhode Island, United States
3University of Missouri, Missouri, United States
4East Tennessee State University, Tennessee, United States
5Englewood Hospital, New Jersey, United States
6Department of Gastroenterology and Hepatology, The Queen’s medical center west Oahu, Hawaii, United States
*Corresponding author: Adham E Obeidat, University of Hawaii, 1356 Lusitana St. UH Tower 7th floor. Honolulu, HI 96813, United States
Received: 07 May 2022; Accepted: 17 May 2022; Published: 20 May 2022
Citation: Adham E Obeidat, Ratib Mahfouz, Landon Kozai, Mahmoud Mansour, Mohammad Darweesh, Ahmad Alqam, Parthav Shah, Traci Murakami. Hypoglycemia is Associated with Increased In-Hospital Mortality in Patients with Liver Cirrhosis; A Nationwide Study. Archives of Clinical and Biomedical Research 6 (2022): 448-461.
View / Download Pdf Share at FacebookAbstract
Aim: Cirrhosis may cause dysregulation of glucose homeostasis due to abnormalities in metabolism and responses to changes in the blood glucose level. Cirrhotic patients may be more prone to hypoglycemia. Hypoglycemia is associated with poor outcomes in cirrhotic patients with sepsis and may indicate a greater severity of illness. This study aims to elucidate the effect of hypoglycemia on in-hospital mortality in cirrhotic patients.
Methods: This review was completed using the National Inpatient Sample (NIS) database from 2016 to 2019. Using ICD-10-CM codes, we identified patients carrying a diagnosis of cirrhosis and hypoglycemia and gathered baseline demographic and clinical data. Odds ratios (OR) for comorbidities were calculated. Diabetic patients were excluded from the study to rule out the possibility of iatrogenic hypoglycemia.
Results: Out of 1,778,829 inpatients with cirrhosis, 31,615 had a diagnosis of hypoglycemia. Total hospital charges were significantly higher in the cirrhosis and hypoglycemia group compared to the non-hypoglycemia group. The mean length of stay (LOS) in the hypoglycemia group was significantly higher. Mortality, vasopressor usage, mechanical ventilation, cardiac arrest, and intensive care unit (ICU) admission were significantly higher in the hypoglycemia group compared to the non-hypoglycemia one.
Conclusion: In patients with cirrhosis, hypoglycemia was associated with higher in-hospital mortality and rate of critical illness as suggested by elevated rates of ICU admission, mechanical ventilation, and vasopressor use. These patients also had longer hospital LOS and higher total hospital charges. Hypoglycemia may reflect advanced liver disease or indicate early sepsis and potentially forbodes a prolonged, complicated hospital cours
Keywords
Cirrhosis; Hypoglycemia; Liver; Mortality; Critical Illness
Article Details
1. Introduction
Chronic liver inflammation causes diffuse hepatic fibrosis which can eventually give rise to liver cirr-hosis and subsequent liver failure [1]. Cirrhosis is the leading cause of liver-related death globally [2]. The liver regulates glucose homeostasis by controlling various pathways of glucose metabolism, including glycogenesis, glycogenolysis, glycolysis and gluco-neogenesis [3]. It functions as a reserve for carbohy-drates, storing glycogen from glucose in postprandial periods and releasing glucose during fasting periods [4]. Liver cell damage in cirrhosis can alter the liver’s metabolism and affect the liver’s ability to regulate the blood glucose level, thereby making cirrhotic patients prone to hypoglycemia. Hypoglycemia is not uncom-mon in cirrhotic patients. Nouel et al. reported hypo-glycemia in 15 out of 30 cirrhotic patients admitted with septicemia [5]. Moreover, hypoglycemia was associated with increased mortality in patients with liver cirrhosis [4, 6-8]. These findings indicate the important impact of hypoglycemia on patients with liver cirrhosis. In this retrospective United States (US) national inpatient-based population study, we evaluated the effect of hypoglycemia on in-hospital mortality as well as other in-hospital complications in patients with liver cirrhosis.
2. Methods
2.1 Data source
This was a retrospective cohort study of patients who were admitted to hospitals in the US between the years 2016 to 2019. The data was extracted from the Health-care Cost and Utilization Project National Inpatient Sample (NIS) database. The NIS is sponsored by the Agency for Healthcare Research Quality (AHRQ) and is considered the largest publicly available inpatient health care database in the US. The database includes data from at least 46 states and covers more than 97% of the US population [9]. A 20% probability sample was collected and subsequently weighted to ensure that the selected population was nationally represent-ed. Each admission in the database was assigned one principal diagnosis, up to 40 secondary diagnoses, and 25 procedures. These variables are defined via the International Classification of Disease, 10th revision, and Clinical Modification (ICD-10-CM) codes.
2.2 Study variables
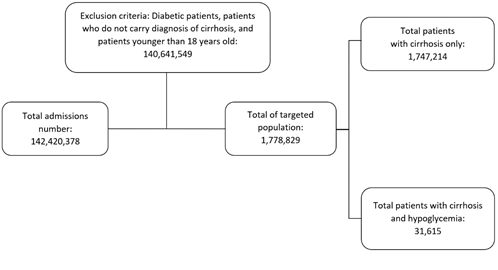
Target population age was 18 years and older. Using ICD-10-CM codes, we identified patients who carried a diagnosis of cirrhosis and hypoglycemia. Patient’s age (in years), gender, race (White, Black, Hispanic, Others), and hospital information (region and bed size) were collected and considered as baseline chara-cteristics. The comorbidities included were chronic kidney disease (CKD), alcoholism, hepatocellular carcinoma (HCC), hypertension (HTN), cachexia, hepatorenal syndrome (HRS), hepatopulmonary synd-rome (HPS), hepatic failure, spontaneous bacterial peritonitis (SBP), ascites, varices, smoking and obesity (body mass index (BMI) of more than 24.9 were considered obese). Diabetic patients were exclu-ded from this study to rule out the probability of iatrogenic hypoglycemia. Intensive care unit (ICU) admission was defined as any patient who had cardiac arrest or needed vasopressors or mechanical ventil-ation. Figure 1 demonstrates the study population (Figure 1).
The statistical analysis was done using STATA software, version 17.0 (StataCorp., College Station, TX, USA). The demographic and clinical charact-eristics of patients with cirrhosis and hypoglycemia and those without hypoglycemia were described using descriptive statistics. In this study, multivariate logistic regression analyses were performed to deter-mine factors associated with in-hospital mortality. Variables that were not statistically significant (p-value < 0.1) on univariate analysis were excluded from the multivariate analysis. The odds ratio (OR) at 95% confidence interval (CI) was used to describe the association between the study and outcome variables. Statistical significance was defined as a two-tailed p-value of < 0.05.
3. Results
3.1 Demographic and clinical characteristics
Out of 1,778,829 patients carrying the diagnosis of cirrhosis, it was found that 31,615 patients (1.78%) were diagnosed with concurrent hypoglycemia during admission (Figure 1). The mean age of patients with cirrhosis and concurrent hypoglycemia was nearly the same but slightly less than patients without hypo-glycemia (57.62 years vs 58.35 years, p-value < 0.001). While male gender was more predominant in both groups, percentage of females in the cirrhosis with hypoglycemia group was more pronounced compared to the cirrhosis without hypoglycemia group (42.65% vs 37.7%, p-value <0.001). The white race percentage was nearly identical and the most prevalent in both groups (~66.6%). Black race prevalence was higher in hypoglycemia group (19.61% vs 10%, p-value <0.001). In terms of hospital characteristics, both groups were more likely to be in a large hospital in the southern region (Table 1). Regarding comor-bidities, the prevalence of CKD, alcoholism, HCC, cachexia, HRS, hepatic failure and SBP were more pronounced in the cirrhosis and hypoglycemia group. HTN, smoking and obesity were more prevalent in the cirrhosis without hypoglycemia group (Table 1).
3.2 Inpatient outcomes
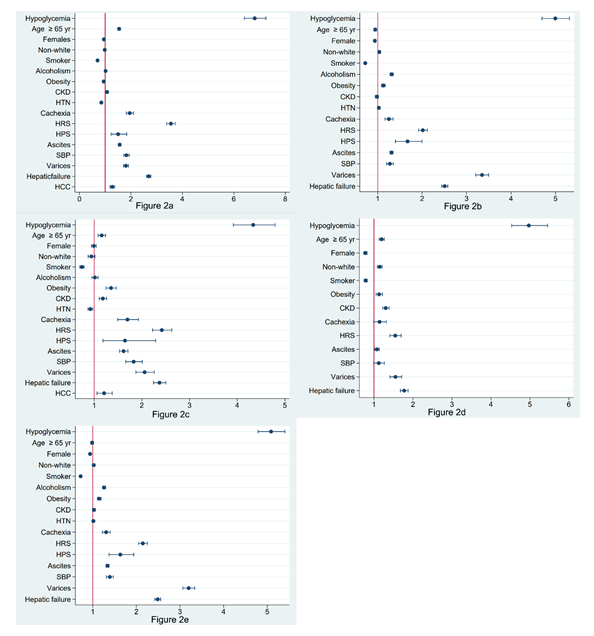
3.2.1 In-hospital mortality: The rate of in-hospital-mortality in the cirrhosis with hypoglycemia group was higher than that in the cirrhosis without hypo-glycemia (Table 2). This was also reflected on the multivariate analysis as patients with cirrhosis and hypoglycemia had a higher chance of dying during hospitalization compared to those without hypogl-ycemia after adjusting for other possible confounders (OR 6.8; CI 95% 6.4-7.24, p-value < 0.001) (Table 3). Moreover, patients older than 65-year-old were 54% more likely to die during hospitalization and females were 5% less likely to die. Non-white patients were 2% less likely to die (p-value 0.290). Interestingly, patients with HRS had the highest chance for in-hospital mortality, followed by hepatic failure. Cachexia, SBP, varices, ascites, HPS, HCC and CKD were associated with a modest increase in odds of in-hospital mortality. On the other hand, HTN, obesity and smoking were associated with a statistically significant decrease in odds of in-hospital mortality (Table 3).
3.2.2 Total hospital charges and length of stay (LOS): Total hospital charges and the mean LOS were significantly higher in the cirrhosis and hypoglycemia group compared to the cirrhosis without hypogl-ycemia. Mortality, vasopressor usage, mechanical ventilation, cardiac arrest and ICU admission were significantly higher in cirrhotic patients with hypoglycemia compared to cirrhotics without hypoglycemia (Table 2).
3.2.3 Other in-hospital complications: Cirrhotic patients with hypoglycemia were more likely to need mechanical ventilation and vasopressors. Moreover, patients with cirrhosis and hypoglycemia had a higher chance of having cardiac arrest during hospitalization and to be admitted to the ICU. Tables 4, 5, 6 and 7 summarize these findings. Figure 2 demonstrates OR plots.
|
Variable |
No Hypoglycemia |
Hypoglycemia |
P value |
|
Age (mean, year) |
58.35 |
57.62 |
<0.001 |
|
Gender (%) |
<0.001 |
||
|
Male |
62.3% |
57.35% |
|
|
Female |
37.7% |
42.65% |
|
|
Race (%) |
<0.001 |
||
|
White |
66.67% |
66.5% |
|
|
Black |
10.09% |
19.61% |
|
|
Hispanic |
14.59% |
12.76% |
|
|
Others |
8.65% |
10.75% |
|
|
Hospital region (%) |
<0.001 |
||
|
Northeast |
16.93% |
13.24% |
|
|
Midwest |
19.18% |
21.4% |
|
|
South |
39.75% |
38.89% |
|
|
West |
24.13% |
24.17% |
|
|
Hospital bed size (%) |
<0.001 |
||
|
Small |
18.3% |
16.12% |
|
|
Medium |
28.34% |
28.93% |
|
|
Large |
53.36% |
54.39% |
|
|
Comorbidities (%) |
|||
|
CKD |
17.85% |
24.8% |
<0.001 |
|
Alcoholism |
56.87% |
60.38% |
<0.001 |
|
HCC |
3.22% |
4.73% |
<0.001 |
|
HTN |
48.86% |
46.24% |
<0.001 |
|
Cachexia |
2.48% |
5.95% |
<0.001 |
|
HRS |
4.33% |
10.82% |
<0.001 |
|
Hepatic failure |
20.7% |
41.48% |
<0.001 |
|
HPS |
0.3% |
0.3% |
0.9688 |
|
Ascites |
30.99% |
44.63% |
<0.001 |
|
SBP |
2.91% |
8.32% |
<0.001 |
|
Varices |
4.84% |
4.7% |
0.5876 |
|
Smoking |
50.92% |
47.18% |
<0.001 |
|
Obesity |
11.02% |
10.99% |
<0.001 |
Abbreviations: CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; SBP, spontaneous bacterial peritonitis
Table 1: Clinical and demographic characteristics of cirrhotic patients with hypoglycemia vs no hypoglycemia.
|
Outcome |
No hypoglycemia |
Hypoglycemia |
P value |
|
Total hospital charge ($) |
70,924 |
109,633 |
<0.001 |
|
LOS (days) |
6.2 |
7.79 |
<0.001 |
|
Death (%) |
6.01% |
35.51% |
<0.001 |
|
Vasopressor use (%) |
1.62% |
9.47% |
<0.001 |
|
Mechanical ventilation (%) |
7.39% |
32.86% |
<0.001 |
|
Cardiac arrest (%) |
1.65% |
9.24% |
<0.001 |
|
ICU admission (%) |
8.09% |
35.54% |
<0.001 |
Abbreviations: LOS, length of stay; ICU, intensive care unit.
Table 2: Clinical outcomes in cirrhotic patients with hypoglycemia vs no hypoglycemia.
|
In-hospital mortality |
Univariate analysis OR (CI 95%) |
P value |
Multivariate analysis aOR (CI 95%) |
P value |
|
Hypoglycemia |
8.61 (8.15-9.1) |
<0.001 |
6.8 (6.4-7.24) |
|
|
Age ≥ 65 years |
1.32 (1.28-1.36) |
<0.001 |
1.54 (1.49-1.59) |
<0.001 |
|
Female |
0.98 (0.95-1.01) |
<0.109 |
||
|
Non-white |
1.05 (1.02-1.08) |
0.003 |
0.98 (0.95-1.01) |
0.290 |
|
CKD |
1.45 (1.4-1.5) |
<0.001 |
1.07 (1.03-1.12) |
<0.001 |
|
Alcoholism |
1.08 (1.04-1.11) |
<0.001 |
1.01 (0.98-1.05) |
0.409 |
|
HCC |
1.71 (1.6-1.82) |
<0.001 |
1.28 (1.19-1.37) |
<0.001 |
|
HTN |
0.8 (0.78-82) |
<0.001 |
0.85 (0.83-0.88) |
<0.001 |
|
Cachexia |
2.68 (2.52-2.85) |
<0.001 |
1.94 (1.81-2.08) |
<0.001 |
|
HRS |
6.2 (5.95-6.45) |
<0.001 |
3.56 (3.4-3.79) |
<0.001 |
|
Hepatic failure |
3.66 (3.56-3.77) |
<0.001 |
2.69 (2.61-2.78) |
<0.001 |
|
HPS |
2.09 (1.75-2.5) |
<0.001 |
1.51 (1.24-1.84) |
<0.001 |
|
Ascites |
2.24 (2.18-2.3) |
<0.001 |
1.56 (1.51-1.61) |
<0.001 |
|
SBP |
3.79 (3.6-3.98) |
<0.001 |
1.82 (1.72-1.93) |
<0.001 |
|
Varices |
1.86 (1.76-1.95) |
<0.001 |
1.81 (1.71-1.9) |
<0.001 |
|
Smoking |
0.63 (0.61-65) |
<0.001 |
0.7 (0.68-0.72) |
<0.001 |
|
Obesity |
0.8 (0.76-0.83) |
<0.001 |
0.85 (0.81-0.89) |
<0.001 |
Note: Variables that were not statistically significant (p-value <0.1) on univariate analysis were excluded from the multivariate analysis
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; SBP, spontaneous bacterial peritonitis.
Table 3: Univariate and multivariate analysis of factors associated with in-hospital mortality in cirrhotic patients with hypoglycemia.
|
Mechanical ventilation |
Univariate analysis OR (CI 95%) |
P value |
Multivariate analysis aOR (CI 95%) |
P value |
|
Hypoglycemia |
6.13 (5.79-6.49) |
<0.001 |
5 (4.7-5.31) |
<0.001 |
|
Age ≥ 65 years |
0.79 (0.77-0.81) |
<0.001 |
0.94 (0.91-0.98) |
0.001 |
|
Female |
0.91 (0.89-0.93) |
<0.001 |
0.94 (0.91-0.96) |
<0.001 |
|
Non-white |
1.1 (1.07-1.14) |
<0.001 |
1.03 (1.01-1.7) |
0.021 |
|
CKD |
1.07 (1.03-1.1) |
<0.001 |
0.98 (0.95-1.02) |
0.289 |
|
Alcoholism |
1.5 (1.46-1.54) |
<0.001 |
1.31 (1.28-1.17) |
<0.001 |
|
HCC |
0.97 (0.9-1.04) |
0.367 |
||
|
HTN |
0.88 (0.85-0.9) |
<0.001 |
1.03 (0.98-1.05) |
0.71 |
|
Cachexia |
1.52 (1.42-1.62) |
<0.001 |
1.25 (1.16-1.35) |
<0.001 |
|
HRS |
3.37 (3.22-3.52) |
<0.001 |
2.02 (1.92-2.12) |
<0.001 |
|
Hepatic failure |
3.22 (3.14-3.31) |
<0.001 |
2.51 (2.44-2.58) |
<0.001 |
|
HPS |
2.07 (1.75-2.45) |
<0.001 |
1.67 (1.4-2) |
<0.001 |
|
Ascites |
1.64 (1.6-1.68) |
<0.001 |
1.31 (1.27-1.35) |
<0.001 |
|
SBP |
2.34 (2.22-2.45) |
<0.001 |
1.27 (1.2-1.35) |
<0.001 |
|
Varices |
3.56 (3.41-3.7) |
<0.001 |
3.35 (3.21-3.5) |
<0.001 |
|
Smoking |
0.71 (0.69-0.73) |
<0.001 |
0.72 (0.7-0.74) |
<0.001 |
|
Obesity |
1.09 (1.05-1.13) |
<0.001 |
1.13 (1.09-1.17) |
<0.001 |
Note: Variables that were not statistically significant (p-value <0.1) on univariate analysis were excluded from the multivariate analysis
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; SBP, spontaneous bacterial peritonitis.
Table 4: Univariate and multivariate analysis of factors associated with mechanical ventilation in cirrhotic patients with hypoglycemia.
|
Vasopressor use |
Univariate analysis OR (CI 95%) |
P value |
Multivariate analysis aOR (CI 95%) |
P value |
|
Hypoglycemia |
6.36 (5.78-6.99) |
<0.001 |
4.33 (3.92-4.79) |
<0.001 |
|
Age ≥ 65 years |
1.05 (0.99-1.12) |
0.104 |
||
|
Female |
1 (0.96-1.06) |
0.789 |
||
|
Non-white |
1.01 (0.94-1.09) |
0.788 |
||
|
CKD |
1.48 (1.39-1.57) |
<0.001 |
1.18 (1.11-1.26) |
<0.001 |
|
Alcoholism |
1.11 (1.05-1.17) |
0.001 |
1.01 (0.95-1.08) |
0.651 |
|
HCC |
1.55 (1.37-1.76) |
<0.001 |
1.21 (1.06-1.38) |
0.005 |
|
HTN |
0.84 (0.8-0.89) |
<0.001 |
0.92 (0.87-0.97) |
0.003 |
|
Cachexia |
2.3 (2.04-2.6) |
<0.001 |
1.7 (1.49-1.93) |
<0.001 |
|
HRS |
4.73 (4.39-5.09) |
<0.001 |
2.42 (2.22-2.63) |
<0.001 |
|
Hepatic failure |
3.36 (3.19-3.54) |
<0.001 |
2.37 (2.24-2.5) |
<0.001 |
|
HPS |
2.27 (1.65-3.13) |
<0.001 |
1.65 (1.18-2.29) |
0.003 |
|
Ascites |
2.27 (2.15-2.39) |
<0.001 |
1.62 (1.53-1.71) |
<0.001 |
|
SBP |
3.81 (3.5-4.15) |
<0.001 |
1.83 (1.66-2.01) |
<0.001 |
|
Varices |
2.14 (1.96-2.35) |
<0.001 |
2.06 (2.24-2.5) |
<0.001 |
|
Smoking |
0.66 (0.62-0.7) |
<0.001 |
0.74 (0.7-0.77) |
<0.001 |
|
Obesity |
1.33 (1.23-1.43) |
<0.001 |
1.35 (1.25-1.46) |
<0.001 |
Note: Variables that were not statistically significant (p-value <0.1) on univariate analysis were excluded from the multivariate analysis
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; SBP, spontaneous bacterial peritonitis.
Table 5: Univariate and multivariate analysis of factors associated with vasopressor use in cirrhotic patients with hypoglycemia.
|
Cardiac arrest |
Univariate analysis OR (CI 95%) |
P value |
Multivariate analysis aOR (CI 95%) |
P value |
|
Hypoglycemia |
6.06 (5.54-6.62) |
<0.001 |
4.97 (4.53-5.45) |
<0.001 |
|
Age ≥ 65 years |
1.16 (1.1-1.22) |
<0.001 |
1.2 (1.13-1.26) |
<0.001 |
|
Female |
0.8 (0.77-0.84) |
<0.001 |
0.78 (0.74-0.82) |
<0.001 |
|
Non-white |
1.22 (1.16-1.29) |
<0.001 |
1.15 (1.09-1.21) |
<0.001 |
|
CKD |
1.51 (1.42-1.6) |
<0.001 |
1.3 (1.22-1.39) |
<0.001 |
|
Alcoholism |
0.95 (0.91-1) |
0.055 |
||
|
HCC |
1.14 (1-1.29) |
0.051 |
||
|
HTN |
1.03 (0.98-1.08) |
0.294 |
||
|
Cachexia |
1.4 (1.22-1.6) |
<0.001 |
1.14 (0.99-1.31) |
0.062 |
|
HRS |
2.33 (2.14-2.53) |
<0.001 |
1.55 (1.41-1.7) |
<0.001 |
|
Hepatic failure |
3.66 (3.56-3.77) |
<0.001 |
1.77 (1.68-1.88) |
<0.001 |
|
HPS |
0.88 (0.54-1.42) |
0.6 |
||
|
Ascites |
1.3 (1.24-1.37) |
<0.001 |
1.08 (1.02-1.14) |
0.008 |
|
SBP |
1.7 (1.52-1.91) |
<0.001 |
1.12 (0.99-1.27) |
0.064 |
|
Varices |
1.61 (1.47-1.78) |
<0.001 |
1.55 (1.41-1.71) |
<0.001 |
|
Smoking |
0.73 (0.7-0.77) |
<0.001 |
0.79 (0.74-0.83) |
<0.001 |
|
Obesity |
1.12 (1.04-1.2) |
0.002 |
1.13 (1.05-1.22) |
0.001 |
Note: Variables that were not statistically significant (p-value <0.1) on univariate analysis were excluded from the multivariate analysis
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; SBP, spontaneous bacterial peritonitis.
Table 6: Univariate and multivariate analysis of factors associated with cardiac arrest in cirrhotic patients with hypoglycemia.
|
ICU admission |
Univariate analysis OR (CI 95%) |
P value |
Multivariate analysis aOR (CI 95%) |
P value |
|
Hypoglycemia |
6.26 (5.92-6.63) |
<0.001 |
5.09 (4.79-5.41) |
<0.001 |
|
Age ≥ 65 years |
0.84 (0.81-0.86) |
<0.001 |
0.99 (0.96-1.02) |
0.349 |
|
Female |
0.92 (0.9-0.94) |
<0.001 |
0.94 (0.92-0.96) |
<0.001 |
|
Non-white |
1.09 (1.06-1.122) |
<0.001 |
1.02 (0.99-1.05) |
0.11 |
|
CKD |
1.14 (1.11-1.18) |
<0.001 |
1.03 (0.99-1.06) |
0.114 |
|
Alcoholism |
1.42 (1.39-1.46) |
<0.001 |
1.26 (1.22-1.3) |
<0.001 |
|
HCC |
1.06 (0.99-1.134) |
0.076 |
||
|
HTN |
0.88 (0.86-0.9) |
<0.001 |
1.01 (0.99-1.04) |
0.274 |
|
Cachexia |
1.58 (1.5-1.7) |
<0.001 |
1.31 (1.22-1.4) |
<0.001 |
|
HRS |
3.62 (3.47-3.78) |
<0.001 |
2.15 (2.04-2.25) |
<0.001 |
|
Hepatic failure |
3.22 (3.14-3.3) |
<0.001 |
2.49 (2.42-2.56) |
<0.001 |
|
HPS |
2.04 (1.73-2.4) |
<0.001 |
1.63 (1.37-1.94) |
<0.001 |
|
Ascites |
1.7 (1.66-1.74) |
<0.001 |
1.34 (1.3-1.38) |
<0.001 |
|
SBP |
2.56 (2.34-2.69) |
<0.001 |
1.39 (1.31-1.47) |
<0.001 |
|
Varices |
3.34 (3.21-3.48) |
<0.001 |
3.2 (3.07-3.34) |
<0.001 |
|
Smoking |
0.7 (0.69-0.72) |
<0.001 |
0.72 (0.7-0.74) |
<0.001 |
|
Obesity |
1.11 (1.08-1.15) |
<0.001 |
1.15 (1.11-1.19) |
<0.001 |
Note: Variables that were not statistically significant (p-value <0.1) on univariate analysis were excluded from the multivariate analysis
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; CKD, chronic kidney disease; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HPS, hepatopulmonary syndrome; SBP, spontaneous bacterial peritonitis.
Table 7: Univariate and multivariate analysis of factors associated with ICU admission in cirrhotic patients with hypoglycemia.
4. Discussion
In this retrospective nationwide inpatient population study, we demonstrated that the occurrence of in-hospital hypoglycemia in patients with cirrhosis is associated with increased in-hospital mortality and worse outcomes compared to patients with cirrhosis who do not experience hypoglycemia. Our study showed higher mortality rates in cirrhotic patients who developed a hypoglycemia episode during hospital-lization compared to patients with normal blood sugar level. This is consistent with what is described in the current literature [4, 6-8]. Pfortmueller et al., in a retrospective study of 312 patients admitted with decompensated liver cirrhosis, showed that patients who developed hypoglycemia had significantly higher chances of ICU admission and mortality, with a lower estimated survival rate compared to normoglycemic and hyperglycemic patients [4]. Our study demon-strated a significantly higher chance of in-hospital mortality and ICU admission even after adjusting for decompensating factors such as ascites, HRS, HPS, SBP, hepatic failure, and HCC.
One population-based retrospective study done in Taiwan by Hung et al., demonstrated an increased 30-day mortality in cirrhotic patients with hypoglycemia compared to normoglycemic patients. This study also showed that the cirrhotic patients with concurrent HCC also had a higher 30-day mortality rate than those without HCC [6]. Compared to Hung et al. study, our study had a larger patient sample. The results of this study are more generalizable, as Hung et al did not comment on race, while the population of our study was multi-racial. The heightened mortality associated with hypoglycemia may be explained by the presence of cirrhosis-related complications and the elevated risk of sepsis. In this study, patients with hypoglycemia were also more likely to present during the hospital-lization with a complication of cirrhosis, such as HRS, hepatic failure, ascites, and SBP. The presence of these comorbidities typically adds complexity to clinical management and compounds the higher mortality rate of compensated cirrhosis [10-11]. This may reflect that hypoglycemia itself is an indicator of advanced cirrhosis and appears concurrently with its known complications. Furthermore, the occurrence of cirr-hosis-related complications is often related to sepsis. Patients with cirrhosis are more susceptible to deve-loping bacterial infection and suffer higher mortality from sepsis [1]. Saiman et al. reported a significantly increased probability of septicemia in cirrhotic patients with blood glucose level < 100 mg/dL. He also reported that blood glucose level < 100 mg/dL in cirrhotic patients with positive blood culture was associated with increased mortality [8]. Hypogly-cemia per se is associated with elevated mortality in the setting of sepsis, regardless of cirrhosis also being present [12-14]. Future studies could focus on the utility of adding hypoglycemia to calculations for risk stratification and prognosis including transplant priority in cirrhotic patients.
In our study, we found that hypoglycemia was associated with greater severity of illness, including increased risk of ICU admission, shock requiring vasopressors, mechanical ventilation, and cardiac arrests. However, the reason for such critical illness was not specified, but it is plausible that sepsis could account for the majority of cases. Hypoglycemia may be an important early predictor of inpatient septicemia and in-hospital mortality in patients with cirrhosis, and may portend the development of severe illness with multiorgan failure leading to ICU admission, and subsequently mechanical ventilation, initiation of vasopressor therapy, and ultimately cardiac arrest [5, 8]. Sepsis itself may cause aberrant glucose homeos-tasis due to systemic inflammation and relative adrenal insufficiency, although this may also be related to impaired hepatic glucose metabolism due to liver fibrosis, cirrhosis-related adrenal insufficiency, or malnutrition [3, 6, 12, 15]. Thus, hypoglycemia may be a poor prognostic indicator of either impending acute clinical deterioration or represent a long-term sequela of chronic liver disease. Hypoalbuminemia is also a common finding in cirrhosis and has been linked to higher mortality in septic patients with hypogly-cemia [14]. However, our data did not include diagnoses of hypoalbuminemia in the patient sample
and thus we were unable to support this finding.
In keeping with the increased likelihood of acute complications related to cirrhosis, hypoglycemia was also associated with a longer length of stay and total hospital charges. This is likely related to the higher incidence of these complications and the additional resources required to manage them. Incorporating an understanding of prognostication in cirrhosis may be important for future development of healthcare policy and inpatient hospital resource utilization. Hypogly-cemia was more likely to occur in females than males. Although this remains unclear, this discrepancy could be related to differences in sex-hormone effects on liver physiology [16]. Furthermore, Black race was also associated with greater incidence of hypogly-cemia. It is possible that genetic, socioeconomic, and other demographic variables contribute to illness complications [17].
This study has several limitations which include the inability to categorize patients based on their MELD score which is used for prognostication and risk of short-term mortality in cirrhosis [18]. Our data may suggest that hypoglycemia tends to occur more frequently in patients with decompensated liver disease. Given that the MELD score correlates with degree of liver dysfunction, it is possible that hypogl-ycemia may correlate similarly [19]. Our study does not account for individuals who had a diagnosis of sepsis, and with the current data we may only hypo-thesize that sepsis could account for a majority of cases with severe illness. Moreover, due to the nature of the NIS database, our observations reflect admiss-ions and not individual patients. Therefore, the unit of analysis is the admission. Given the inability to account for multiple admissions for a given patient in the NIS, our conclusions may be confounded by the risk of repeat hospitalization. Thus, our reported rates may be viewed as over-estimates of a per patient admission rate. Mortality rates, however, are unlikely to be affected. Under-or over-coding can lead to mis-classification, although the large number of patients in the database strongly mitigates against substantial misclassification bias. NIS undergoes data quality assessment annually to ensure the internal validity of the data.
In conclusion, hypoglycemia in a nationwide diverse population of hospitalized non-diabetic inpatients with cirrhosis was associated with higher 30-day mortality and rate of critical illness as suggested by an elevated rate of ICU admission, mechanical ventilation, and shock requiring vasopressor medication. In addition, these patients had a longer length of hospital stay and higher total hospital charges. These outcomes were observed even when controlling for various comor-bidities and complications due to decompensated cirrhosis. Hypoglycemia in cirrhotic patients may be an important prognostic factor as an early indicator of sepsis and could forebode a prolonged hospital stay due to critical illness, hence such patients should be monitored carefully for signs of worsening clinical status.
Funding
The authors did not receive support from any organization for the submitted work.
Conflicts of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethics Approval
This retrospective chart review study was done using the NIS deidentified database. Therefore an IRB approval was not obtained.
References
- Gines P, Krag A, Abraldes JG, Sola E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet 398 (2021): 1359-1376.
- Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5 (2020): 245-266.
- Hye-Sook Han, Geon Kang, Jun Seok Kim, Byeong Hoon Choi, Seung-Hoi Koo. Regula-tion of glucose metabolism from a liver-centric perspective. Exp Mol Med 48 (2016): e218.
- Carmen Andrea Pfortmueller, Christoph Wiemann, Georg-Christian Funk, Alexander Benedikt Leichtle, Georg Martin Fiedler, Aristomenis Konstantinos Exadaktylos, et al. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care 29 (2014): 316.e7-12.
- Nouel O, Bernuau J, Rueff B, Benhamou JP. Hypoglycemia. A common complication of septicemia in cirrhosis. Arch Intern Med 141 (1981): 1477-1478.
- Hung TH, Tseng CW, Tsai CC, Lee HF. Prognosis of hypoglycemia episode in cirrhotic patients during hospitalization. BMC Gastro-enterol 21 (2021): 319.
- Hagel S, Bruns T, Herrmann A, Stallmach A, Schmidt C. Abnormal glucose tolerance: a predictor of 30-day mortality in patients with decompensated liver cirrhosis. Z Gastroenterol 49 (2011): 331-334.
- Saiman Y, Mahmud N. Hypoglycemia is an early, independent predictor of bacteremia and in-hospital death in patients with cirrhosis. Eur J Gastroenterol Hepatol 33 (2021): e693-e9.
- Healthcare Cost and Utilization Project. The National (Nationwide) Inpatient Sample (NIS) (2016).
- Kapil Gupta, Abhishek Bhurwal, Cindy Law, Scott Ventre, Carlos D Minacapelli, Savan Kabaria, et al. Acute kidney injury and hepatorenal syndrome in cirrhosis. World J Gastroenterol 27 (2021): 3984-4003.
- Kumar R, Mehta G, Jalan R. Acute-on-chronic liver failure. Clin Med (Lond) 20 (2020): 501-504.
- Wang J, Zhu CK, Yu JQ, Tan R, Yang PL. Hypoglycemia and mortality in sepsis patients: A systematic review and meta-analysis. Heart Lung 50 (2021): 933-940.
- Sunghoon Park, Dong-Gyu Kim, Gee Young Suh, Jun Goo Kang, Young-Su Ju, Yong-Jae Lee, et al. Mild hypoglycemia is independently associated with increased risk of mortality in patients with sepsis: a 3-year retrospective observational study. Crit Care 16 (2012): R189.
- Furukawa M, Kinoshita K, Yamaguchi J, Hori S, Sakurai A. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern Emerg Med 14 (2019): 539-548.
- Trifan A, Chiriac S, Stanciu C. Update on adrenal insufficiency in patients with liver cirrhosis. World J Gastroenterol 19 (2013): 445-456.
- Kur P, Kolasa-Wolosiuk A, Misiakiewicz-Has K, Wiszniewska B. Sex Hormone-Dependent Physiology and Diseases of Liver. Int J Environ Res Public Health (2020).
- Desai AP, Mohan P, Roubal AM, Bettencourt R, Loomba R. Geographic Variability in Liver Disease-Related Mortality Rates in the United States. Am J Med 131 (2018): 728-734.
- Roth JA, Chrobak C, Schädelin S, Hug BL. MELD score as a predictor of mortality, length of hospital stay, and disease burden: A single-center retrospective study in 39,323 inpatients. Medicine (Baltimore) 96 (2017): e7155.
- Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut 52 (2003): 134-139.