Huaier Regulates Cell Fate by the Rescue of Disrupted Transcription Control in the Hippo Signaling Pathway
Article Information
Tomoo Tanaka1, 11, *†, Takayoshi Suzuki2, Jun Nakamura2, Yoshiaki Kawamura3, Sotaro Sadahiro4, Hiroshi Kijima5, Itaru Yamamoto6, Nicole Vo6, Masamitsu Yamaguchi6, Yasuhiro Irino7, 9, Masakazu Shinohara8, 9, Ding Wei10, ‡ and Manami Tanaka11, †, ‡
1Division of Applied Medical Sciences, Tokai University School of Medicine, 143 Shimokasuya, Isehara, Kanagawa 259-1193, Japan
2Division of Gastroenterology and Hepatology, Department of Internal Medicine. Tokai University Tokyo Hospital, Yoyogi, Tokyo 151-0053, Japan
3Department of Urology, Tokai University School of Medicine, 143 Shimokasuya, Isehara, Kanagawa 259-1193, Japan
4Department of Surgery, Tokai University School of Medicine, 143 Shimokasuya, Isehara, Kanagawa 259-1193, Japan
52nd Department of Pathology, Hirosaki University Medical School, Hirosaki, Aomori 036-8562, Japan
6Department of Applied Biology, The Center for Advanced Insect Research, Kyoto Institute of Technology, Matsugasaki, Sakyo-Ku, Kyoto 606-8585, Japan
7Division of Evidence-Based Laboratory Medicine, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-Cho, Chuo-Ku, Kobe 650-0017, Japan
8Division of Epidemiology, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-Cho, Chuo-Ku, Kobe 650-0017, Japan
9The Integrated Center for Mass Spectrometry, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-Cho, Chuo-Ku, Kobe 650-0017, Japan
10Japan Kampo NewMedicine, Co., Ltd., 2-8-10 Kayaba-Cho, Chuo-Ku, Tokyo 103-0025, Japan
11Bradeion Institute of Medical Sciences, Inc., 2-22-9 Fujimino, Hiratsuka, Kanagawa 259-1211, Japan
† These authors contributed equally to this work.
‡ Requests for Huaier extract and commercially available granules.
*Corresponding Author: Tomoo Tanaka, Division of Applied Medical Sciences, Tokai University School of Medicine, 143 Shimokasuya, Isehara, Kanagawa 259-1193, Japan
Bradeion Institute of Medical Sciences, Inc., 2-22-9 Fujimino, Hiratsuka, Kanagawa 259-1211, Japan
Tel: +81-463-93-1121 (ext. 2602);
Received: 16 June 2017; Accepted: 20 June 2017; Published: 26 June 2017
View / Download Pdf Share at FacebookAbstract
Background: Among historical nature remedies in Eastern Asia, Huaier (Trametes robiniophila murr) has long been reported for its significant efficacy on longevity and health maintenance, and more importantly, on cancer. The target cancers of Huaier show the almost complete overlap with those strongly influenced by the Hippo pathway function, which is also well known as a tumor suppressor mechanism.
Objective: We have initiated the present study in order to prove the hypothesis that the Hippo signaling pathway is a main molecular mechanism of Huaier effects on cancer and on multiple physiological disorders.
Methods: We performed in vivo study using Drosophila flies genetically disrupted transcriptional control in the Hippo pathway with overexpressing non-phosphorylatable Yorkie (Yki:V5S168A) as a cancer model. In addition, metabolome analysis was performed using those cancer-model mutants with a comparison to healthy controls.
Results: The administration of Huaier clearly resulted in the recovery of rough eye formation of the mutants, indicating the rescue of tumor suppressor mechanism disrupted by the genetically inoculated overabundant transcriptional activators. These improvements occurred in a dose-dependent manner, just as shown in clinical observations. Simultaneously, the GC/MS-based metabolome analysis revealed that Huaier uptake lead the metabolic profile of the mutant flies strongly shifted to that of the early developmental stage.
Conclusion: The present study emphasizes that a broad spectrum of Huaier effects was based on the rescue of the disrupted Hippo signaling pathway control, especially on the recovery in transcriptional dysregulation. The metabolome profile shift would explain the molecular basis of the tissue recovery after a long course of Huaier treatment, by the potential to mo
Keywords
Huaier (Trametes Robiniophila Murr); Complementally Cancer Therapy; Hippo Signaling Pathway; YAP/TAZ (Yorkie); Metabolome Analysis
Article Details
1. Introduction
Administration of Huaier has been reported significantly effective on the broad range of cancers [1], such as breast [2], lung [3], ovarian [4], stomach, pancreas, liver [5-7], colorectal [8], and prostate cancers, including fibrosarcoma [9]. In addition, not only anti-proliferative effect on tumor and consequent angiogenesis, Huaier inhibits metastasis [4], induction of drug resistance and caner stem cells [8-10], and induces autophagic cancer cell death [2, 11], and immunomodulation [12]. Not only in cancer, Huaier also demonstrated typical curative effects, especially on dysfunction in liver, kidney, and on skin lesion.
Recent advances in cancer research enabled to prevent cancer progression by non-invasive dissection of early stages of cancer [13, 14], yet the conventional therapy has many limits on stage 3 or more advanced cases. The effective complementally therapy is required, and that with no toxicity and side effects. Although a rapid progress in the methodology in the identification and verification of cancer-targeting molecules [15, 16], current technology has not yet succeeded to solve this task, and it is considered partly because mono-targeted agents should have certain limitations against diseases originated from multiple factors and environmental stresses throughout a lifelong time [17].
On the other hand, natural herbs and products, target multiple cellular events, and are anticipated to play an important role in developing new therapeutics for many diseases [18, 19]. Not only on cancer, we observed multi-dimensional efficacies of Huaier, especially on liver dysfunction, nephritis, bronchiectasis, constipation and skin lesions in a dose-dependent manner (summarized in Figure 1). Based on these clinical significance, it is extremely difficult to determine the simple molecular mechanism of Huaier on those multiple diseases and disorders. In addition, the natural herbs of medical importance have extremely difficult to prepare in quantity with standardized quality, which long prevents the common use and wide distribution to public. Recent advancement in technology enabled to cultivate those useful species in quantity, and quality controlled distribution in the conventional granule forms has begun since 1970’s [7, 19], and currently Huaier is recognized widely as the patent anti-cancer drug in China (Chinese administration license No. Z-20000109), especially against hepatoma and breast cancer [1, 2, 5-7, 10, 11].
The effective ingredient of Huaier was proteoglycan, which consists of 41.53% polysaccharides, 12.93% amino acids and 8.72% water [1]. The proteoglycan is the most effective anticancer element among all of the isolated ingredients of Huaier extract, which was confirmed on breast cancer MCF-7, liver cancer H22, lung cancer Lewis and sarcoma murine S180 cells (unpublished data). More importantly, Huaier has no toxicity or side effects (LD50=?), and promises a broad spectrum of usage to any healthy problems by dose-dependent manner, and without any disturbance to the conventional therapy.
We have already confirmed the drastic elimination of tumor mass in both mice and rats inoculated with cancer cells, but the detailed mechanism was yet unknown. As for the molecular basis of anti-cancer effects of Huaier, many reports using aqueous Huaier extract have indicated various independent systems and factors correlating specific cancer cell death [1, 3-5, 7-11]. Those in vitro examinations indicated multiple molecular mechanisms responsible for cancer-specific apoptosis, autophagic cell death, and efficacy even on the metastatic lesions with specific immunomodulation [1, 12]. We searched for the candidate pathway, which may explain these multiple events observed in the clinical features. It should have a character to integrate multiple signal cascades to modulate tissue- and organ-homeostasis, and that in multiple organs and tissues.
In conclusion, we focused on the Hippo signaling pathway according to the tissue- and cell-type specificity, and also from its function on cancer progression. Since the discovery of the Wrts kinase in Drosophila in 1995, rapid progress of investigation in the Hippo signaling pathway has emphasized its important contribution to tissue regeneration through self-renewal of cells, and cancer development in a wide-range of organs and cell-types [20]. The core signaling cascade from Hippo (mammalian Mst1/2) to Yorkie (mammalian YAP) is well understood [20-22]. Thus Hippo pathway and its regulators provide good therapeutic targets in diseases such as (neuro) degeneration and cancer [21].
The Hippo signaling pathway consists of a highly conserved kinase cascade (MAT and Lats), and downstream transcription co-activators (YAP and TAZ) [23], which plays a key role in tissue homeostasis by regulating tissue-specific stem cells. Dysregulation of Hippo pathway is associated with cancer development, as reported in liver, lung, colorectal, ovarian, and prostate, and including metastasis, which were completely overlapping targets of Huaier. Although there is a complex network of upstream inputs that modulate Hippo pathway activity, it is hypothesized that Hippo pathway integrates all of these inputs from multiple signaling pathways to generate a concerted cellular response.
Consequently, We initiated molecular approach in vivo by utilizing the transgenic mutant Drosophila in which overexpressing non-phosphorylatable form Yorkie (Yki:V5S168A ; described ykiS168A hereafter) enhances cell proliferation by transcriptional stimulation [24]. Phosphorylated YAP/TAZ (Yorkie) localizes to the cytosol, decreasing tumor growth, whereas unphosphorylated YAP/TAZ (Yorkie) is localized mainly in the nucleus and promotes cell and tumor signaling is associated with tumor progression [2, 21, 22]. Dysregulation of transcription control can be observed by severe overgrowth phenotype in the eye imaginal disc (rough eye formation) [24, 25].
The administration of Huaier clearly resulted in the recovery of rough eye formation of the mutants, which indicates the rescue of tumor suppressor mechanism. These improvements occurred in a dose-dependent manner, just as shown in clinical observations. The GC/MS-based metabolome analysis changed the metabolic profile of the treated mutants to that of the early developmental stage, whereas the control flies showed the normal maturation profile. Thus Huaier proved its efficacy on cancer and transcriptional dysregulation in the Hippo signaling pathway, which may suggest a broad spectrum of its effects on the other diseases caused by transcriptional dysregulation in the Hippo pathway. In addition, it is suggested that Huaier might play a key role to reset the tissue potential observed in such as embryonic stem cells and various progenitor cells [20-22], and to restart the normal cell proliferation and specification process in a long course of treatment.
2. Materials and Methods
2.1 Clinical evaluation of anti-cancer effect of Huaier on cancer lesion
The clinical data analysis, CT, PET and Photo images demonstrated in the present study was obtained and analysed in Tokai University Hospital with an informed consent of each patient.
2.2 Preparation of the conventional Huaier granule (Trametes robiniophila murr)
Commercially available Huaier granule (Trametes robiniophila murr) was kindly provided by the Japanese supplier (Japan Kampo NewMedicine, Co., Ltd. Tokyo, Japan). This is the same content as commonly used in public was used in the present study, and no artificial modification or extraction was performed. The effective ingredient of Huaier extract was proteoglycan, which consists of 41.53% polysaccharides, 12.93% amino acids and 8.72% water.
2.3 Drosophila stocks and dietary condition
Fly stocks were maintained at 25°C on standard food containing 0.65% agar, 10% glucose, 4% dry yeast, 5% corn flour and 3% rice powder. The flies carrying w; UAS-ykiS168A:V524 were obtained from Bloomington Drosophila Stock Center at Indiana University. GMR-GAL4 used in this study was described previously [22, 24]. Standard food contains 0.65% agar, 10% glucose, 4% dry yeast, 5% corn flour and 3% rice powder, and used to feed the hatched Drosophila fly larvae. The desired concentration of Huaier granule was mixed thoroughly with standard food, and it takes about 7 days to become adult stage, and used for the following analysis. Yeast paste with blue color (FD&C Blue Dye No.1, Sigma Aldrich) was used to check the feeding behavior.
2.4 Viability assay of flies
Viability assay was carried out to evaluate the effect of Huaier on development of Drosophila [24]. For viability analyses, first instar larvae were collected. Ten larvae were reared at 25°C in a single plastic vial containing 1 ml of Drosophila instant medium (Formula 4-24, Blue, Carolina Biological Supply Company, Burlington, NC, USA) with or without indicating amount of Huaier. Number of pupae and eclosed adult flies were counted. Six independent experiments for each line were carried out. The intake of food containing Huaier was confirmed by inspecting blue color in larval and adult intestines. P-values were calculated using the student t-test and the error bars represent Standard Errors from Means. All data are shown as means ± SEM. The GraphPad Prism version 6.02?XY analyses?software was used. Comparable results were considered statistically significant when the P-value < 0.05.
2.5 Huaier effect on rough eye phenotype assessment by scanning electron microscopic analysis
In order to qualitatively and quantitatively evaluate morphological changes of adult compound eyes, adult flies fed from first instar larvae were anesthetized, mounted and observed under a scanning electron microscope (Keyence VE-7800, Keyence Corp., Osaka, Japan) in high vacuum mode. In every experiment, the eye phenotypes of at least five newly eclosed adult male flies of each line were simultaneously examined by scanning electron microscopy. Six independent experiments for each line were carried out.
2.6 Evaluation of Huaier effect on disrupted Yorkie transcriptional control by 5’-ethynyl-2’-deoxyuridine (EdU) labeling
To evaluate cell proliferation in the eye imaginal disc after Huaier feeding, EdU assays were performed as reported previously [24]. Detection of cells in S phase was performed using EdU-labeling kits from Invitrogen (Click-iT EdU Alexa Flour 488 and 594 Imaging Kit, Thermo Fisher Scientific Inc., Waltham, MA, USA). Third instar larvae were dissected in PBS and the imaginal discs were suspended in Grace’s insect medium in the presence of 10mM EdU for 60 min at 25ºC. The samples then were fixed with 3.7% Paraformaldehyde in PBS adjusting to pH 7.4 for 20 min at 25ºC. After fixing, samples were washed with 3% BSA in PBS and were permeabilised in 0.5% Triton X-100 in PBS for 20 min at 25ºC. Then, samples were washed with 3% BSA in PBS and incubated with Click-iT reaction cocktails for 30 min at 25ºC (following the manufactures’ instructions). After further washing with 3% BSA in PBS and PBS, samples were stained with Hoechst 33342 (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) for labeling DNA, and finally samples were mounted and observed with a confocal laser-scanning microscope (Olympus FLUOVIEW FV10i and FV1000, Tokyo, Japan).
2.7 Metabolome analysis
Newly hatched Drosophila samples were treated 7 days with 167 mg/ml. Huaier were designated to be directly frozen in liquid N2 immediately and stored at -20?C until the extraction [24, 26]. Used strains were Canton S, a wild type strain of Drosophila melanogaster, and transgenic mutant strain carrying w; UAS-ykiS168A.
2.8 Sample extraction and derivatization for GC-MS
Extraction and derivatization for GC/MS were performed as previously described [27]. Briefly, the freeze-dried sample was crushed using a ball mill for 5 min at 20 Hz before extraction to increase the extraction efficiency. Afterwards, 5 mg of each sample (approximately 20 freeze-dried adult male flies) was extracted with 1 ml extraction solvent, which consisted of methanol/water/chloroform (2.5:1:1). 60 µl ribitol (0.2 mg/ml) was added subsequently as internal standard. After centrifugation at 16,000 x g for 3 min at 4?C, 900 µl of the supernatant was transferred to a 1.5 ml micro tube and mixed with 400 µl distilled water (Wako, Tokyo, Japan). After repeating centrifugation, 400 µl of the polar phase was transferred into a fresh 1.5 ml microfuge tube. Then, the solvent was removed using a centrifugal concentrator (CC-105, Tomy Co., Tokyo, Japan) for 2 hours and sample was subsequently freeze-dried overnight. Derivatization of the samples was done by oximation using methoxyamine hydrochloride (Sigma Aldrich, St. Louis, MO, USA) in pyridine (50 µl, 10 mg/ml) at 30?C for 90 min, followed by silylation using 25 µl of N-methyl-N- (trimethylsilyl) trifluoroacetamide (MSTFA) (GL Sciences, Tokyo, Japan) at 37?C for 30 min.
2.9 GC-MS analysis
Gas chromatography quadrupole mass spectrometry (GC-Q/MS) analysis was performed on GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan) with a CP-SIL 8 CB low-bleed column (0.25 mm x 30 m, 0.25 µm, Varian Inc., Palo Alto, CA, USA) and an AOC-20i/s autosampler (Shimadzu, Kyoto, Japan), as previously described [28]. Tuning and calibration of the mass spectrometer was done prior to analysis. One microliter of derivatized sample was injected in split mode, 25:1 (v/v), with an injection temperature of 230?C. The carrier gas (He) flow was 1.12 ml/min with a linear velocity of 39 cm/s. The temperature was held at 80?C for 2 min, increased by 15?C/min to 330?C, and then held for 6 min. The transfer line and ion source temperatures were 250 and 200?C, respectively. Ions were generated by electron ionization (EI) at 70eV. Spectra were recorded at 10,000 unit/second over the mass range m/z 85-500.
2.10 Data processing
The raw chromatographic data were converted into ANDI files (Analytical Data Interchange Protocol, *.cdf) using GC-MS Solution software package (Shimadzu). The data were imported to MetAlign software (Wageningen UR, The Netherlands, available for free at the website http://www.pri.wur.nl/UK/products/MetAlign/) for peak selection and alignment. The peak intensity of each compound was normalized based on the ribitol internal standard. AIoutput2 (version 1.29) was used as annotation software. The retention indices of all detected metabolites were calculated based on the standard alkane mixture and tentative identification of metabolites was done by comparing the retention indices with our in-house library [26].
3. Results
3.1 Clinical Significance of anti-cancer effects of Huaier
It is about two decades since Huaier (Trametes robiniophila murr) has been introduced in the clinics in Japan, and showed significant efficacy on various phases of cancer [1]. The present study was based on the clinical observation of anti-cancer significance of Huaier, with or without conventional cancer therapy. Typical features were summarized in Figure 1. Complete elimination of cancer lesion was observed in breast cancer with lymph node metastasis (Figure 1A). No metastasis or recurrence has been so far detected without chemotherapy for 5 years now.
The prevention of recurrence and metastasis without chemotherapy were also observed in a case of adrenal tumor (Figure 1B), and this case was happened to be associated with a cure of infertility within a year after surgical dissection of tumor mass. Huaier administration showed more significant therapeutic effects up to stage 3 cancers originated from secretory and reproductive organs, such as adrenal tumor, prostate cancer, ovarian cancer, and breast cancer. Anti-cancer effect of Huaier was strongly dose-dependent, and 20g per day for 4 weeks to cause complete elimination, shrinkage, or necrosis in the lesions, which resulted in the significant improvement of patients’ prognosis (Figure 1A and 1B).
An anti-cancer effect of Huaier was significant not only on the primary cancer lesion or cancer cells, but also on the massive metastatic lesions (Figure 1C) in the late stage of colorectal cancer. In this case, it is recommended 60g of Huaier per day with conventional chemotherapy, but even 20 g of daily dose could prevent the induction of side effect of chemotherapy and induction of drug resistance [9,11].
In addition, not only anti-proliferative effect on tumor and consequent angiogenesis (Fig 1B), Huaier demonstrated typical curative effects especially on skin lesion (Figure 1D). Eczema and severe itching with liver dysfunction (familial) vanished away within 4 weeks, and we observed immunoactivation in the lesions about 7 days after
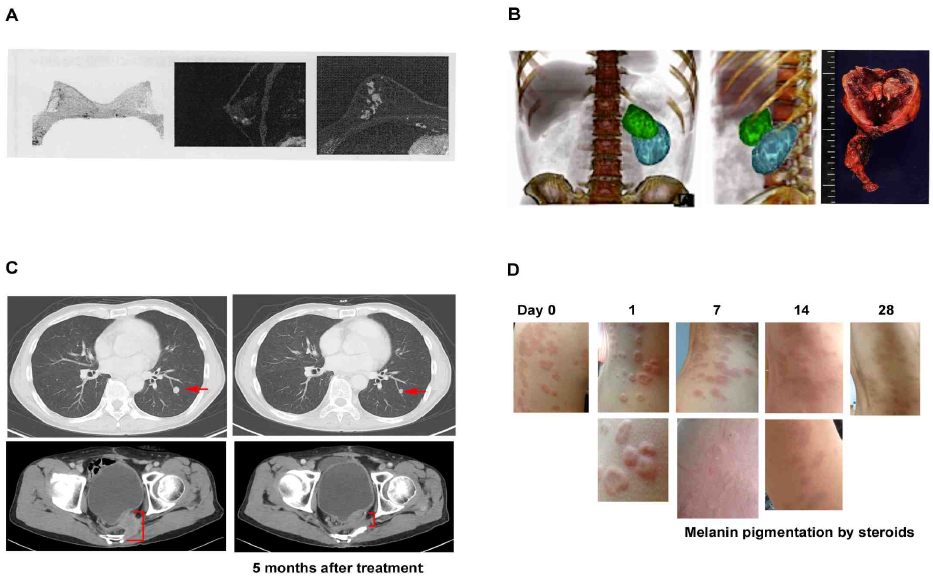
Figure 1: Anti-proliferative effect of Huaier on cancer and consequent angiogenesis, metastasis, and induces autophagic cancer cell death.
(A) PET image of 49 years’ old left breast cancer patients (stage 3), with invasion to adjacent lymph nodes detected by biopsy. Huaier administration 20g per day began at 4 weeks before surgical dissection and continued for one year. No cancer cells were detected in dissected lesions and lymph nodes by pathological analysis
(B) CT image of 35 years’ old male with left adrenal tumor (pheochromocytoma), CD56 (+), chromogranin A (+), synaptophysin (+) with lymph nodes invasion. Huaier was administrated 20g per day from 12 weeks before surgical dissection by laparoscope, and continued for total 18 months. Three dimensional video can be seen in the Youtube (https://www.youtube.com/watch?v=ScaBDkrEGKc). Green mass is adrenal tumor and blue mass is kidney. Tumor consisted of multiple cysts, inside of which were full of massive hemorrhagic necrosis. The size of tumor did not change after Huaier administration, and significant angiogenesis was not observed.
(C) CT image shows the improvement of metastatic lesion in short span of 68 year old male patient of colorectal caner (stage 4). 8 years have passed since surgical operation. Huaier 20g per day for 3 months: (upper) lung metastatic lesion, (lower) bone metastasis.
(D) Extensive eczema from abdominal to back area with liver dysfunction, steroid cream applied for 4 months. Huaier was administered with 3g per day for 4 weeks. Note the inflammatory edema in rush on day 1. Eczema and severe itching vanished away within 4 weeks, except pigmentation caused by long application of steroid cream, and no relapse reported.
administration of 3g of Huaier per day. The patients were fully recovered from the skin lesion as well as liver
dysfunction (familial) within 3 months.
Cancer patients were treated up to 2 years with a gradual decrease of Huaier dose, 20g to 3g, and so far no metastasis or recurrence were observed. More importantly, the quality of life of those patients were maintained well enough, no restriction in the usual activities of daily life. The prolongation and prognosis in severe cases of stage 4 cancer were significant (3-5 years or more), and that without any pains or physiological disturbance in sleep behavior, in digestive and excretory functions. However, we have to emphasize that Huaier was not effective on the infectious diseases, and pain-control from the physiological damages.
3.2 Verification of Drosophila model for the experimental design
In order to prove the hypothesis that the Hippo signaling pathway is the principal integrate molecular mechanism caused by Huaier administration, we planed and performed a ‘simple and clear’ demonstration using Drosophila model. Transgenic Drosophila fly was an appropriate model for our purpose, with decreased lifespan and uncontrolled cell proliferation [17, 24]. The transgenic mutant Drosophila in which overexpressing non-phosphorylatable form Yorkie (Yki:V5S168A; described ykiS168A hereafter) enhances cell proliferation by transcriptional stimulation [21-24]. The assumed (and later proved) effective sites of Huaier were shown in Figure 2. The original schematic figure was used with a courtesy of Prof. Dr. Kun-Liang Guan, from the publication in EMBO Rep. 25, 642-656 (2014) [20]. Phosphorylated YAP/TAZ (Yorkie) localizes to the cytosol, decreasing tumor growth, whereas unphosphorylated YAP/TAZ (Yorkie) is localized mainly in the nucleus and promotes cell and tumor signaling is associated with tumor progression [20-23]. Dysregulation of transcription control can be observed by severe overgrowth phenotype in the eye imaginal disc (rough eye formation) [24, 25].
In order to analyse the effect of Huaier, we first confirmed the toxicity and/or side effects by the drug feeding experiments [24]. In Japan, National center provides the on-demand experimental services, especially for the molecular researches of human diseases. However, even with the detailed analysis of molecular basis, no successful proof was provided so far using candidate chemical compounds for the disease control.
In the present study, we have chosen to use the commercially available form of Huaier granules for the possible reproduction of clinical significance even in the in vivo experiments. Huaier granule has been reported to have more effects than aqueous extracted form of Trametes robiniophila murr, and/or the accumulation of the effects from each component [1, 12].
The following examinations have been continuously performed for three years at the National Drosophila Center attached to Kyoto Institute of Technology, Kyoto, Japan. The experiments have been performed continuously for 3.5 years, with a strict reconfirmation by the Laboratory staffs in charge as described previously [24, 28]. Each experiment was performed at least in triplicates, and three independent researchers confirmed the data with Laboratory members attached to the National Center. Yeast paste with blue color (FD&C Blue Dye No.1, Sigma Aldrich) was use to check the feeding behavior, and no detectable differences between wild type and transgenic mutant flies as described [17, 24].
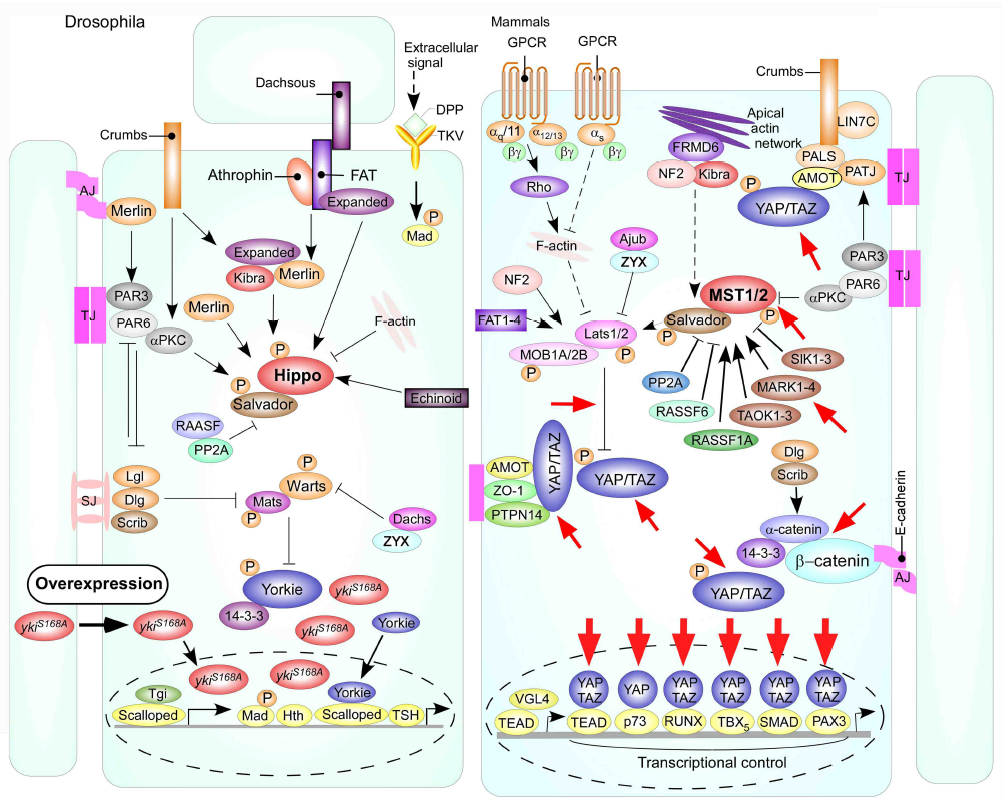
Figure 2: Schematic models of the Hippo signaling pathway in Drosophila and mammals as shown by Mo, J. S. et al. [20].
Red-circled ykiS168 indicates the changes in Drosophila mutant used in the present study. Cells are shown with respective cellular junctions; adherence junction (AJ), tight junction (TJ) and septate junction (SJ). Hippo pathway components in Drosophila and mammals are shown in various colors, with arrows and blunt lines indicating activation and inhibition, respectively. The yellow spheres indicate phosphorylation of target proteins by kinase. Continuous lines indicate known interactions, whereas dashed lines indicate unknown mechanisms. Red arrows indicate the presumed points of Huaier effect. The presumed points of Huaier effect was judged from the results obtained in the present study and from previous reports [1-12, 21-24, 26-28].
The influence of Huaier at a various doses was examined with wild-type larvae (Figure 3) to confirm the toxicity of the Huaier compounds to the experimental model. Feeding of Huaier to wild type Canton S and transgenic flies did not show any difference in the pupariation rates up to 167 mg/ml (Figure 3A and 3B). Similarly, no influence or changes on the eclosion rate up to 333 mg/ml, either (Figure 3C and 3D).
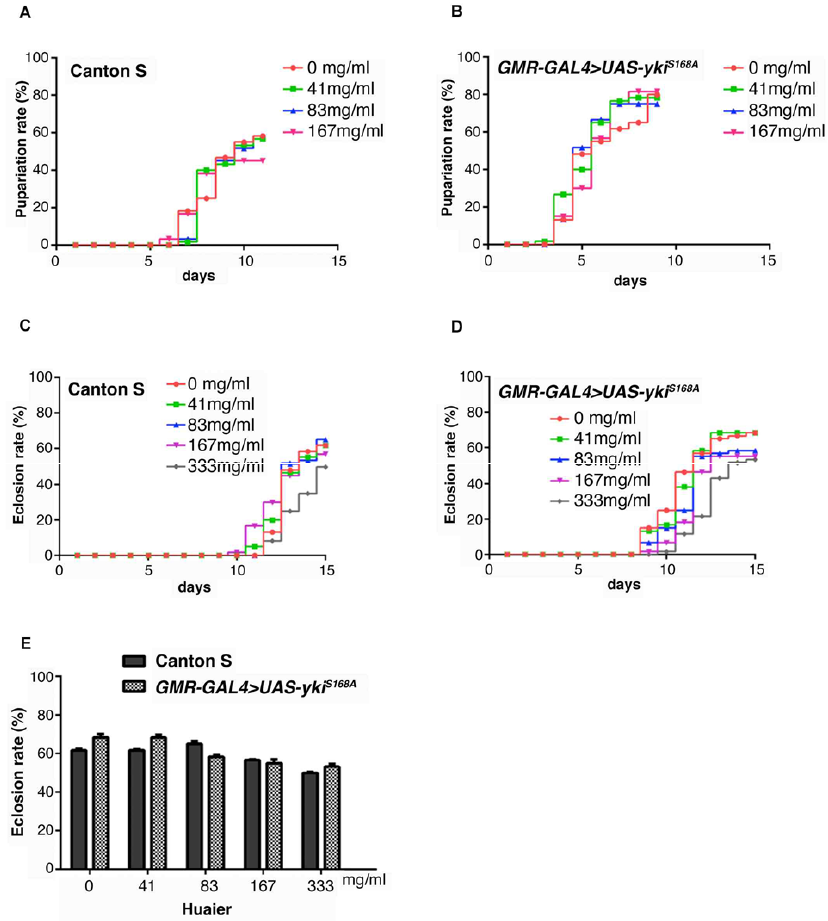
Figure 3: Huaier administration has no toxicity, and no changes in viability of both normal and transgenic Drosophila.
(A) Pupae formation rate of Canton S. Drosophila was fed with the indicated amount of Huaier.
(B) Pupae formation rate ofthe transgenic fly with overabundant non-phosphorylatable Yorkie (ykiS168), GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A).
(C) Eclosion rate of Canton S. Drosophila was fed with the indicated amount of Huaier.
(D) Pupae formation rate of the transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A).
(E) Survival rate of adult flies from Canton S and the transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A). No significant difference between Canton S and the transgenic flies. P-value < 0.05. n=60. No effect on food intake was confirmed by using blue color (FD & C Blue Dye No.1, Sigma Aldrich, St, Louis, MO, USA). Drosophila was fed with the indicated amount of Huaier.
The summary of the comparison was shown in Figure 3E. Huaier administration had no difference between wild-type and transgenic flies was observed on food intake of larvae, and both in morphology and in feeding behavior. The results indicated that, just as the results from clinical observations, there is no toxic effect of Huaier on Drosophila development by the evaluation of the pupariation rates and eclosion rates.
3.3 Huaier recovered rough eye formation in transgenic mutants by the reconstruction of tumor suppressor mechanism in the Hippo signaling pathway
Thus, the toxicity of Huaier on transgenic flies was determined to be negligible, next we examined Huaier effect on rough eye formation. Overabundance non-phosphorylatable Yorkie (ykiS168A) induces severe rough eye formation of adult fly (Figure 4A and 4B). The significance of this transgenic mutants were that, 1) genetic link of the effect can be easily observed by the morphological chances in eye discs, and 2) the evaluation of the experimental effects can be specifically focused on the transcriptional regulation points among the abundant molecules and functions involved in, and/or related to the Hippo pathway [22, 24].
In comparison to wild type Canton S (Figur 4A), we observed morphological abnormality in transgenic mutants (Figure 4B), GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A) as previously described [20, 22, 24]. Administrating Huaier for 7 days after hatching clearly suppressed this overgrowth eye phenotype. This improvement of morphological abnormality was observed in dose dependent manner, and almost complete suppression the rough eye formation was shown at 333 mg/ml density.
As a confirmation of recovery in transcription control of Hippo pathway, the EdU assay in the eye imaginal discs of third instar larvae was performed (Figure 4C). The mutant disc caused a significant ectopic DNA synthesis (red color by EdU staining) in the posterior region (white border line region) compared to the control which only overexpressed GFP (green color).
On the other hand, at a dose of 167 mg/ml, a folded pattern in the eye discs was demonstrated, suggesting the partial rescue of the ectopic EdU cells (Figure 4C, right low). These results indicated that Huaier treatment inhibited ectopic DNA synthesis, which resulted in the efficient rescue of rough eye formation [24].
These results succeeded to prove our original hypothesis by a simple and clear demonstration. Huaier recovered the disrupted transcription control by overabundant non-phosphorylatable Yorkie (ykiS168A), which resulted in normal tissue rescue against cancer progression. This is reproducible evidence observed in cancer patients, typically in many breast cancer and hematoma [1, 2, 5-7, 10, 11]. In addition, this is the first successful result using Drosophila disease model in the National Drosophila Center, Kyoto, Japan, with a good reproducibility with the clinical observation. Although we tried many candidate chemical synthesized compounds especially correlating with a transcriptional control, the results showed strong cytotoxicity than efficacy even with pg level. As noted before, these results suggest that natural products with crude mixture of effective elements have more efficacy than mono-targeted agents, which have certain limitations against diseases originated from multiple factors [17].
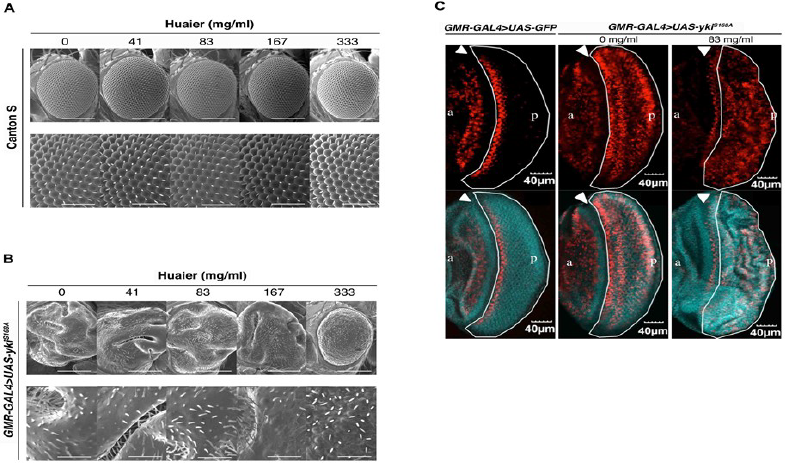
Figure 4: Huaier administration recovers the rough eye formation caused by overabundant non-phosphorylatable Yorkie (ykiS168A) in transgenic flies.
(A) No significant changes in adult compound eye morphology with the different concentration of Huaier on hatched larvae for 7 days. Scanning electron micrographs of newly eclosed adult male compound eyes were shown. From the left column to right; Canton S fed with no Huaier; Canton S fed with 41 mg/ml; Canton S fed with 83 mg/ml Huaier; Canton S fed with 167 mg/ml Huaier; Canton S fed with 333 mg/ml Huaier granule concentration. No significant variation in eye phenotype was observed among the examined flies. Anterior is to the left and dorsal to the top. The flies were developed at 25°C. The lower column indicates enlarged images of the upper. The bars indicate50µm (upper) and 14.2µm (lower), respectively.
(B) Scanning electron micrographs using transgenic mutants. From the left column to right; transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A), fed with no Huaier; transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A), fed with 41 mg/ml Huaier; transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+(GMR-GAL4>UAS-ykiS168A), fed with 83 mg/ml Huaier; transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+(GMR-GAL4>UAS-ykiS168A), fed with 167 mg/ml Huaier; transgenic fly, GMR-GAL4/y; +; UAS-ykiS168A:V5/+(GMR-GAL4>UAS-ykiS168A), fed with 333 mg/ml Huaier granule concentration. The eye phenotype of at least five newly eclosed adult male flies was examined. No significant variation in eye phenotype was observed among the five individuals. Anterior is to the left and dorsal to the top. The flies were developed at 25°C. The bars indicate50µm (Upper column) and 14.2µm (lower column), respectively.
(C) Feeding of Huaier suppressed the ectopic DNA synthesis in eye imaginal discs of non-phosphorylatable Yorkie (ykiS168A) overexpressing flies. Upper column: The eye imaginal discs were labeled with EdU (red). Lower column: Merged with DAPI. Left column; the eye imaginal discs of the control fly [24], GMR-GAL4/y; UAS-GFP/+; + (GMR-GAL4>UAS-GFP), middle and right; the eye imaginal discs of ykiS168Amutant, GMR-GAL4/y; +; UAS-ykiS168A:V5/+ (GMR-GAL4>UAS-ykiS168A). a indicates anterior and p indicates posterior. The bar indicates 40µm.
3.4 Huaier uptake caused metabolome profile shift to developmental/ early embryonic stage
In order to obtain the detailed information which contributed to the morphological changes by Huaier uptake, GC/MS-based metabolome analysis were performed for the profiling of low molecular weight hydrophilic metabolites, including sugars, amino acids, and organic acids [26, 28]. The basic and detailed metabolic profile of Drosophila melanogaster, also provided from the National Drosophila Center, Kyoto, Japan, was reported with a consideration of developmental embryonic stages [28]. The same samples were used in the present study as controls. Thus the precise metabolome analysis and evaluation could be obtained by matching the metabolome composition to any stages of development, from the hatching time to adult stage [28].
The resulting metabolome data matrix was subjected to PCA (Figure 5). The results showed that in the principal component (PC) space formed by PC1 (68.6%) and PC3 (18.4%), the data of 4 different samples were subsequently separated into four distinct clusters (Figure 5A). The grouping was almost in complete agreement with the type of Drosophila (the wild type or ykiS168A transgenic mutants), and with or without administration of Huaier. Compared with the standard metabolome matrix published previously [28], Huaier administration group clearly made the metabolome profile shifted from matured/adult stage to early developmental stage (clusters moved counter-clockwise rotation), whereas Huaier administrated normal flies showed the maturation shift (clockwise rotation). Cancer model (ykiS168A transgenic mutants) showed a slight counter-clockwise rotation as predicted with undifferentiated cell clusters, and then Huaier administration group clearly made the significant counter-clockwise rotation, just the same as observed in early stage (0-4 hours after egg laying) (Figure 5A).
The score plot interpretation, we focused on the distribution of metabolites in the loading plot based on their distance to the origin (Figure 5B and 5C). Several key metabolites were found important for the therapeutic effect of Huaier. Specifically, trehalose and glucose had highly positive contribution, just as shown in early embryonic stages in Drosophila embryonic development, while glutamine had negative contribution to the separation on PCI (Figure 5B). On the other hand, glycine, alanine, homoserine, and lysine levels are high, whereas malic acid, citric acid, arabitol are low (Figur 5B). These metabolites are inversely correlated, which made the treated groups separated from the untreated groups according to PC3. Since these metabolites, related to sugar and amino acid metabolisms, are very important energy resources of the cell proliferation and specification, Huaier administration may emerge the energy metabolism into that required in the early developmental stage [28].
As shown in Figure 5C, there were 3 patterns of typical changes of the metabolics. First, significant increase after Huaier treatment was observed in arabinose-5-phosphate and arabitol, as well as in glutamine and asparagine. Second, significant decrease was demonstrated with lysine and homoserine. The others showed slight but significant decrease after Huaier administration were observed such as alanine, glycine, uracil, xanthine, xylitol and DOPA. In addition, the importance of alanine as a carbon source was emphasized to be associated with tumor suppressor mechanism especially in cancer stem cells [28].
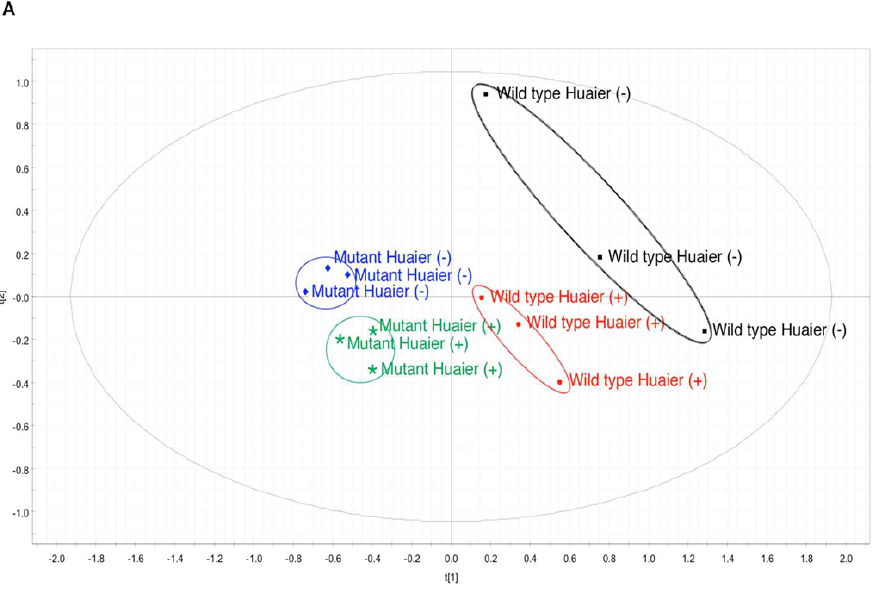
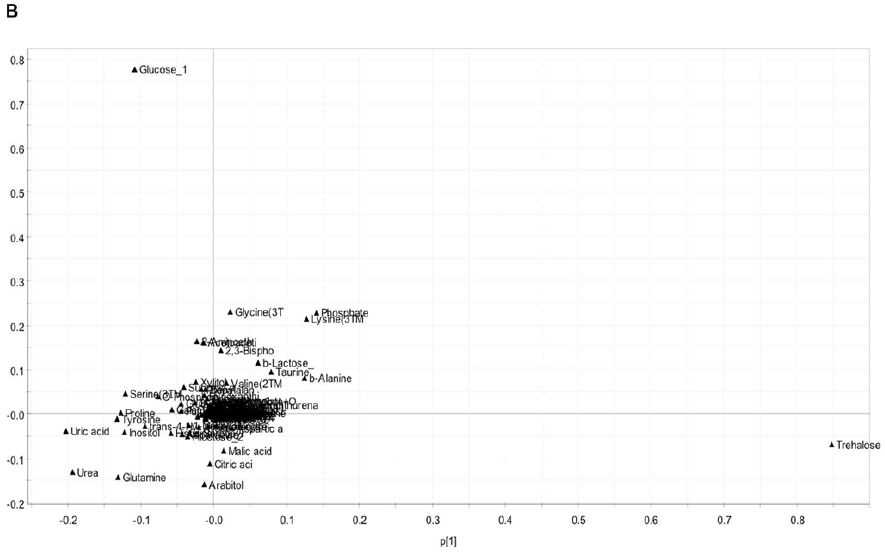
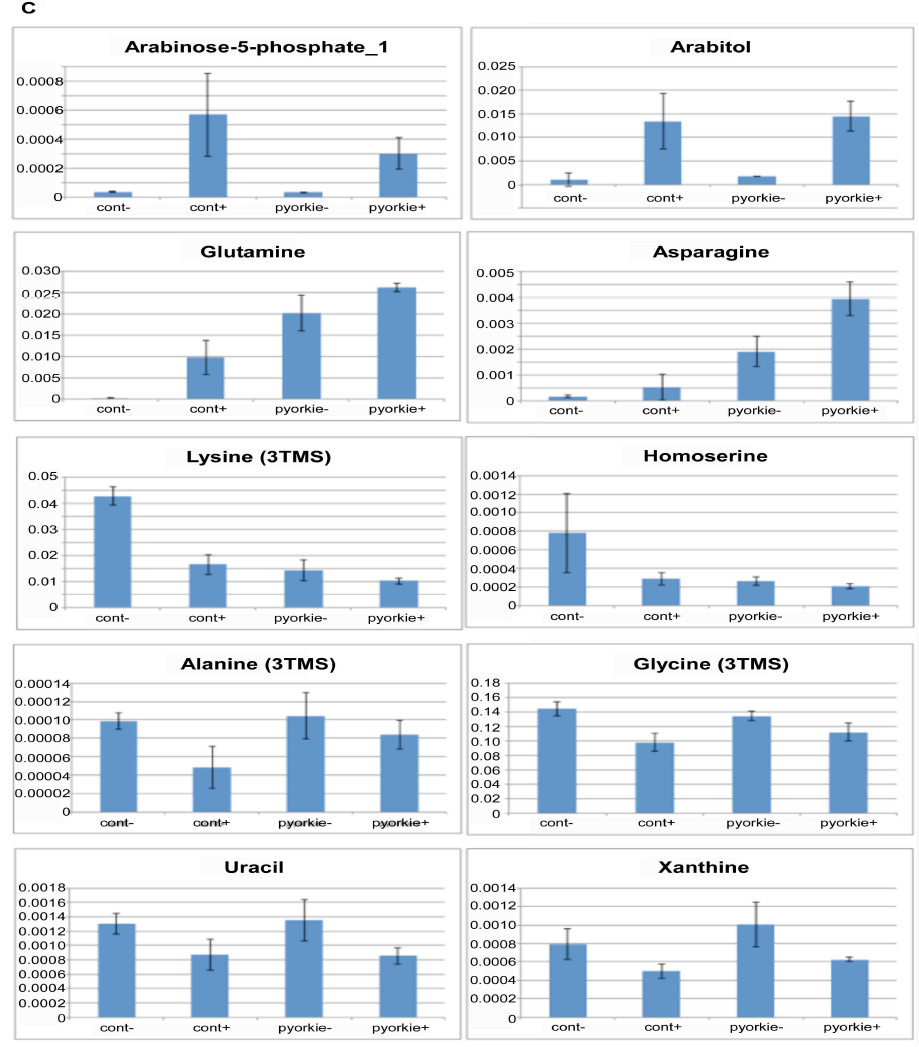
Figure 5: There is a high coincidence between the composition of metabolome and Huaier intake (167 mg/ml mixture).
(A) PCA score plot shows independent clusters.
(B) The loading plot shows the contribution of each metabolite on the discrimination in score plot according to the distance to the origin.
(C) Important changes in metabolites by 167 mg/ml Huaier administration for 7 days. Samples were newly eclosed adult male flies, and classified into 4 clusters; normal Canton S, Canton S+ Huaier, ykiS168Atransgenic flies, ykiS168A transgenic flies + Huaier. All samples were analyzed in triplicates (n=10-14, total 5 mg in dry weight).
Trehalose is also reported to be present as an energy source in the Drosophila hemolymph as early as the larval stage. There has been reported that there is a strong correlation between the increase of trehalose level and neurogenesis in Drosophila embryo [28]. The change of amino acid metabolism also suggests that Huaier administration shifted the requirement of metabolomes according to the cell potential at the developmental stage, or at the time of embryogenesis. We also analyzed non-aqueous small protein debris after the extraction of sample materials, and so far no changes were detected by the Western blots.
4. Discussion
Thus the present study demonstrates a simple and clear proof to the hypothesis that the molecular basis of Huaier effects was based on the effect on the Hippo signaling pathway. The significant points of action were summarized in Figure 2, with a consideration of the reported candidate mechanisms together [1, 3-5, 7-11, 20]. Judging from both in vitro and in vivo examinations, it is thus indicated that the effect of Huaier was based on the recovery of Hippo pathway control to retain tissue homeostasis from the degenerative changes specifically occurred in cancer (summarized in Figure 6). More importantly, it is demonstrated that Huaier provides not only as a complementally anti-cancer tool together with conventional therapy, but also an effective tool to regulate and restore the dysfunction of Hippo pathway. The transgenic flies used in the present study were specifically useful to evaluate the effect of Huaier on the recovery of the transcriptional control of Hippo pathway [8, 21-24].
As a key downstream effectors of Hippo pathway, YAP/TAZ (Yorkie ortholog) is involved in embryonic stem cells as well as tissue-specific stem cell self-renewal, and tissue regeneration and homeostasis of multiple organs, such as the liver, intestine, and pancreas (cancer from all of these organs are Huaier effective), skin, heart, and central nervous system [20, 29, 30]. YAP/TAZ (Yorkie ortholog) is also suggested to play a key role in cancer stem cells [20, 23, 27]. Thus the results obtained here indicated the potential of Huaier revitalizing the general and normal function and activity of the Hippo signaling pathway. As shown in Figure 2 [20], it is suggested that Huaier could modulate and restore the damages in the Hippo pathway toward the normalization of transcriptional control in the cell. For this purpose, the process would begin with the induction of the intracellular pluripotency, and also cell fate specification.
The results obtained by metabolome analysis indicated the strong shift to the embryonic stage of the whole body of the Drosophila samples, which might be an aspect of the process to recover tissue homeostasis. Huaier
administration to Drosophila samples showed the clear separation of metabolome matrix changes from the untreated
flies, both in wild type and transgenic mutants, and demonstrated a drastic shift to the early developmental stage in which the Hippo pathway has most potential to interact with other pathways to promote and maintain pluripotency [20, 21].
Huaier influences not only intracellular molecule-to-molecule interaction, but also tissue recovery process from massive cancer cell death. Obviously Huaier causes significant improvement of physiological conditions after elimination of tumor within a couple of years, which suggests that the damaged tissue and its altered homeostasis simultaneously should be restored. For this purpose, the required condition might be first to invert the ageing process of the cells toward the condition such as observed in the stem cells or induced pluripotent stem cells [20], followed by the normal cellular specification process. These speculations, however, still remains as one of a variety of speculations, and it could not be proved here because of the many biological limitations in our in vivo model system. More attention should be paid to solve these questions on Huaier effects based on the clinical evidence, such as the strong inhibition of recurrence, metastasis, and drug resistance, and also significant improvement of prognosis and prolongation of lifetime without severe symptoms and disturbance.
The overabundant ykiS168A used in the present study mimics the intracellular situation of Hippo pathway observed in embryonic stem cells. YAP/TAZ (Yorkie) are the major downstream mediators of the Hippo pathway, and interacts with other transcription factors to mediate the transcription of a diverse array of genes (Figure 2). Thus Hippo pathway and transcriptional control of YAP/TAZ (Yorkie) stands as a core system to integrate and control the other molecular mechanisms. With these considerations, Huaier seems to modulate and reconstruct the disrupted Hippo pathway using YAP/TAZ (Yorkie) potential, which consequently resulted in the appropriate regulation of cell specification and specification.
We have tried many drug candidates influencing transcriptional control in nucleus in vitro, all of which resulted in the complete cell death because of a strong toxicity at pg level. Huaier provides a simple and clear proof that can control and modulate transcriptional dysregulation in the Hippo signaling pathway, and that with no toxicity or side effects. There are many degenerative diseases and disorders correlated with dysfunctional transcription system in the Hippo pathway, to which Huaier can be applied and tested. We happened to apply Huaier to one case of Parkinson’s disease, which resulted in drastic improvement of the symptoms.
Surprisingly, the metabolome analysis provided the important information relating to the treatment process of a long course (2-5 years) of Huaier administration. First, the metabolome profile of cancer mutants was not simply returned to be the normal type after the rescue of the Hippo pathway. Second, the profile shift was back to that just as shown in early developmental stages. Together with other results obtained by the metabolome analysis, Huaier administration seemed to induce the intracellular pluripotency, and also cell fate specification, via the reconstruction and modification of the Hippo signaling pathway in damaged cells. In reality, it is recommended to surgically dissect at most cancer cells and lesions. The prevention of the recurrence was most apparent with Huaier treatment at four weeks’ advance, and the recovery of the patients judged from the data by body weight, motor function, daily behavior, and from the other blood test is significantly better by a couple of weeks compared with the patients treated by conventional therapy only.
5. Conclusion
The predicted Huaier effects on cancer and other physiological disorders were summarized in Figure 6. The present study thus provides the molecular basis of Huaier effect, which will suggest the possibility of its medical contribution to more target organs and disorders strongly influenced by the Hippo pathway function, such as heart,
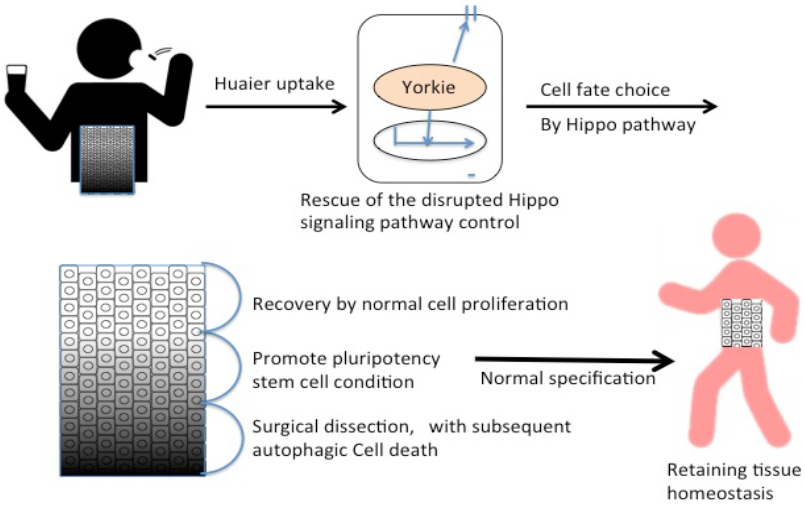
Figure 6: Summary of Huaier effects on the rescue and repair of tissue homeostasis.
skeletal muscles, and central nervous system [30]. Thus, Huaier will provide a valuable tool to overcome the difficulty in the treatment of many degenerative diseases, and that in a dose-dependent manner.
6. Acknowledgements
The authors wish to thank many healthy volunteers, and patients kindly collaborated with the present study. This project was supported by grants from the Grant-in-Aid for Scientific Research (KAKENHI), grant number 16K09258 by Japan Society for the Promotion of Science (JSPS) to TT, TS, HK and MY. This study was supported by the Grant for Joint Research Project of The Center for Advanced Insect Research Promotion, Kyoto Institute of Technology (TT, MY, DW and MT).
7. Author contributions
TT and MT designed the study from the clinical observation of the cancer patients with Huaier treatment (as a complemental therapy), and the medical and genetic analysis of the experimental data, and drafted the manuscript. TS, JN, YK, SS and HK contributed to the study design by clinical observations with conventional therapy and biochemical and pathological analysis using the clinical specimens. IY, NV and MY carried out the Drosophila experiments. YI and MS carried out metabolome analysis. DW contributed to the provision of Huaier granules and other herbs, and the summary of the available clinical data from the clinics in China and Japan.
8. Conflict of interest
The authors have no competing interest to declare.
References
- Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review). Oncol Rep 34 (2015): 12-21.
- Zhang, N, Kong X, Yan S, Yuan C, Yang Q. Huaier aqueous extract inhibits proliferation of breast cancer cells by inducing apoptosis. Cancer Sci 101 (2010): 2375-2383.
- Wu T, Chen W, Liu S, Lu H, Wang H, et al. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS Lett 588 (2014): 2107-2114.
- Yan X, Lyu X. Yun A, Wei Y, Yang Q, et al. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3beta/beta-catenin pathway. Plos One 8 (2013): e63731.
- Zheng J, Li C, Wu X, Liu M, Sun X, et al. Huaier polysaccharides suppresses hepatocarcinoma KHCC97-H cell metastasis via inactivation of EMT and AEG-1 pathway. In J Biol Macromo 64 (2014): 106-110.
- Li C. Wu X, Zhang H, Yang G, Hao M, Sheng S, Sun Y, et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumor Biol 36 (2014): 1739-1745.
- Wang X. Wang N, Cheung F, Lao L, Li C, Feng, Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integ Med 13 (2015): 142-164.
- Zhang T, Wang K, Zhang J, Wang X, Chen Z, Ni C, Qiu F, et al. Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via down regulation of the Wnt/?-catenin pathway. Oncol Lett 5 (2013): 1171-1176.
- Cui Y, Meng H, Liu W, Wang H, Liu Q. Huaier aqueous extract induces apoptosis of human fibrosarcoma HT1080 cells through the mitochondrial pathway. Oncol Lett 9 (2015): 1590-1596
- Wang X, Zhang N, Huo Q, Sun M, Dong L, et al. Huaier aqueous extract inhibits stem-like characteristics of MCF7 breast cancer cells via inactivation of hedgehog pathway. Tumor Biol 35 (2014): 10805-10813.
- Wang X, Qi W, Li Y, Zhang N, Dong L. Sun M, et al. Huaier extract induces autophagic cell death by inhibiting the mTOR/S6K pathway in breast cancer cells. PLoS ONE 10 (2015): 0131771.
- Sun Y, Sun T, Wang F, Zhang J, Li C, et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydr Polym 92 (2013): 577-582.
- Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, et al.Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 366 (2012): 697-706. Erratum (2016): Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 374: 1898.
- Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, et al. (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366: 687-696.
- Tanaka M, Tanaka T, Matsuzaki S, Seto Y, et al. Rapid and quantitative detection of a mammalian septin Bradeion as a practical diagnostic method of colorectal and urologic cancers. Med Sci Monit 9 (2003): 61-68.
- Tanaka M. Tanaka T, Kijima H, Itoh J, Matsuda T, Hori S, Yamamoto M.Characterization of tissue- and cell type-specific expression of a novel human septin family gene, Bradeion. Biochem Biophys Res Commun286 (2001): 547-553.
- Chongtham A, Agrawal N. Crucumin modulates cell death and it protective in Huntington’s disease model. Sci Rep 6 (2016):18736.
- Da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol 1 (2001): 364-369.
- Li L, Ye S, Wang Y. Progress on experimental research and clinical application of Trametes robiniophila. Bull Chin Cancer 16 (2007): 110-113.
- Mo JS, Park JW, Guan, KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 25 (2014): 642-656.
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 13 (2013): 246-257.
- Zhao B, Wei X, Li W, Udan RS, Yang Q, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21 (2007): 2747-2761.
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28 (2008): 2426-2436.
- Vo N, Horii T, Yanai H, Yoshida H, Yamaguchi M. The Hippo pathway as a target of the Drosophila DRE/DREF transcriptional regulatory pathway. Sci Rep 4 (2014): 7196.
- Oh H, Irvine KD. In vivo analysis of Yorkie phosphorylation sites. Oncogene 28 (2009): 1916-1927.
- Tsugawa H, Bamba T, Shinohara M, Nishiumi S, Yoshida M, et al. Practical non-targeted gas chromatography/mass spectrometry-based metabolomics platform for metabolic phenotype analysis. J Biosci Bioeng 11 (2011): 292-298.
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci 103 (2006): 12405-12410.
- Thuy An PN, Yamaguchi M, Bamba T, Fukusaki E. Metabolome analysis of Drosophila melanogaster during embryogenesis. PLoS ONE 9 (2014): 519-526.
- Joardar A, Menzl J, Podolsky TC, Manzo E, Estes PS, et al. PPAR gamma activation is neuroprotective in a Drosophila model of ALS based on TDP-43. Hum Mol Genet 24 (2015): 1741-1754.
- Liu QF, Lee JH, Kim YM, Lee S, Hong YK, et al. In vivo screening of traditional medicinal plants for neuroprotective activity against Aß42 cytotoxicity by using Drosophila models of Alzheimer's disease. Biol Pharm Bull 38 (2015): 1891-1901.
