Hypertrophic Obstructive Cardiomyopathy with SAM Phenomenon: A Case Report and Literature Review
Article Information
Mei-lian Cai1,2*, Guo-qiang Zhong1
1Department of Cardiology, the First Affiliated Hospital of Guangxi Medical University, Nanning City, China
2Department of Cardiology, the Second Affiliated Hospital of Guilin Medical University, Guilin City, China.
*Corresponding author: Mei-lian Cai, Department of Cardiology, the First Affiliated Hospital of Guangxi Medical University, Nanning City, China
Received: 14 November 2022; Accepted: 21 November 2022; Published: 28 November 2022
Citation:
Mei-lian Cai, Guo-qiang Zhong. Hypertrophic Obstructive Cardiomyopathy with SAM Phenomenon: A Case Report and Literature Review. Cardiology and Cardiovascular Medicine 6 (2022): 515-522.
View / Download Pdf Share at FacebookAbstract
Background: Hypertrophic cardiomyopathy (HCM) is defined by the presence of left ventricular hypertrophy (LVH) in the absence of other potentially causative cardiac, systemic, syndromic, or metabolic diseases [1]. It is the most common genetic abnormality of the myocardium, with an anaesthetized prevalence ranging from 1:500 to as high as 1:200 [2-4]. It is the primary cause of sudden cardiac death (SCD) among teenagers and athletes.
Patient: A 56-year-old man presented with chest tightness and palpitations which had been occurring post-activity for the previous 6 months. The patient was advised to be admitted. He underwent echocardiography, cardiac magnetic resonance (CMR), coronary angiography (CAG) examination, and left ventriculography. He was diagnosed with hypertrophic obstructive cardiomyopathy (HOCM) with systolic anterior motion (SAM) phenomenon.
Results: Echocardiography results showed that the interventricular septal thickness was 14-16 mm and that there were 2 degrees of SAM of the mitral valve. This resulted in severe stenosis of the left ventricular outflow tract (LVOT) and moderate to severe mitral insufficiency. Left ventriculography confirmed mitral regurgitation (MR) associated with HOCM with SAM phenomenon. Under the protection of a permanent pacemaker, the patient was treated with alcohol septal ablation (ASA). After discharge, the symptoms of chest tightness and palpitation did not recur.
Conclusion: Beneficial effects were observed when patients with HOCM and SAM were treated with ASA under the condition of a permanent pacemaker.
Keywords
Alcohol Septal Ablation; Hypertrophic Cardiomyopathy; Hypertrophic Obstructive Cardiomyopathy; Systolic Anterior Motion Phenomenon
Alcohol Septal Ablation articles; Hypertrophic Cardiomyopathy articles; Hypertrophic Obstructive Cardiomyopathy articles; Systolic Anterior Motion Phenomenon articles
Article Details
Abbreviations:
ASA- Alcohol Septal Ablation; ApHCM- Apical Hypertrophic Cardiomyopathy; CAG- Coronary Angiography; CCBs- Calcium Channel Blockers; CHD- Coronary Heart Disease; CMR- Cardiac Magnetic Resonance; CT- Computed Tomography; DCG- Dynamic Electrocardiogram; ECG- Electrocardiogram; EF- Ejection Fraction; HCM- Hypertrophic Cardiomyopathy; HOCM- Hypertrophic Obstructive Cardiomyopathy; LAD- Left Anterior Descending; LBBB- Left Bundle Branch Block; LCX- Left Circumflex Artery; LV- Left Ventricular; LVH- Left Ventricular Hypertrophy; LVOT- Left Ventricular Outflow Tract; LVOTO- Left Ventricular Outflow Tract Obstruction; LVOTG- Left Ventricular Outflow Tract Gradient; MR- Mitral Regurgitation; MVOHCM- Midventricular Obstruction Hypertrophic Cardiomyopathy; NT-proBNP- N-terminal pro-B-type natriuretic peptide; NYHA- New York Heart Association; RBBB- Right Bundle Branch Block; AVB- Atrioventricular Block; SAM- Systolic Anterior Motion; SCD- Sudden Cardiac Death; SRT- Septal Reduction Therapy; SSM- Surgical Septal Myectomy; TTE- Transthoracic Echocardiography; TEE- Transoesophageal Echocardiography
1. Introduction
Hypertrophic cardiomyopathy (HCM) is mainly an autosomal dominant inherited heart disease. In adolescents and adults, 40%~60% of HCM is caused by gene mutations encoding myocardial sarcomere proteins, 5%~10% is caused by other gene mutations or nongenetic diseases, and 25%~30% cases of HCM have unknown causes [5,6]. Clinical diagnosis of HCM is made by imaging with two-dimensional echocardiography. The most important haemodynamic consequence of this condition is left ventricular outflow tract (LVOT) obstruction (LVOTO), in which 70% of patients develop a dynamic LV outflow gradient of 30 mmHg or greater [7]. LVOTO is typically associated with HCM and is the third most frequent cause of unexplained hypotension [8]. In addition, there are many wide clinical manifestations, such as chest pain, chest tightness, anhelation, palpitation, syncope, and sudden cardiac death (SCD).
2. Case Presentation
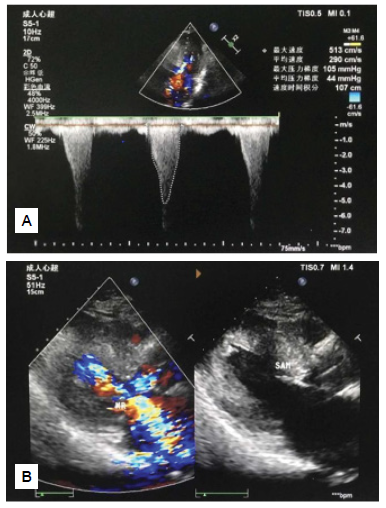
On July 7, 2022, a 56-year-old man presented with chest tightness and palpitations which had been occurring post-activity for the previous 6 months. The patient suffered from slight chest tightness and palpitation, which was a paroxysmal dull pain that was located in the lower section of the sternum and lasted from more than a couple of minutes to one hour with connection to physical activity. The symptoms were accompanied by chest pain and shortness of breath, but not by dizziness, syncope or haemoptysis. He had not received treatment. The patient had smoked five cigarettes a day for 20 years. He denied the use of any alcohol and did not have any known chronic diseases (such as coronary heart disease (CHD), hypertension, diabetes, etc.). There were no similar diseases or any other genetic history of disease in his family. The patient’s vital signs were normal upon admission. On physical examination, the patient’s body temperature was 36.5°C, pulse rate was 74 beats/min, respiratory rate was 18 breaths/min, blood pressure was 126/88 mmHg, weight was 66 kg, and height was 1.68 metres. The patient had a clear mind, upright posture, no cyanosis of lips, no dilation of jugular veins, and there was no negative sign of hepatic jugular venous reflux. The respiratory sounds of both lungs were clear, and there were no dry or wet rales. The cardiac boundary was normal, with a heart rate of 74 beats/min. The heart rhythm was uniform, and there was a systolic ejection murmur in the fourth intercostal space on the left edge of the sternum. The abdomen was flat and soft, without tenderness and rebound pain, and the bowel sound was normal. There was no oedema in either of the lower limbs. Auxiliary examination results showed no detectable changes in myocardial enzymes, myoglobin, serum troponin T, blood routine, coagulation function, liver and kidney function, serum glucose, electrolytes, blood lipids, N-terminal pro-B-type natriuretic peptide (NT-proBNP) or chest X-ray. The patient refused genetic testing for HCM. Electrocardiographic recordings during sinus rhythm showed an ST change, ST segment depression and T-wave inversion in leads V2 to V6. Dynamic electrocardiogram (DCG) showed the same results as electrocardiography (ECG), except for occasional atrial premature contraction and ventricular premature contraction. Transthoracic echocardiography (TTE) revealed the following (Figures 1A and 1B): 1. Significant hypertrophy (16 mm) and protrusion (15 mm) of the basal segment of the ventricular septum + II° systolic anterior motion (SAM) of the mitral valve resulted in severe obstruction of the LVOT (the narrowest inner diameter was 7 mm, peak gradient of LVOT 105 mmHg, average gradient of LVOT 44 mmHg) and moderate to severe mitral regurgitation (MR) (max regurgitating velocity 7.3 m/second, max regurgitating gradient 213 mmHg, instantaneous backflow 57 ml). 2. The echo light spots of the ventricular septum and left ventricular (LV) myocardium were slightly thickened and enhanced. (HCM?) 3. Mild aortic valve regurgitation was observed. 4. The LV diastolic function was poor (FS: 38%), and there were no obvious abnormalities in the LV overall contractile function (EF: 68%).
3. Admission Diagnosis
The patient was diagnosed with the following: 1. Hypertrophic Obstructive Cardiomyopathy (HOCM)? 2. Coronary Heart Disease (CHD)? Cardiac magnetic resonance (CMR) showed significant hypertrophy of the ventricular septum wall of the LV, with the thickest location being approximately 14 mm (Se17 Img 25), and II° SAM of the mitral valve. Myocardial blood flow perfusion was without defect. Myocardial enhancement was delayed. CMR indicated the diagnosis of HOCM. Coronary angiography (CAG) showed that some plaques were observed in the left anterior descending (LAD) artery with 30% stenosis. There was a TIMI flow of grade 3. No plaques or occlusion were observed in the left trunk, left circumflex artery (LCX) or right coronary artery. The blood stream was unobstructed. The left ventricular septal branch was not observed providing collateral blood supply to other areas of the myocardium. Left ventriculography showed that the interventricular septum under the mitral valve and the middle part of the interventricular septum were significantly hypertrophied. The guide conduit was sent to the left coronal opening. The guide wire passed through the first septal branch to the distal end, and then the first septal branch was blocked by balloon test. At the same time, the MR decreased from 50 ml to 15 ml. This confirmed that the MR was related to the SAM phenomenon in HOCM.
4. Definite Diagnosis
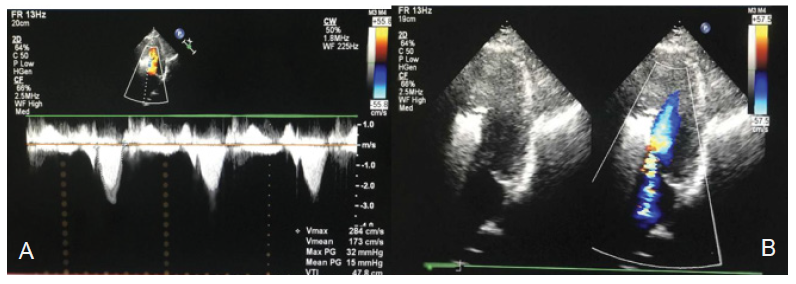
The patient was diagnosed with HOCM, New York Heart Association (NYHA) class II and coronary arteriosclerosis. During his hospitalization, the patient was treated with metoprolol succinate sustained release tablets (47.5 mg, qd) and atorvastatin tablets (20 mg, qn). The patient was advised to quit smoking and to avoid competitive sports activities, large meals, dehydration and excess alcohol. The patient should maintain a healthy body mass index and a healthy lifestyle [9]. Next, alcohol septal ablation (ASA) was planned, and permanent dual chamber pacemaker implantation was performed before the operation. Permanent dual chamber pacemaker implantation was performed on July 26, 2022. The patient improved and was discharged on August 1, 2022. The patient was hospitalized in our department on September 12 once more and was treated with ASA on September 15. During the operation, the TTE results showed that the peak gradient of LVOT was 89 mmHg, the average gradient of LVOT was 42 mmHg, and the MR was 41 ml. The left ventricular septal branch was blocked by 3 ml of medicinal anhydrous alcohol injected slowly from the over-the-wire (OTW) balloon. The patient immediately felt mild chest tightness and chest pain, and was immediately injected with 5 mg of morphine, causing relief of the symptoms. After observation for 5 minutes, part of the pipe was flushed. Postoperatively, TTE showed the following (Figures 2A and 2B): 1. Increased blood flow velocity in the LVOT + I° SAM of the mitral valve (velocity 2.8 m/second, peak gradient of LVOT 32 mmHg, average gradient of LVOT 15 mmHg). 2. Mild to moderate MR (instantaneous backflow 18 ml). Two days later, the patient generally had no chest tightness, chest pain, or palpitations after activities, but his heart rate was 66 beats/min. The patient was advised to continue taking metoprolol succinate sustained release tablets (95 mg, qd) and atorvastatin tablets (20 mg, qn). The patient improved and was discharged on September 17, 2022. He was advised to be evaluated according to ECG, TTE, and CMR after 6 months.
5. Results and Discussion
We presented a case of HOCM with a demonstrated SAM of the mitral valve with a peak instantaneous gradient of 219 mmHg across the basal LV cavity. Postoperative SAS and TTE revealed a significant reduction in the LVOT gradient, improvement in SAM, and reduction in the MR grade. He had no symptom recurrence since he was discharged. In patients with HCM, approximately 30%-35% are LVOTO, which is considered significant with a peak gradient ≥30 mmHg; in another 30%-35% of HCM patients, the obstruction is latent and is inducible only by provocative manoeuvres (e.g., Valsalva or amyl nitrite inhalation). It can also be diagnosed by exercise echocardiography [10]. The remaining 30%-40% of patients do not have either a resting or provoked LVOT gradient and are classified as having nonobstructive HCM [11,12]. The former two are called HOCM. LVOTO was distinguished mainly by two-dimensional and Doppler echocardiography. Notably, LVOTO in HCM is a labile and dynamic phenomenon that can vary depending on LV afterload, preload, and contractility [13,14]. The presence of a resting peak LVOT gradient ≥30 mmHg is associated with an increased risk of SCD and progression to NYHA Class III or IV symptoms of heart failure [15]. In an adult, HCM is defined by a wall thickness ≥15 mm in one or more LV myocardial segments—as measured by any imaging technique (echocardiography, CMR imaging or computed tomography (CT)). Genetic and nongenetic disorders can present with lesser degrees of wall thickening (13-14 mm); in these cases, the diagnosis of HCM requires evaluation of other features, including family history, noncardiac symptoms and signs of ECG abnormalities, laboratory tests and multimodality cardiac imaging [9]. SAM was described for the first time in the 1960s in patients with asymmetrical HCM, and it was initially believed to be one of the pathognomonic signs of this disease entity [16]. LVOTO has been associated with SAM of the mitral valve, which is observed in 30–60% of patients with HCM [16-18]. However, there have been reports confirming that the following have been associated with SAM phenomenon: acute obstruction of the interventricular branch of the left coronary artery, mitral valve repair surgeries and ventricular septal hypertrophy in hypertension (under conditions of absolute hypovolemia), which can lead to LVOTO [8, 19]. TTE is the most common method for diagnosing HCM, while CMR is the most accurate method for diagnosing HCM; at the same time, CMR is the preferred noninvasive imaging method for evaluating myocardial fibrosis [20, 21]. Sometimes, it is very important to diagnose HCM with CMR. Sharrack et al found that a man with Marfan syndrome who underwent urgent echocardiography was suggestive of HCM, but cardiovascular magnetic resonance identified features of acute myocarditis. Repeated imaging 4 months later showed resolution of septal thickness, confirming acute myocarditis [22]. If TTE is not performed to identify patients with HCM, it is recommended that transoesophageal echocardiography (TEE) be performed. Commonly, TEE is performed before surgical septal mastectomy (SSM). Ventriculography is performed to identify different types of HCM. Ventriculography shows the “Spade A sign”, indicating apical hypertrophic cardiomyopathy (ApHCM). Recently, there have been three treatments for HOCM, including medical therapies, SSM, and ASA. SSM and ASA are called septal reduction therapy (SRT). Resting or provoked gradients of ≥50 mm Hg are considered a threshold for invasive therapy in patients who exhibit drug-refractory symptoms [1, 23]. Medical therapies are the basic treatment for HOCM, including β-receptor blockers, first-in-class targeted inhibitors of cardiac myosin, calcium channel blockers (CCBs), and class Ia antiarrhythmic drugs. β-Receptor blockers are a first-line treatment for symptomatic HOCM [9, 24-25], and they inhibit myocardial contractility, reduce the LVOT gradient, especially the LVOT gradient in exercise, reduce LVOTO, decrease the heart rate, improve ventricular diastolic filling, and significantly improve patients' cardiac function and quality of life [26-28]. The patients are required to control their heart rate at 55 to 60 beats per minute. β-Receptor blockers may also be used in children with HCM. Mavacamten and aficament are first-in-class targeted inhibitors of cardiac myosin, which are used in patients with symptomatic HOCM. Mavacamten has been shown to improve symptom burden and exercise capacity by reducing the left ventricular outflow tract gradient (LVOTG) in the EXPLORER-HCM phase 3 study [29]. Mavacamten was the first and only approved myocardial globulin inhibitor approved by the US Food and Drug Administration (FDA) in April 2022 and can be used in adults with symptomatic NYHA functional class II~III HOCM to improve functional capacity and symptoms. Among severely symptomatic drug-refractory HOCM patients meeting guideline criteria of eligibility for SRT, receiving mavacamten drug therapy for 16 weeks significantly reduced or obviated the need for SRT using clinically driven endpoints [30]. Compared with placebo, aficament can significantly reduce LVOTG and levels of NT-proBNP [31]. CCBs are also commonly used to treat symptomatic (i.e., reduced muscle strength and frequency) HOCM. Contraindications such as sick sinus syndrome, hypotension, and LVOTG ≥80-100 mmHg should be excluded. Propiramide and sibenzozoline are class ?a antiarrhythmic drugs. If patients still have symptoms related to LVOTO after using β-receptor blockers or CCBs, it is recommended to add propiramide or sibenzozoline by titrating gradually to the maximum tolerable dose. Propiramide can reduce the SAM phenomenon, the MR grade, and LVOTG [32-33]. SRT: NYHA functional class III or IV, the peak of systolic gradient in LVOT ≥50 mmHg, is related to left ventricular hypertrophy (LVH) or SAM phenomenon. The septal interval thickness can be operated safely and effectively. At present, both SSM and ASA are effective ways to treat HOCM, and there is no significant difference between them in reducing LVOTO, improving symptoms or quality of life, or in short- or long-term mortality [34]. Between ASA and SSM, the long-term survival rate and the clinical outcome are comparable [35]. However, the incidence of residual obstruction after ASA is higher, and the probability of reintervention may be higher. An analysis of 3859 patients with HOCM showed that, after adjusting for age, sex and complications, the 10-year all-cause mortality risk of ASA increased by 68% compared with SSM [36]. SSM was reported earliest by Morrow et al. in 1961 [37] and was the standard surgical procedure for HOCM to open-chest, aortic-incision septal myocardectomy. Later, the Morrow procedure expanded the scope of septal resection, extending distal to the mitral septal contact site to the base of the papillary muscle and even the apex, which is termed extended ventricular septal myocardectomy or modified Morrow procedure. Currently, in addition to ventricular septal hypertrophy leading to myocardectomy of the ventricular septum via predominantly the aortic incision, other special types of HOCM, such as ApHCM, may be treated by myocardectomy via apical incision [38], and mid-ventricular obstruction hypertrophic cardiomyopathy (MVOHCM) may be performed by myocardectomy via apical incision or aortic incision [39]. The operative-related mortality rate for SSM alone is currently less than 1% [40], whereas the mortality rate for SSM combined with mitral valve surgery is 3%-5%. Therefore, SSM is recommended for HOCM combined with mitral valve disease requiring surgical treatment. However, patients with RBBB are prone to complicate ?° atrioventricular block (AVB) after SSM. ASA for the treatment of HOCM was introduced as a percutaneous alternative to surgical myectomy in 1995 [41]. The rate of major adverse cardiac events after ASA is less than 2% [42]. Perioperative complications mainly included AVB and right bundle branch block (RBBB), so postoperative patients more often require pacemaker implantation [43]. Patients with HOCM following ASA are at an increased risk of developing AVB after SSM [44]. Repeated septal reduction therapy after ASA is not associated with a higher risk of major cardiovascular events over a long-term follow-up period, but patients more often require pacemaker implantation [45]. AVB or RBBB was prevented with permanent dual chamber pacemaker implantation before ASA (Class IIb recommendation and level C). Liebregts et al. suggested that ASA in younger patients with HOCM was safe and effective for the relief of symptoms at long-term follow-up. They proposed that the indication for ASA can be broadened to younger patients [43]. Lucon et al. illustrated that the effects of very long-term DDD pacing in patients with symptomatic HOCM with NYHA functional class ≥II were beneficial [46].
6. Conclusion
- LVOTO has been associated with the SAM phenomenon, which occurs not only in HOCM but also in other diseases.
- HCM is diagnosed by two-dimensional echocardiography or CMR.
- Prior to SAS, the patient received permanent dual chamber pacemaker implantation to avoid AVB or
- LVOTO in HCM is a labile and dynamic phenomenon that requires dynamic evaluation.
Declarations
At approximately the 2-month outpatient follow-up, the patient stated that he took the medicine regularly.
Study Limitations
The patient refused genetic testing for HCM. The most important limitation of this study is its lack of long-term follow-up, and the subsequent inability to assess the patient's prognosis.
Funding
Funding information is not applicable.
Conflicts of Interest
The authors declare that they do not have any conflicts of interest.
Ethics Approval
Written informed consent was obtained from the patient.
Consent to Participate
The work was approved by all the authors.
Consent to Publication
The work has been approved for publication by all the authors.
Availability of Data and Material
Some or all data and material generated or used during the study are available in a repository or online (provide full citations that include URLs or DOI).
Code Availability
Some or all code generated or used during the study are available in a repository or online (provide full citations that include URLs or DOI).
Author Contributions
Conceptualization: Mei-lian Cai, Data curation: Mei-lian Cai. Investigation: Mei-lian Cai, Resources: Mei-lian Cai. Supervision: Guo-qiang Zhong, Writing- original draft: Mei-lian Cai. Writing-review & editing: Mei-lian Cai.
References
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [J]. J Thorac Cardiovasc Surg 162 (2021): e23-e106.
- Maron BJ, Gardin JM, Flack JM, et al. Prev-alence of hypertrophic cardiomyopathy in agene ralpopulation of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Developmentin (Young) Adults [J]. Circulation 92 (1995): 785-789.
- MaronB J, Mathenge R, Casey SA, et al. Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities [J]. J Am Coll Cardiol 33 (1999): 1590-1595.
- Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy [J]. J Am Coll Cardiol 65 (2015): 1249-1254.
- Authors/Task Force members, Elliott PM, Anastasakis A, et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) [J]. Eur Heart J 35 (2014): 2733-2779.
- Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives[J]. J Am Coll Cardiol 60 (2012): 705-715.
- Albano BB, Fadreguilan EC, Chua JM, et al. Treating a Structural Heart Disease Using a Non-structural Approach: Role of Cardiac Pacing in Hypertrophic Cardiomyopathy [J]. Cardiol Res 8 (2017): 20-25.
- Sobczyk D. Dynamic left ventricular outflow tract obstruction: underestimated cause of hypotension and hemodynamic instability [J]. J Ultrason 14 (2014): 421-42
- Authors/Task Force members, Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) [J]. Eur Heart J 35 (2014): 2733-2779.
- Rowin EJ, Maron BJ, Olivotto I, et al. Role of exercise testing in hypertrophic cardiomyopathy [J]. JACC Cardiovasc Imaging 10 (2017): 1374-1386.
- Maron BJ, Ommen SR, Semsarian C, et al. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine [J]. J Am Coll Cardiol 64 (2014): 83-99.
- Wigle ED. Cardiomyopathy: The diagnosis of hypertrophic cardiomyopathy [J]. Heart 86 (2001): 709-714.
- Adams JC, Ommen SR, Klarich KW, et al. Signifificance of postprandial symptom exacerbation in hypertrophic cardiomyopathy [J]. Am J Cardiol 105 (2010): 990-992.
- Geske JB, Sorajja P, Ommen SR, et al. Left ventricular outflow tract gradient variability in hypertrophic cardiomyopathy [J]. Clin Cardiol 32 (2009): 397-402.
- Maron MS, Olivotto I, Betocchi S, et al. Effect of left ventricular outflflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy [J]. N Engl J Med 348 (2003): 295-303.
- Luckie M, Khattar RS. Systolic anterior motion of the mitral valve--beyond hyperthrophic cardiomyopathy [J]. Heart 94 (2008): 1383-1385.
- Luckner G, Margreiter J, Jochberger S, et al. Systolic anterior motion of the mitral valve with left ventricular outflow tract obstruction: three cases of acute perioperative hypotension in noncardiac surgery [J]. Anesth Analg 100 (2005): 1594-1598.
- Ibrahim M, Rao C, Ashrafifi an H, et al. Modern management of systolic anterior motion of the mitral valve [J]. Eur J Cardiothorac Surg 41 (2012): 1260-1270.
- Maslow AD, Regan MM, Hearing JM, et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease [J]. J Am Coll Cardiol 34 (1999): 2096-2104.
- Nagueh SF, Phelan D, Abraham T, et al. Recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: anupdate from the American Society of Echocardiography, in collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography [J]. J Am Soc Echocardiogr 35 (2022): 533-569.
- Cardiovascular Physicians Branch of Chinese Medical Doctor Association, Editorial Committee of Chinese Journal of Cardiovascular Diseases. The Chinese expert consensus on the clinical application of magnetic resonance imaging in cardiomyopathy [J]. The Chinese Journal of Cardiovascular Disease 43 (2015): 673-681.
- Sharrack N, Poenar AM, Simms AD, et al. Acute Myocarditis Mimicking Hypertrophic Cardiomyopathy in Marfan Syndrome and Morphologically Abnormal Mitral Valve [J]. JACC Case Rep 4 (2022): 105-110.
- Sherrid MV, Shetty A, Winson G, et al. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with beta-blockade or verapamil [J]. Circ Heart Fail 6 (2013): 694-702.
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [J]. J Am Coll Cardiol 76 (2020): 3022-3055.
- Kitaoka H, Tsutsui H, Kubo T, et al. Japanese Circulation Society Joint Working Group. JCS/JHFS 2018 Guideline on the Diagnosis and Treatment of Cardiomyopathies [J]. Circ J 85 (2021): 1590-1689.
- Dybro AM, Rasmussen TB, Nielsen RR, et al. Randomized trial of metoprolol in patients with obstructive hypertropic cardiomyopathy [J]. J Am Coll Cardiol 78 (2021): 2505-2517.
- Dybro AM, Rasmussen TB, Nielsen RR, et al. Effects of metoprolol on exercise hemodynamics in patients with obstructive hypertrophic cardiomyo pathy [J]. J Am Coll Cardiol 79 (2022): 1565-1575.
- Monda E, Lioncino M, Palmiero G, et al. Bisoprolol for treatment of symptomatic patients with obstructive hypertrophic cardiomyopathy. The BASIC (bisoprolol AS therapy in hypertrophic cardiomyopathy) study [J]. Int J Cardiol 354 (2022): 22-28.
- Ho CY, Olivotto I, Jacoby D, et al. Study Design and Rationale of EXPLORER-HCM: Evaluation of Mavacamten in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy[J]. Circ Heart Fail 13 (2020): e006853.
- Desai MY, Wolski K, Owens A, et al. Study design and rationale of VALOR-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy who are eligible for septal reduction therapy [J]. Am Heart J 239 (2021): 80-89.
- Owens AT, Masri A, Abraham TP, et al. Efficacy and safety of aficamten and disopyramidecoad ministrationin obstructive hypertrophic cardiomyopathy: results from REDWOOD-HCM cohort 3 [J]. J Am Coll Cardiol 79 (2022): 244.
- Sherrid MV, Shetty A, Winson G, et al. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with β-blockade or verapamil [J]. Circ Heart Fail 6 (2013): 694-702.
- Adler A, Fourey D, Weissler-Snir A, et al. Safety of outpatient initiation of disopyramide for obstructive hypertrophic cardiomyopathy patients [J]. J Am Heart Assoc 6 (2017): e005152.
- Nguyen A, Schaff HV, Hang D, et al. Surgical myectomy versusal cohol septal ablation for obstructive hypertrophic cardiomyopathy: Apropensity score-matched cohort [J]. J Thorac Cardiovasc Surg 157 (2019): 306-315.
- Meng X, Wang WY, Gao J, et al. Hypertrophic Obstructive Cardiomyopathy: Comparison of Outcomes After Myectomy or Alcohol Ablation [J]. Front Cardiovasc Med 9 (2022): 755376.
- Cui H, Schaff HV, Wang S, et al. Survival following alcohol se ptal ablation or septal myectomy for patients with obstructive hypertrophic cardiomyopathy [J]. J Am Coll Cardiol 79 (2022): 1647-1655.
- Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy [J]. Ann Surg 154 (1961): 181-189.
- Schaff HV, Brown ML, Dearani JA, et al. Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy [J]. J Thorac Cardiovasc Surg 139 (2010): 634-640.
- Hang D, Schaff HV, Ommen SR, et al. Combined transaortic and transapical approach to septal myectomy in patients with complex hypertrophic cardiomyopathy [J]. J Thorac Cardiovasc Surg 155 (2018): 2096-2102.
- Maron BJ, Dearani JA, Ommen SR, et al. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction [J]. J Am Coll Cardiol 66 (2015): 1307-1308.
- Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 346 (1995): 211-21
- Chinese Heart Failure Association of Chinese Medical Docter Association, National Heart Failure Committee, Editorial Board of Chinese Journal of Heart Failure and Cardiomyopathy. 2022 Chinese guildeline on hypertrophic cardimyopathy [J]. Chin J Heart Fail & Cardiomyopathy 6 (2022): 80-103.
- Liebregts M, Faber L, Jensen MK, et al. Outcomes of Alcohol Septal Ablation in Younger Patients With Obstructive Hypertrophic Cardiomyopathy. JACC Cardiovasc Interv 10 (2017): 1134-1143.
- Yang Q, Zhu C, Cui H, et al. Surgical septal myectomy outcome for obstructive hypertrophic cardiomyopathy after alcohol septal ablation. J Thorac Dis 13 (2021): 1055-1065.
- Veselka J, Faber L, Liebregts M, et al. Long-term outcome of repeated septal reduction therapy after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: insight from the Euro-ASA registry. Arch Med Sci 16 (2020): 1239-1242.
- Lucon A, Palud L, Pavin D, et al. Very late effects of dual chamber pacing therapy for obstructive hypertrophic cardiomyopathy. Arch Cardiovasc Dis 106 (2013): 373-3