Human Herpesvirus 6 Reactivation: A Rare Case of Acute Liver Failure and Literature Review
Article Information
Hannah Wozniak1*, Jeanne-Laure Vionnet1*, Manuel Schibler2, Claudia Paula Heidegger1, Jérôme Pugin1, Laurent Spahr3, Sara Cereghetti1
1Division of Intensive Care, Department of Acute Medicine, Geneva University Hospitals and University of Geneva, Geneva, Switzerland
2Division of Infectious Diseases, Department of Medicine, Geneva University Hospitals and University of Geneva, Geneva, Switzerland
3Division of Gastroenterology and Hepatology, Department of Medicine, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland
*contributed equally.
*Corresponding Author: Sara Cereghetti, Division of Intensive Care, Department of Acute Medicine, Geneva University Hospitals and University of Geneva, Geneva, Switzerland.
Received: 02 December 2022; Accepted: 30 December 2022; Published: 16 January 2023
Citation: Hannah Wozniak, Jeanne-Laure Vionnet, Manuel Schibler, Claudia Paula Heidegger, Jérôme Pugin, Laurent Spahr, Sara Cereghetti. Human Herpesvirus 6 Reactivation: A Rare Case of Acute Liver Failure and Literature Review. Archives of Clinical and Medical Case Reports 7 (2023): 01-04.
View / Download Pdf Share at FacebookAbstract
Acute hepatitis is a frequent cause of admission to intensive care unit (ICU). The differential diagnosis is broad and determining the etiology can be challenging. We report a 68-year-old immunocompromised patient who developed acute liver failure associated with pericardial effusion who was diagnosed with human herpesvirus 6 (HHV-6) reactivation. The diagnosis was made by a positive real-time PCR (rPCR). After an extensive hepatitis workup with viral, toxic and autoimmune investigations and after exclusion of an inherited chromosomally integrated HHV-6 by rPCR of the fingernails, no convincing alternative diagnosis was found. The evolution of transaminases and viremia monitoring by quantitative PCR under antiviral treatment is described. Although HHV-6 reactivation is a very rare cause of acute hepatitis and is difficult to diagnose, fulminant hepatitis is one of the most common complications of HHV-6 reactivation. Since acute liver failure can lead to specific medical interventions, to liver transplantation, or can be fatal, the diagnosis of HHV-6 should be systematically considered, and early detection can lead to a better prognosis.
Keywords
HHV-6 Reactivation; Hepatitis; Immunocompromised; Pericardial Effusion
HHV-6 Reactivation articles; Hepatitis articles; Immunocompromised articles; Pericardial Effusion articles
HHV-6 Reactivation articles HHV-6 Reactivation Research articles HHV-6 Reactivation review articles HHV-6 Reactivation PubMed articles HHV-6 Reactivation PubMed Central articles HHV-6 Reactivation 2023 articles HHV-6 Reactivation 2024 articles HHV-6 Reactivation Scopus articles HHV-6 Reactivation impact factor journals HHV-6 Reactivation Scopus journals HHV-6 Reactivation PubMed journals HHV-6 Reactivation medical journals HHV-6 Reactivation free journals HHV-6 Reactivation best journals HHV-6 Reactivation top journals HHV-6 Reactivation free medical journals HHV-6 Reactivation famous journals HHV-6 Reactivation Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Hepatitis articles Hepatitis Research articles Hepatitis review articles Hepatitis PubMed articles Hepatitis PubMed Central articles Hepatitis 2023 articles Hepatitis 2024 articles Hepatitis Scopus articles Hepatitis impact factor journals Hepatitis Scopus journals Hepatitis PubMed journals Hepatitis medical journals Hepatitis free journals Hepatitis best journals Hepatitis top journals Hepatitis free medical journals Hepatitis famous journals Hepatitis Google Scholar indexed journals Ultrasound articles Ultrasound Research articles Ultrasound review articles Ultrasound PubMed articles Ultrasound PubMed Central articles Ultrasound 2023 articles Ultrasound 2024 articles Ultrasound Scopus articles Ultrasound impact factor journals Ultrasound Scopus journals Ultrasound PubMed journals Ultrasound medical journals Ultrasound free journals Ultrasound best journals Ultrasound top journals Ultrasound free medical journals Ultrasound famous journals Ultrasound Google Scholar indexed journals Immunocompromised articles Immunocompromised Research articles Immunocompromised review articles Immunocompromised PubMed articles Immunocompromised PubMed Central articles Immunocompromised 2023 articles Immunocompromised 2024 articles Immunocompromised Scopus articles Immunocompromised impact factor journals Immunocompromised Scopus journals Immunocompromised PubMed journals Immunocompromised medical journals Immunocompromised free journals Immunocompromised best journals Immunocompromised top journals Immunocompromised free medical journals Immunocompromised famous journals Immunocompromised Google Scholar indexed journals Laryngoplasty articles Laryngoplasty Research articles Laryngoplasty review articles Laryngoplasty PubMed articles Laryngoplasty PubMed Central articles Laryngoplasty 2023 articles Laryngoplasty 2024 articles Laryngoplasty Scopus articles Laryngoplasty impact factor journals Laryngoplasty Scopus journals Laryngoplasty PubMed journals Laryngoplasty medical journals Laryngoplasty free journals Laryngoplasty best journals Laryngoplasty top journals Laryngoplasty free medical journals Laryngoplasty famous journals Laryngoplasty Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals SRAS-CoV-2 articles SRAS-CoV-2 Research articles SRAS-CoV-2 review articles SRAS-CoV-2 PubMed articles SRAS-CoV-2 PubMed Central articles SRAS-CoV-2 2023 articles SRAS-CoV-2 2024 articles SRAS-CoV-2 Scopus articles SRAS-CoV-2 impact factor journals SRAS-CoV-2 Scopus journals SRAS-CoV-2 PubMed journals SRAS-CoV-2 medical journals SRAS-CoV-2 free journals SRAS-CoV-2 best journals SRAS-CoV-2 top journals SRAS-CoV-2 free medical journals SRAS-CoV-2 famous journals SRAS-CoV-2 Google Scholar indexed journals Pericardial Effusion articles Pericardial Effusion Research articles Pericardial Effusion review articles Pericardial Effusion PubMed articles Pericardial Effusion PubMed Central articles Pericardial Effusion 2023 articles Pericardial Effusion 2024 articles Pericardial Effusion Scopus articles Pericardial Effusion impact factor journals Pericardial Effusion Scopus journals Pericardial Effusion PubMed journals Pericardial Effusion medical journals Pericardial Effusion free journals Pericardial Effusion best journals Pericardial Effusion top journals Pericardial Effusion free medical journals Pericardial Effusion famous journals Pericardial Effusion Google Scholar indexed journals Treatment articles Treatment Research articles Treatment review articles Treatment PubMed articles Treatment PubMed Central articles Treatment 2023 articles Treatment 2024 articles Treatment Scopus articles Treatment impact factor journals Treatment Scopus journals Treatment PubMed journals Treatment medical journals Treatment free journals Treatment best journals Treatment top journals Treatment free medical journals Treatment famous journals Treatment Google Scholar indexed journals
Article Details
1. Background
Acute hepatitis is a frequent cause of admission to intensive care unit (ICU). The differential etiologic diagnosis of acute hepatitis is wide, and pinpointing the actual cause can be challenging. Classical causes of acute hepatitis are viral infections, drugs, alcohol and immune diseases [1]. HHV-6 is a rare but sometimes severe cause of acute hepatitis, and is observed mainly either in young children in the setting of primary infection, or in immunosuppressed pediatric and adult patients following uncontrolled viral reactivation. While some antiviral treatments have been proposed for HHV-6 hepatitis management, the optimal therapy is not well defined. We present here a case of acute HHV-6 hepatitis in a patient on immunosuppressive treatment with a review of the literature on this topic.
2. Case Presentation
A 68 year-old man with a history of seronegative polyarthritis was admitted to the emergency room with dyspnea, nausea, vomiting and diarrhea for two days. His medical treatment consisted of prednisone 30 mg daily (tapering regimen) and of recently introduced methotrexate 15 mg daily. His vital signs showed tachycardia of 100 beats per minute, normal blood pressure and temperature, and decreased oxygen saturation requiring oxygen therapy (4l/min via nasal cannulae). Clinical examination revealed lower limb edema and disseminated skin rash. Blood tests revealed leukocytosis (22.6 G/l), thrombocytopenia (68 G/l), elevated C-reactive protein (CRP, 132mg/l), acute renal failure (KDIGO3, creatinine 223umol/l, eGFR 23ml/min/m2), elevated liver enzymes (ASAT > 7000U/l, ALAT 5121 U/l, GGT 340U/l) and normal bilirubin. Coagulation tests indicated reduced factor V levels (12%), increased INR (2.59), low Quick (24%), and prolonged PTT (47,7 seconds). The arterial blood gas analysis showed metabolic acidosis (pH 7.28, lactates 8 mmol/l). Abdominal ultrasound showed signs of a congestive liver with hyperechogenic hepatomegaly, modulated portal vein flow and reflux into the suprahepatic veins. The CT-scan also showed an enlarged liver with discrete heterogeneous parenchyma, periportal edema and vesicular wall infiltration (Figure 1). It also revealed a circumferential pericardial effusion up to 14 mm thick around the left ventricle and a moderate pleural effusion. A subsequent echocardiography was performed, which confirmed the pericardial effusion of 15 mm, without signs of compression of the right heart cavities and a normal biventricular function (Figure 2). A diagnosis of acute liver failure (ALF) of yet undetermined origin was retained. A broad ALF investigation workup including virological, autoimmune and toxic tests was performed. The differential diagnosis of hypoxic hepatitis was also considered. N-acetyl-cysteine and empirical IV acyclovir treatment (10mg/kg twice daily) were initiated and the patient was admitted to the ICU. He rapidly developed multi-organ failure with impaired consciousness requiring orotracheal intubation and mechanical ventilation, anuria with hypervolemia treated with continuous veno-venous hemofiltration, and arterial hypotension requiring vasopressor medication. 24 hours after admission, an echocardiography showed an increase of the pericardial effusion with compression of the right cavities. A pericardial drainage allowed the release of 60 ml of serum. The results of the biological examination revealed a positive HHV-6 real-time PCR (rPCR) (quantified at 44000 copies/ml). HHV-6 serology was positive for IgG and negative for IgM, indicating past infection. The rPCR of HHV-6 from a serum sample taken 28 days earlier was negative, as was the HHV-6 rPCR performed on the patient's fingernails, thus ruling out inherited chromosomally integrated HHV-6 (iciHHV-6). HHV-6 detection by rPCR in the hemorrhagic pericardial fluid was positive (CT value 39.3). Investigations for other causes of viral hepatitis as well as paracetamolemia and the autoimmune workup were negative. HHV-6 reactivation associated with the newly introduced immunosuppressive drug (i.e. methotrexate) in addition to ongoing corticosteroid therapy leading to ALF, was retained. Antiviral treatment was changed to ganciclovir 2,5mg/kg/12h (dosage adapted to renal function), and the methotrexate treatment was interrupted. The clinical and biological evolution was excellent within five days of starting ganciclovir therapy (Figure 3). Indeed, the patient regained consciousness, allowing extubation, and liver enzymes, coagulation parameters and kidney function returned to normal. The total duration of treatment was three weeks. The rheumatologists suggested reducing prednisone therapy in view of this infectious event and recommended starting anti-IL-6 therapy six weeks after this acute event.
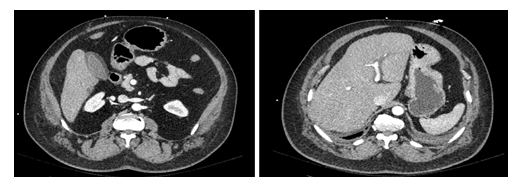
Figure 1: CT-scan with IV contrast injection showing an enlarged liver with discretely heterogeneous parenchyma, periportal edema and vesicular wall infiltration.
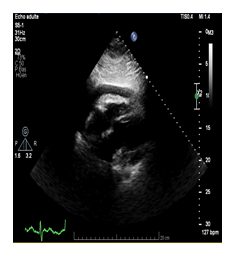
Figure 2: Transthoracic echocardiography-subcostal four-chamber view: pericardial effusion of 14 mm, without signs of compression of the right heart cavities and normal biventricular function.
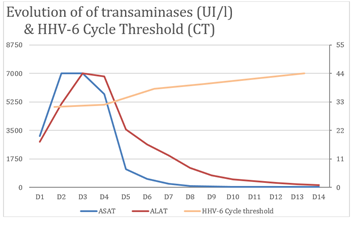
Figure 3: HHV-6 viral load assessed by rPCR CT values and liver tests over time. Ganciclovir treatment was started on day 1 (D1).
3. Discussion
In this paper, we describe the case of a patient who developed ALF in the context of the introduction of immunosuppressive therapy with methotrexate. This case illustrates how difficult it is to make a clear diagnosis in some cases of ALF, as the cause is sometimes due to several mechanisms. The definitive diagnosis in our case report probably results from a mixed origin: on the one hand, a viral hepatitis component following reactivation of HHV-6 in the context of immunosuppression and, on the other hand a hypoxic hepatitis component as a consequence of the pericardial effusion. There are only a few case descriptions of HHV-6 ALF in the literature [2]. The present case is unique in that it is the only one to describe the association of ALF with concomitant pericardial effusion and to show the evolution of viremia under treatment. HHV-6 belongs to the Herpesviridiae family and the Betaherpesviriniae subfamily, to the Roseolovirus genus, and is divided into two distinct species, HHV-6A and HHV-6B. It shares a lot of genomic and phenotypic similarities with CMV [3]. HHV-6 is worldwide prevalent and is mainly acquired in early childhood. Studies report rates of more than 90% in children over 2 years of age [4,5]. The exact mode of transmission is not yet fully understood, but close contact, saliva and urine are supposed to be part of the spreading [5]. Primary infection in children is usually asymptomatic or causes only mild disease (Roseola infantum, or sixth disease) [3]. The virus infects various cells, such as brain and liver tissue, salivary glands, endothelium, T cells and macrophages [6]. The virus subsequently persists lifelong in a latent form, as do herpesviruses in general [3]. Viral reactivations are usually controlled by cellular immunity, but can manifest clinically in various forms in the context of immunosuppression [6,7]. Current literature describes cases of encephalitis, retinitis, temporal lobe epilepsy or neurocognitive disorders in the context of HHV6 reactivation [8,9]. Cases of myocarditis [10,11] have also been described, as well as gastro-intestinal disorders, hepatitis [12,13], gastroenteritis [7)] or colitis. Some patients developed pneumonitis or drug-induced hypersensitivity syndrome [14]. All these presentations can be fatal [6]. Importantly, these severe courses occur mainly in severely immunosuppressed patients, typically hematopoietic transplant and solid organ recipients. The severity of HHV-6 reactivation in this case of an apparently moderately immunosuppressed patient (methotrexate and prednisone therapy) is therefore surprising. In the case of a positive HHV-6 PCR result that is not due to a primary infection, it must be first determined whether or not it is explained by inherited chromosomally integrated HHV-6 (iciHHV-6). In fact, HHV-6 is capable of chromosomal integration into the telomeric region of a germline host cell, resulting in the Mendelian inheritance of the viral DNA, of which at least one copy is present in all nucleated cells [15,16]. Fingernails or hair follicles can be tested by qualitative polymerase chain reaction (PCR) to identify iciHHV-6 as direct expression of host germ cell DNA [3,15,17]. When iciHHV-6 has been ruled out, it is then necessary yet sometimes tricky to assess the causality between HHV-6 replication and the patient’s clinical picture. In our case, the patient’s hepatitis has been linked to HHV-6 reactivation, since this virus is known to cause hepatic injury in the context of immunosuppression, and because no convincing alternative cause was identified despite an extensive etiologic work-up. Regarding antiviral therapy, because of the similarity with the CMV, antivirals with a similar spectrum of activity are usually used [3]. There are three molecules which have been studied to treat HHV-6 active infections: cidofovir, ganciclovir and foscarnet [3,6]. Considering their safety profile and in vitro activity, foscarnet 90 mg/kg IV every 12 hours and ganciclovir 5 mg/kg IV every 12 hours for three to four weeks, which must be adapted to renal function, are the first-line treatments chosen in Switzerland [3,19,20]. This treatment options have been used for HHV-6 reactivation mainly in transplanted patients, and there is little literature on non-transplant patients. Since these infections are rare and a final causality by HHV-6 is often difficult to establish, it remains unclear when and how an antiviral treatment against HHV-6 should be started. In a recent literature review [3] the authors suggest initiating treatment when 1) viral load is high and ici-HHV-6 is not present, 2) there is a context of immunosuppression, 3) HHV-6 appears to be the cause of the observed clinical findings, and 4) there is no other more likely cause. In addition to clinical response, monitoring viral load by quantitative PCR allows to confirm the antiviral’s efficacy, or on the contrary to suspect antiviral resistance [3]. In addition to antiviral treatment, immunosuppressive therapy should be reduced whenever possible [3].
4. Conclusion
Although reactivation of HHV-6 is a rare cause of acute liver failure, it should be considered as a cause in immunocompromised patients with acute liver failure of undetermined origin. There is little literature on the most appropriate antiviral treatment for HHV-6-induced ALF. This case illustrates the potential usefulness of ganciclovir for a favorable clinical outcome.
Disclosure Statement
No financial support and no other potential conflict of interest relevant to this article was reported.
References
- Samuel PI and D. Etiology and Prognosis of Fulminant Hepatitis in Adults. Liver Transplant 13 (2008): 767-768.
- Charnot-Katsikas A, Baewer D, Cook L, et al. Fulminant hepatic failure attributed to infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman: A case report and review of the literature. Clin. Virol 75 (2016): 27-32. Elsevier B.V.
- Bonnafous HAP, Gautheret-Dejean AA. Human Herpesviruses 6A, 6B, and 7. Spectr 4 (4).
- Dockrell DH, Smith TF, Paya CV. Human herpesvirus 6. Mayo Clin. Proc74(1999): 163-170. Mayo Foundation for Medical Education and Research.
- Daniel K. Braun GD, PEP. Human herpesvirus 6. Expert Rev. Mol. Med 1 (1997): 1-17.
- De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Microbiol. Rev 18 (2005): 217-245.
- Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Microbiol. Rev 28 (2015): 313-335.
- Voigt M, Sinn K, Malouhi A, et al. HHV-6 encephalitis in a non-transplanted adult acute myeloid leukemia patient. Hematol100 (2021): 1895-1897.
- Berzero G, Campanini G, Vegezzi E, et al. Human Herpesvirus 6 Encephalitis in Immunocompetent and Immunocompromised Hosts. Neuroimmunol. neuroinflammation 8 (2021): 1-10.
- Fukae S, Ashizawa N, Morikawa S, et al. A fatal case of fulminant myocarditis with human herpesvirus-6 infection. Med. 39 (2000): 632-636.
- Chang YL, Parker ME, Nuovo G, et al. Human herpesvirus 6-related fulminant myocarditis and hepatitis in an immunocompetent adult with fatal outcome. Pathol 40 (2009): 740-745. Elsevier Inc.
- Härmä M, Höckerstedt K, Lautenschlager I. Human herpesvirus-6 and acute liver failure. Transplantation 76 (2003): 536-539.
- Cacheux W, Carbonell N, Rosmorduc O, et al. HHV-6-related acute liver failure in two immunocompetent adults: Favourable outcome after liver transplantation and/or ganciclovir therapy. Intern. Med 258 (2005): 573-578.
- Ichiche M, Kiesch N, De Bels D. DRESS syndrome associated with HHV-6 reactivation. J. Intern. Med 14 (2003): 498-500.
- Pantry SN, Medveczky PG. Latency, integration, and reactivation of human herpesvirus-6. Viruses 9 (2017) : 1-12.
- Trempe F, Morissette G, Gravel A, et al. L’herpèsvirus humain de type 6 et l’intégration chromosomique: Conséquences biologiques et médicales associéVirologie 15 (2011): 381-393.
- Sedlak RH, Cook L, Huang ML, et al. Identification of chromosomally integrated human herpesvirus 6 by droplet digital PCR. Chem 60 (2014): 765-772.
- Human Herpesvirus 6 - HHV6. ARUP Consult® (2022).
- Ward KN, Hill JA, Hubacek P, et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica104 (2019): 2155-2163.
- Hill JA. Human herpesvirus 6 in transplant recipients: an update on diagnostic and treatment strategies. Curr Opin Infect Dis 32 (2019): 584-590.
