Huaier Therapy for Successful Recovery of Cancer and Health Maintenance: Steady Progress and the End of Failed Promise
Article Information
Manami Tanaka1*, Tomoo Tanaka1, Fei Teng2, Hong Lin2, Ning Li3, Zhu Luo3, Sotaro Sadahito4, Toshiyuki Suzuki5, Ding Wei6 and Zhengxin Lu7
1Bradeion Institute of Medical Sciences, Co., Ltd, Kanagawa 259-1145, Japan
2BGI-Shenzhen, Building NO.7, BGI Park, Shenzhen 518083, China
3BGI-Japan, Kobe 650-0047 Japan
4Department of Surgery, Kameda-Morinosato Hospital, Kanagawa 243-0122, Japan
5Department of Surgery, Oiso Hospital, Tokai University School of Medicine, Kanagawa 259-0198, Japan
6Japan Kampo NewMedicine, Co. Ltd., Tokyo 103−0025, Japan
7QiDong Gaitianli Medicines Co. Ltd., Jiangsu Province, China
*Corresponding author: Manami Tanaka, Bradeion Institute of Medical Sciences, Co., Ltd, Kanagawa 259-1145, Japan
Received: 30 May 2021; Accepted: 11 June 2021; Published: 23 June 2021
Citation: Manami Tanaka, Tomoo Tanaka, Fei Teng, Hong Lin, Ning Li, Zhu Luo, Sotaro Sadahito, Toshiyuki Suzuki, Ding Wei and Zhengxin Lu. Huaier Therapy for Successful Recovery of Cancer and Health Maintenance: Steady Progress and the End of Failed Promise. Archives of Clinical and Biomedical Research 5 (2021): 457-483.
View / Download Pdf Share at FacebookAbstract
The progress in cancer research has been processed by the discovery of molecules, genes, and mechanisms to cause carcinogenesis. Even with the advent of a novel technologies, the consequent drug discovery is still not enough to meet the demands, with the limited numbers of choice for anti-cancer strategy are not satisfactory. It is considered partly because mono-targeted agents should have certain limitations against diseases originated from multiple factors and environmental stresses throughout a life-long time. These defects in the current anti-cancer strategy of cancer therapy chiefly are caused by the lack of information required for cancer recovery. It is extremely required to obtain minimum-essential information to define the molecular basis for cancer recovery, with a comparison to that in the process of carcinogenesis. For this purpose, we should have successful agents possibly and reliably to cure the disease.
We have identified anti-cancer effects of Huaier, and its unique characteristics of 1) no side effects or toxicity, 2) in dose dependent manner, 3) causing specific cancer cell death with simultaneous tissue regeneration, 4) miRNA-mediated transcriptional control on the rescue of multiple signal transduction, by dependent on individual genomic potential. Here we summarize the results of our genome scope project on clinical research of Huaier therapy on cancer patients. MEGA-DATA analysis in the process of cancer recovery by total RNA and small non-coding RNA sequencing. The results revealed significant differences in the behavior and coordination of RNA editing events, biomolecules, and multiple signaling pathways which beyond any speculations from the findings in the process of carcinogenesis.
The time has come to change a point of view of “cancer therapy”, from the mere killing of cancer cells to regenerate the disrupted biophysiological functions through ageing and stress-accumulation in a l
Keywords
Huaier (Trametes robiniophila murr) ; Cancer therapy; MEGA-DATA analysis; miRNA-medicated transcription control; Tissue regeneration; Epigenetics; Neurodegenerative diseases
Huaier (Trametes robiniophila murr) articles; Cancer therapy articles; MEGA-DATA analysis articles; miRNA-medicated transcription control articles; Tissue regeneration articles; Epigenetics articles; Neurodegenerative diseases articles
Article Details
1. Long History, Short Distance for the Encounter with Huaier
Medicinal herbs have recently attracted evoked more and more attentions among world-wide audiences, especially as a supplement therapy to the diseases like cancer, and also for the health maintenance. Among historical nature remedies in Asia, Huaier (Trametes robiniophila murr) has long been reported for significant efficacy on longevity, health maintenance, and cancer [1-3].
The difficulty in the herbal medicine exists in a lack of quantitative preparation with standardized quality, which long prevents the common use and distribution to public. Recent advancement in technology enabled to cultivate those useful species in quantity, and quality-controlled distribution in the conventional granule forms has begun since 1970’s, and currently Huaier is recognized widely as the patent anti-cancer drug in China (Chinese administration license No. Z-20000109) [2, 4, 5]. A couple of decades has been required for Japanese patients to be applied for Huaier therapy. Enormous numbers of reports, mainly from China, indicated as much molecular mechanisms responsible for cancer-specific apoptosis, autophagic cell death, and efficacy even on the metastatic lesions with specific immunomodulation by using in vitro cultured cancer cells [2-15]. However, the reality of Huaier effects remained unsolved even after the decades of sequential research works.
At the beginning of 21st century, Huaier therapy was introduced in Japan at Ariake Hospital attached to the National Cancer Institute, to the severe advanced stage cancer patients who could not have more conventional chemotherapy or surgical dissection. Surprisingly, the significant improvement of prognosis and Quality of Life (QOL) assessments were observed by a dose-dependent manner, especially on hepatocellular carcinoma and breast cancer. Thereafter, accumulated successful results were enough plausible to the medical doctors as many patients together, to recognize anti-cancer effects of Huaier. We ourselves have confirmed the drastic improvements not only in cancer patients, but also in the other disorders in skin, liver, kidney diseases and disorders, and more importantly, neurodegenerative diseases such as Parkinson’s disease [16]. In China, many investigations revealed the effects by experiments using in vitro cultured cells and animal models ino culated with cancer cells, but the detailed mechanism was yet unknown.
The effective ingredient of Huaier was proteoglycan, which consists of 41.53% polysaccharides, 12.93% amino acids and 8.72% water. The proteoglycan is the most effective anticancer element among all of the isolated ingredients of Huaier extract, which was confirmed on breast cancer MCF-7, liver cancer H22, lung cancer Lewis and sarcoma murine S180 cells (unpublished data) [6-15]. More importantly, Huaier has no toxicity or side effects (LD50=∞), and promises a broad spectrum of usage to any health problems at any situation without any disturbance to the conventional therapy.
We had to wait until we launched into the era of the systematic MEGA-DATA analysis with enough specialists to handle human bioinformatics [17-20]. We initiated clinical research with an approval by the Japanese Medical Association on 5th March, 2018 (ID: JMA-IIA00335). Through total RNA- and small non-coding RNA-sequencing of the volunteer cancer patients, we identified striking numbers of genomic and genetic changes which indicated drastic genomic flexibility and possibility in the process of cancer recovery [16]. The resulting enormous number of sequences hasThe NCBI GEO (GSE157086), and continuously up-loaded with newly identified sequences throughout the project period [4-10]. It is interesting to add Huaier as a novel choice for cancer therapy in 21st century, which was the result from the expedition in search for the natural products (herbs, fruit, flowers, and etc.) for immortality organized by Qin Shi Huang, the first Emperor in Qin dynasty, at B.C. 247-210.
2. Proved Hypothesis: Identification of Hippo Signaling Pathway Rescue by Huaier
Not only in cancer cases, we observed multi-dimensional efficacies of Huaier, especially on liver dysfunction, nephritis, bronchiectasis, constipation and skin lesions in a dose-dependent manner [16]. It is thus extremely difficult to determine the simple molecular mechanisms of Huaier effect on those multiple diseases and disorders. We searched for the hypothetical candidate pathway which may explain these multiple events observed in the clinical features. It should have a character to integrate multiple signal cascades to modulate tissue- and organ-homeostasis.
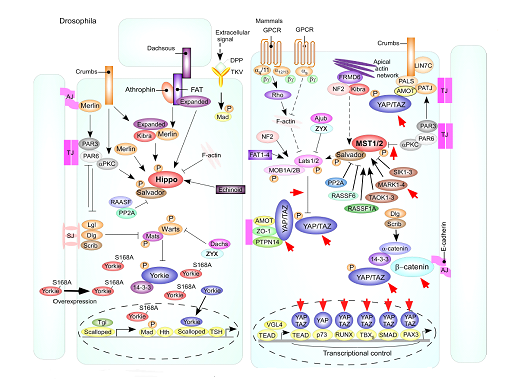
The target cancers of Huaier overlap with those strongly influenced by the oncogenic potential of Hippo signaling pathway [21, 22] as shown in Figure 1, (with a courtesy of Prof. Dr. Mo, San Diego, CA, USA [21]), which is well known as a control mechanism on cell fate and tissue homeostasis. Dysregulation of Hippo pathway is associated with cancer development, as reported in liver, lung, colorectal, ovarian, and prostate, and including metastasis, which were completely overlapping targets of Huaier. We made a hypothesis that Hippo signaling pathway might play as a main molecular mechanism of Huaier effects, and tried to prove this by simple experiments.
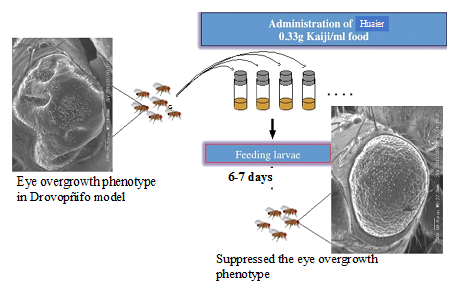
We used transgenic Drosophila flies with overexpressing non-phosphorylatable Yorkie (Yki: V5S168A) as a cancer model and just feed hatched larvae of mutants with a sequential concentration of Huaier granule for a week to be adult form. Our simple experiments to identify Huaier effect on mutant Drosophila with disrupted Hippo signaling pathway succeeded at the first trial. Huaier clearly rescued the function expressed as a rough eye formation within 7 days (Figure 2), with a dose-dependent manner, just as clinical observations [16]. This modulation in the Hippo pathway was accompanied with a strong shift of the metabolome profile to the early embryonic pattern to “rejuvenescence” form, as predicted in B. C. 220.
Figure 1: Schematic models of the Hippo signaling pathway in Drosophila and mammals shown by Mo, J. -S. et al., [21] whereas red-circled ykiS168 indicates the changes in Drosophila mutant used in the present study (left). Red arrows in mammalian cascades (right) indicate the presumed points of Huaier effect. Cells are shown with respective cellular junctions; adherence junction (AJ), tight junction (TJ) and septate junction (SJ). Hippo pathway components in Drosophila and mammals are shown in various colors, with arrows and blunt lines indicating activation and inhibition, respectively. The yellow spheres indicate phosphorylation of target proteins by kinase. Continuous lines indicate known interactions, whereas dashed lines indicate unknown mechanisms [16].
Thus, we succeeded to prove our hypothesis that the molecular mechanism of anti-cancer effects of Huaier was based on the modulated transcriptional dysregulation in the Hippo signaling pathway [21, 22], and that possibly influences cell fate, not only in cancer, but also in many diseases by the Hippo pathway control. However, examinations using experimental models had significant differences and limitations compared with the reality in human biosystems, of course, which urged us to consider clinical research the next stage experimental design.
In contrast, many in vitro investigations followed to our experiments in order to confirm the results, to provide the evidence that dysregulation in Hippo signaling pathway, disruption of transcription control is the major course of carcinogenesis [23-29]. However, without the observations to recover from the disease by Hippo signaling rescue, these reports were mostly the repetition of the past history. Although we sincerely hoped to have the additional successful controlling materials which contributes for cancer recovery, we could not yet detect any success. In addition, no additional findings rather than Hippo signaling pathway rescue for the possible mechanisms for cancer recovery.
3. The Opening of Genome Scope Project: Clinical Research for Thorough Understanding of Molecular Events after Huaier Administration
Here we need two years to prepare for the clinical research. We initiated clinical research and our genome scope project for thorough understanding of molecular basis of Huaier effects [30-34]. As a first step, we analysed 92 blood samples from 31 patients, average generating over 7.4 Giga bases per sample to start with. We repeat, this analysis could be performed with 1) accumulated information of human genes and their molecular aspects, 2) tremendous efforts of the researchers to organize the quantitative information in order (with supercomputers9, and most importantly, 3) distinguished specialists and experts in BGI Kobe and Shenzhen to accomplish the task.
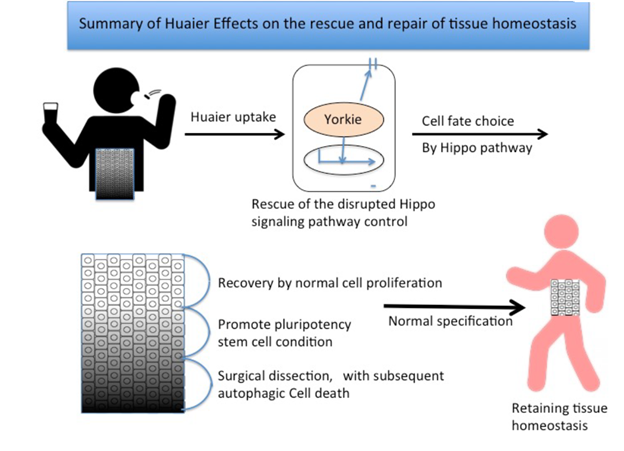
The representing figure on our hypothesis were shown in Figure 3 [16]. Figure 3 demonstrate the hypothetic Huaier efficacy for cancer treatment. With an intensive link of Hippo signaling pathway, we paid special remarks on the multi-functional control enzyme, DYRK1A (dual-specificity tyrosine-regulated kinase 1A [35]) from 2017, especially on its striking feature for maintenance coordination of the biophysiological function. In October 2020, we finally identified and proved the significant similarity of the effects defined in both DYRK1A and Huaier. In fact, we succeeded to identify the compensation of the mutated EGFR and other receptor tyrosine kinases (c-MET/erbB-2) by Huaier, too [31]. We have identified abundant transcriptome mutations in many cancer patients; however, it is noteworthy that there were no mutations in DYRK1A [35]. It suggests the importance of DYRK1A function is superior to any functions coordinated by major oncogenes, and tumor suppressor genes, and other reported genes reported (see the next section, Table 1).
Figure 3: Summary of anti-cancer effects of Huaier by the rescue of disrupted Hippo signaling pathway. Anti-proliferative effect of Huaier on cancer and consequent angiogenesis, metastasis, and induces autophagic cancer cell death in the lower left. The regeneration mechanism of damaged tissues was highlighted in lower right [16].
Table 1: The major oncogenes and tumor suppressor genes with the alteration of expression. Red highlights: up-regulated altered genes, Blue highlights: down-regulated altered genes. Black letters indicate genes with no quantitative changes [31].
Huaier influenced almost all the molecules essential for living and survival [30-34]. The typical findings for the rescue and compensation of disrupted signaling pathways were derived from transcriptional control, via miRNA- and piRNA-mediated control [36-38], through the attachment of the transcriptional factors (RNA editing events), and all these from the alteration and modification of the genome-wide transcription and translation [34]. At first, high ratio of SNP variants as A-G transitions (3,237,169241,896 in total, 37.0%) and A-C transversions (43,213 in total, 6.5 %), with skipped-exon (51%), which influenced significant changes in transcriptional factors and corresponding gene expression in multiple signal transduction pathways [30-34]. Huaier administration contributed the rescue of defected cell communication systems by massive down-regulation in a wide variety of signal transferring pathways [32]. In addition, Huaier seemed to take part in the epigenetic post-transcriptional control for the prevention in carcinogenesis and tumorigenesis for whole family members [31, 32].
Which cancer strategy or conventional chemotherapy could cover all these required genomic and genetic alterations in any of cancer patients? This much quantitative alteration in the genome and genes has never been reported before, by in vitro experiments and in vivo trials using experimental models including Drosophila strains.
4. Sky-High View of Rescued Function in Multiple Genes and Signaling Pathways Required for Cancer Recovery
Thus, we should have a sky-high point of view to see the reality of the results, to accomplish the aim of cancer therapy to any patients, at any stage, and any origin. The results obtained was striking and beyond any expectations and imaginations we have had, and only a few researchers could comprehend the real meaning of them. The sequential publications [16, 30-34] demonstrated the reality of the meaning of “cancer recovery” in genetic code language. Thanks to the KEGG signaling pathway characterization by Kyoto University, Japan [39], the altered genes and functions were clearly shown in these publications.
First of all, it is obvious that mono-targeted strategy could not be enough to treat cancer (Table 1) [40]. The resulting alterations in multiple signaling pathways were shown in Table 2 [31]. As show in these tables, only hundreds of molecules targeting was far to be enough for cancer recovery. We should reconsider cancer strategy from the fact that human biology can go through this much dynamic alterations during the long process of complete recovery from cancer, with compensation of the readjustment in multiple signaling pathways. As described, mono-targeted agents, such as FOLFOX, FOLFIRINOX, and cisplatin, commonly used in the conventional chemotherapy, should have certain limitations against diseases originated from multiple factors and environmental stresses throughout a life-long time [32].
Table 2: The signal transfer pathways with genetic alterations described in table 1. The pathways were in two categories; down-regulated and up-regulated. The typical related functions of each pathway were indicated and highlighted by bold letters. Down-regulation was indicated by blue color and up-regulation by red color. The changes of the regulation were written by the time course of Huaier administration [31].
We completely agree with the surgical dissection of cancer lesion(s), tumor mass should be the first choice, if applicable [31, 32]. However, even with the successful dissection of the lesion, it still remains possibility of occult metastasis, and more importantly, (cancer) stem cell control is required for long years to prevent relapse or recurrence [30]. At longest, we experienced the case of recurrence of breast cancer at 39 years after surgical dissection.
As emphasized above, the best figures to comprehend the process of recovery were transcriptional factor (TF)-differentially expressed genes (DEG) network, which can only be obtained by total RNA-sequencing [31-34]. Each molecule titration is far to be enough to monitor the patients. It became more popular to use this kind of MEGA-DATA analysis among all over the world, and recently involving SARS-COV2 and other virus infections as emphasized before. Our data detected multi-virus infections such as papilloma virus, HIV-1 infection, and other DNA virus infection, too.
In contrast, as compared to conventional chemotherapy using platinum (II) complex caused significant down-regulation of the most of the transcripts in every signaling pathway, especially in signal transfer in central and peripheral nervous systems [32]. Long-range treatment with Cisplatin: CDDP resulted in the complete destruction of RNA synthesis in the newly-borne cells, which inhibits spontaneous material synthesis, and the reproduction of damaged cells and tissues in the end. Thus, Huaier therapy is useful even for the patients with severe advanced stage of cancer, and more careful and precise genomic monitoring is required to control the effects of platinum (II) complex treatment.
5. Huaier Effects on Tissue Regeneration and Stem Cell Control (Ips/ES Cell Control)
Significant impact of Huaier treatment is tissue regeneration observed simultaneously with cancer cell death and active elimination of those damaged cell debris. Huaier effects on prevention of cancer progression in the patients with high risks, and simultaneous tissue regeneration after the dissection of damaged lesions by transcription regulation of pluripotency in induced pluripotent stem (iPS) / embryonic stem (ES) cells [30]. MEGA-DATA analysis of total transcriptomes and non-coding RNAs revealed the rescue of dysfunction in multiple signaling pathways in those patients, including Notch, NFKB, and Wnt signaling pathway with regulatory transcription factors in those pathways. These rescued control systems influenced to, the regulation of stem cell transformation which regulates the proliferation and differentiation of newly-borne cells. The induced normal tissue regeneration were the results of transcriptional control of iPS/ES cell production (see Figure 3).
Among the gene families to control iPS/ES production, especially c-myc expression level played a major role among the other genes or gene families [30]. These results provide a clue to clarify the Huaier effects not only for recovery from cancer, but also for the prevention of many related diseases and disorders caused by daily accumulation of environmental stresses and ageing by controlling normal tissue regeneration by stem cell control. In addition, conventional chemotherapy has been regarded as inefficient to (cancer) stem cell control. It is considered to be one cause of relapse and recurrence even with continuous chemotherapy, especially in breast cancer [15]. Huaier demonstrates significant superiority in this point, for the long-term prevention of cancer relapse.
6. Epigenetic Insights Into Pathogenesis with Mutated Transcriptomes and Compensation by Huaier
Considering carcinogenesis and stem cell control, epigenetic consideration on patients with hereditary mutated tyrosine kinase and receptor kinases should be performed. Epigenetics is the study of heritable phenotype changes that do not involve alterations in the DNA sequence. Epigenetics most often involves changes that affect gene activity and expression, and such effects on cellular and physiological phenotype traits may result from external or environmental factors DNA damage can also cause epigenetic changes [41-43]. The severe damage in DNA damage repair system has been reported for epigenetic influence on carcinogenesis and a loss of stem cell control. Actually, DNA damage is very frequent, occurring on average about 60,000 times a day per cell of the human body.
These damages are usually largely repaired, but at the site of a DNA repair, epigenetic changes can remain. It has been reported that epigenetic silencing of DNA repair gene MLH1 increase the levels of DNA repair enzymes such as MGMT and MLH1 and p53 [31, 33]. However, as reported, we observed many mutations and disrupted functions related to DNA mismatch repair especially in the cancer patients with hereditary mutated EGFR and other receptor tyrosine kinases (c-MET/erbB-2) [31]. In these patients, epigenetic inheritance systems were designated to influence memory and signal transfer inter/intra central and peripheral neurons. Neurological consideration of Huaier effects will be more discussed by considering the strong similarity of DYRK1A function.
Schematic models of the significant linkage of DYRK1A to essential biosystems shown by Fernandez-Martinez P, Sahonero C, and Sanchez-Gomez P [35]. Double-edged regulation of tumorigenesis by DYRK1A. DYRK1A has been associated with pre-tumoral activity by activating known oncogenic precursor by inhibiting tumor suppressors. However, an antitumoral capacity of DYRK1A has been also described through its activation of tumor suppressors or its inhibition of oncogenic proteins. To add complexity to DYRK1A function, some of the known substrates of this kinase can have both oncogenic and tumor suppressor activities, depending on the cellular context and the developmental stage. Again, it is emphasized that there are many important molecules and signaling pathways controlled by DYRK1A, which has never been observed mutations in our clinical researches, whereas the other main oncogenes and tumor suppressor genes were mutated. In addition, there seemed to be hierarchy of tyrosine kinases and receptor kinases according to the importance and necessity in biological systems.
7. Huaier Compensates Impaired Signal Transfer Inter/Intra Neurons in Central and Peripheral Nervous Systems
When we used transgenic Drosophila flies as a cancer model [3], this mutant was revealed to be useful to screen the candidate compound for amyotrophic lateral sclerosis (ALS). However, nearly 5 years’ researches resulted in vain. Simultaneously we observed impaired signal transfer in the central and peripheral nervous systems in the patient successfully recovered from pancreatic cancer, with FOLFIRINOX treatment [32].
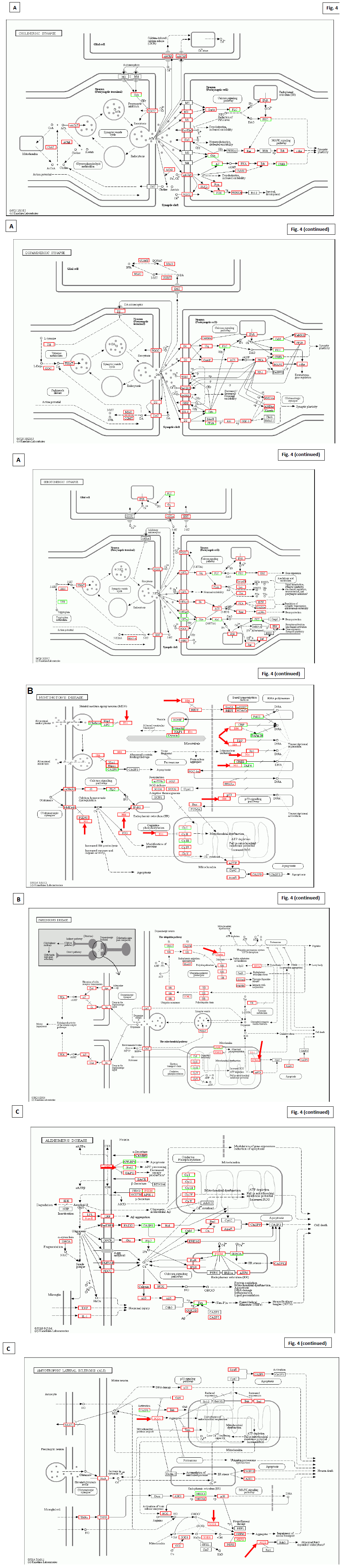
Recently we identified molecular systems to compensate impaired neural transmission, especially by mutated transcripts correlated with neurodegenerative alterations (Figure 4) [45]. These significant rescue of signal transfer inter/intra neurons was observed in the patients with the hereditary mutated EGFR, and receptor tyrosine kinases such as c-MET, HER2/neu (erbB2), Htt, Parkin, APP, SOD1, ALS2, and many oncogenes and tumour suppressor genes.
Figure 4: KEGG characterization of signal transfer intra/inter neurons [45]. Panel A; From Up to bottom, the rescue of neurotransmission in; cholinergic synapse, Dopaminergic synapse, and serotonergic synapse. Quantitative regulation of signal transfer was highlighted in abundant molecules by red box. Panel B; KEGG characterization on molecular modifications in Huntington’s disease and Parkinson’s disease. Note the mutations in Htt (Huntington’s disease) and Parkin (Parkinson’s disease) indicated by red arrows. Panel C; KEGG characterization on molecular modifications in Alzheimer’s disease and amyotrophic lateral sclerosis. Note the mutations in APP (glycoprotein amyloid-beta precursor protein for Alzheimer’s disease), ApoE (apolipoprotein E), SOD1 (superoxide dismutase gene), and ALS2 (Alsin GTPase underlying in hereditary amyotrophic lateral sclerosis for amyotrophic lateral sclerosis) indicated by red arrows.
Huaier treatment prevented those patients from pathogenesis, but influenced to cause mild depression. The epigenetic potential seems to influence the pathogenicity in these hereditary mutations, and that typically observed in the defects in DNA mismatch repair systems. With KEGG pathway characterization, Huaier showed significant effects on the retrieval of normal function inter/intra neurons [44]. Although we have observed only one case successfully recovered from Parkinson’s disease, the further roles of each representative molecules have not yet defined, and no changes identified in the mechanism of mutations in translation and transcription processes. The present study demonstrated detailed molecules and signaling pathways involved in the onset of neurodegenerative diseases, and the significant effects of Huaier to retain and rescue the impaired neurotransmission and signal transfer. Neurodegenerative damages are inevitably caused by chemical administration, and also by ageing process. However, it is emphasized that, 2 years’ of Huaier treatment, Huaier could cope with the severity and onset of the diseases.
Together with the former paper [32], Huaier shows significant efficacy on the compensation of the impaired functions relatted to cell-to-cell communication systems in the central and peripheral nervous systmes by influencoing a wide variety of signal transferring pathways. The treatment focused on specified target molecules should have a certain limitation to influence all these alterations, some with up-rebulation, and the others with down-regulation at a time. There are scarce medicine or candidate compounds even for mutated EGFR and receptor tyrosine kinases. The present study thus provides a clue for the demand for successful treatment and prevention of neurodegenerative diseases by Huaier.
8. The End of Failed Promise, Solution First
The time has come to end the unsuccessful sufferings and conflicts on cancer strategy. We have long published many papers full of promises for effective medicine development, currently without fulfillment. Huaier therapy is one of successful strategy to provide more choices, especially on the advanced stage of cancer with many metastatic lesions. Huaier is also useful to prevent cancer progression in small dose of 3g/day, too. Huaier provides more choices to cancer treatment according to the stages, origins, and individual characteristics of each case. It existed from over 2,000 years ago, and we were too busy to pursue a novel molecular targeting potential without learning from the ancient wisdom. In the end, there are so many failed promises and as much loss of human life. The research results on carcinogenesis, especially obtained by in vitro experiments, showed a wide difference from those observed as clinical evidences. It should be a matter of course, however, there exists significant quantitative differences in molecular aspects detected in cancer patients by quantitative analysis by total RNA sequencing. The molecular basis for cancer recovery contained massive quantitative changes from RNA editing events to transcriptional control by miRNA- and piRNA-modifications.
At first, we should remember what to be considered when we initiate the treatment of cancer; 1) to promote cancer specific cell death inside the pathogenic lesion; 2) to discard the resulting, damaged cell debris; 3) to repair damaged and/or dissected tissues with normal cells; 4) to prevent the relapse and recurrence together with adjacent and distinct metastasis. It is obvious that surgical dissection of tumor mass is the first choice [1, 2], if applicable, however, it is just the beginning to overcome the disease like cancer. Anti-cancer effects of Huaier are; 1) to promote cancer specific cell death inside the pathogenic lesion; 2) to discard the resulting, damaged cell debris; 3) to repair damaged and/or dissected tissues with normal cells; 4) to prevent the relapse and recurrence together with adjacent and distinct metastasis.
Our genome scope project proved that many “normal” individuals were not normal from a point of view of molecular and biophysiological functions [30-34]. Many normal persons showed drastic genomic and genetic alterations for the rescue of the latent, invisible disruptions of multiple signaling pathways after Huaier administration. Even with no symptoms, there was an enormous daily risk of cancer caused by accumulation of environmental stresses and ageing process. Early diagnostic technology has some limitations for correct and precise evaluation of the risk [33, 34], and excessive, frequent medical examinations using endoscopy and radiographic measures in contrast increase risks for carcinogenesis [33]. We would like to emphasize Huaier effects for compensation in signal transfer, communication transfer in the central and peripheral nervous systems, for the supplemental therapy to neurodegenerative diseases.
Acknowledgments
The authors wish to thank cancer patient volunteers and many healthy volunteers kindly collaborated with the present study. We also wish to thank Prof. Dr. Tongbiao Zhao, Professor of Stem Cell and Immunology, Institute of Zoology, Chinese Academy of Sciences, China, for critical review and the comments on the project scheme; the reviewing committee for the medical ethics and safety monitoring of the project. We express special thanks to Prof. Dr. Mo. San Diego, CA, USA, and Prof. Dr. P. Sanchez-Comez, Madrid, Spain, for kind approval to use the figures appeared in the review articles hired in this review. The present study was grant-in-aid from QiDong Gaitianli Medicines Co., Ltd. and Japan Kampo NewMedicine, Co., Ltd.
Conflict of Interest
The authors have no competing interest to declare.
References
- Da Rocha AB, Lopes RM. Schwartsmann G. Natural products in anticancer therapy. Curr Opin. Pharmacol 1 (2001): 364-369.
- Li L, et al. Progress on experimental research and clinical application of Trametes robiniophila. Bull. Chin. Cancer 16 (2007): 110-113.
- Sun Y, Sun T, Wang F, Zhang J, Li C, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr. Polym 92 (2013): 577-582.
- Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review). Oncol. Rep 34 (2015): 12-21.
- Qi T, Dong Y, Gao Z, Xu J. Reearch Progress on the Anti-Cancer Molecular Mechanisms of Huaier. Onco Targets Ther 13 (2020): 12587-12599.
- Wang X, Wang N, Cheung F, Lao L, Li C, et al. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med 13 (2015): 142-164.
- Zhang N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci 101 (2010): 2375-2383.
- Wu T, et al. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett 588 (2014): 2107-2114.
- Yan X, Lyu X, Yun A, Wei Y, Yang Q, et al. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3beta/beta-catenin pathway. Plos One 8 (2013): e63731.
- Zheng J, Li C, Wu X, Liu M, Sun X, et al. Huaier polysaccharides suppresses hepatocarcinoma KHCC97-H cell metastasis via inactivation of EMT and AEG-1 pathway. Int J Biol Macromol 64 (2014): 106-110.
- Li C, Wu X, Zhang H, Yang G, Hao M, et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumor Biol 36 (2014): 1739-1745.
- Zhang T, Wang K, Zhang J, Wang X, Chen Z, et al. Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via downregulation of the Wnt/β-catenin pathway. Oncol Lett 5 (2013): 1171-1176.
- Cui Y, Meng H, Liu W, Wang H, Liu Q. Huaier aqueous extract induces apoptosis of human fibrosarcoma HT1080 cells through the mitochondrial pathway. Oncol Lett 9 (2015): 1590-1596.
- Wang X, Zhang N, Huo Q, Sun M, Dong L, et al. Huaier aqueous extract inhibits stem-like characteristics of MCF7 breast cancer cells via inactivation of hedgehog pathway. Tumor Biol 35 (2014): 10805-10813.
- Wang X, Qi W, Li Y, Zhang N, Dong L, et al. Huaier extract induces autophagic cell death by inhibiting the mTOR/S6K pathway in breast cancer cells. PLoS ONE 10 (2015): 0131771.
- Tanaka T, Suzuki T, Nakamura J, Kawamura Y, Sadahiro S, et al. Huaier Regulates Cell Fate by the Rescue of Disrupted Transcription Control in the Hippo Signaling Pathway. Arch Clin Biomed Res 1 (2017): 179-199.
- Peng Z, Cheng Y, Tan BC, Kang L, Tian Z, et al. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol 30 (2012): 253-260.
- Ramsköld D, Luo S, Wang Y, et al. Full-Length mRNA-Seq from single cell levels of RNA and individual circulating tumor cells. Nat Biotechnol 30 (2013): 777-782.
- Song Y, Li L, Ou Y, Li R, Deng Q, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509 (2014): 91-95.
- Jiang Y, Sun A, ZhaoY, Ying W, Sun H, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 567 (2019): 257-261.
- Mo JS, Park JW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 25 (2014): 642-656.
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat. Rev. Cancer 13 (2013): 246-257.
- Zhang H, Lang TY, Zou DL, Zhou L, Lou M, et al. miR-520b Promotes Breast Cancer Stemness Through Hippo/YAP Signaling Pathway. Onco Targets Ther 12 (2019): 11691-11700.
- Wu L, Yang X. Targeting the Hippo Pathway for Breast Cancer Therapy. Cancers (Basel) 10 (2018): 422-437.
- Wei C, Wang Y, Li X. The role of Hippo signal pathway in breast cancer metastasis. Onco Targets Ther 11 (2018): 2185-2193.
- Feng X, Zhang M, Wang B, Zhou C, Mu Y, et al. CRABP2 regulates invasion and metastasis of breast cancer through hippo pathway dependent on ER status. J Exp Clin Cancer Res 38 (2019): 361-368.
- Shen J, Cao B, Wang Y, Ma C, Zeng Z, et al. Hippo component YAP promotes focal adhesion and tumour aggressiveness via transcriptionally activating THBS1/FAK signalling in breast cancer. J Exp Clin Cancer Res 37 (2018): 175-191.
- Qiao K, Ning S, Wan L, Wu H, Wang Q, et al. LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515-5p/MARK4Hippo signaling pathway. J Exp Clin Cancer Res 38 (2019): 418-432.
- Wang S, Su X, Xu M, Xiao X, Li X, et al. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther 10 (2019): 117-128.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. ? Huaier inhibits cancer progression and induces tissue regeneration by transcriptional regulation of pluripotency of stem cells. J Alternative Compl Integr Med 7 (2021): 162-172.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier inhibits cancer progression correlated with the mutated EGFR and other receptor tyrosine kinases (c-MET/erbB-2) by down-regulation of multiple signal transduction pathways. Arch Clin Biomed Res 5 (2021): 262-284.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Complete remission of the severe advanced stage cancer by miRNA-mediated transcriptional control of Bcl-xL with Huaier therapy compared to the conventional chemotherapy with platinum (II) complex. Arch Clin Biomed Res 5 (2021): 230-261.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Anti-cancer effects of Huaier on prostate cancer; miRNA-mediated transcription control induced both inhibition of active progression and prevention of relapse. J. Alternative Compl Integr Med 7 (2021): 146-155.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier Induces Cancer Recovery by Rescuing Impaired Function of Transcription Control Based on the Individual Genomic Potential. Arch Clin Biomed Res 4 (2020): 817-855.
- Fernandes-Martinez P, Sahonero C, sanchez-Gomez P. DYRK1A: the double-edged kinase as a protagoist in cell growth and tumorigenesis. Mol cell Oncol 2:1 (2015): e-970048.
- Nourse J, raun J, Lackner K, Hüttelmaier S, Danckwardt S. Large-scale identification of functional microRNA targeting reveals cooperative regulation of the hemostatic system. J Thromb Haemos 16 (2018): 2233-2245.
- Seto AG, Kingston RE, Lau, NC. The Coming of Age for Piwi Proteins. Mol Cell 26 (2007): 603-609.
- Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell 136 (2009) 215-233.
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res 36 (2008): 480-484.
- Chongtham A, Agrawal N. Crucumin modulates cell death and it protective in Huntington’s disease model. Sci Rep 6 (2016): 18736.
- Esteller M, Garcia-Foncillas J, Andion E, Goodman, SN, Hidalgo OF, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343 (2000): 1350-1354.
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature 429 (2004): 457-463.
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349 (12003): 2042-2054.
- Verma M, Rogers S, Divi RL, Schully SD, Nelson S, et al. Epigenetic research in cancer epidemiology: trends, opportunities, and challenges. Cancer Epidemiology, Biomarkers & Prevention 23 (2) (2014): 223-233.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier compensates impaired signal transfer inter/intra neurons in central and peripheral nervous systems. submitted to Arch Clin Biomed Res 2021 June.