Huaier Inhibits Cancer Progression Correlated with the Mutated EGFR and Other Receptor Tyrosine Kinases (c-MET/erbB-2) by Down-Regulation of Multiple Signal Transduction Pathways
Article Information
Manami Tanaka1*, Tomoo Tanaka1, Fei Teng2, Hong Lin3, Ning Li3, Zhu Luo3, Ding Wei4, and Zhengxin Lu5
1Bradeion Institute of Medical Sciences, Co., Ltd., Itado 433-1, Isehara, Kanagawa 259-1145, Japan.
2BGI-Shenzhen, Building NO.7, BGI Park, No.21 Hongan 3rd Street, Yantian District, Shenzhen 518083, China
3BGI-Japan, Kobe KIMEC Center BLDG. 8F 1-5-2 Minatojima-minamimachi, Chuo-ku, Kobe 650-0047 Japan.
4Japan Kampo NewMedicine, Co., Ltd. , 2-8-10 Kayaba-Cho, Chuo-Ku, Tokyo 103−0025, Japan.
5QiDong Gaitianli Medicines Co., Ltd., No.88, Heping South Road, Qidong, Jiangsu Province, China.
*Corresponding author: Manami Tanaka, Bradeion Institute of Medical Sciences, Co., Ltd., Itado 433-1, Isehara, Kanagawa 259-1145, Japan/p>
Received: 30 March 2021; Accepted: 08 April 2021; Published: 15 April 2021
Citation:
Manami Tanaka, Tomoo Tanaka, Fei Teng, Hong Lin, Ning Li, Zhu Luo, Ding Wei, and Zhengxin Lu. Huaier Inhibits Cancer Progression Correlated with the Mutated EGFR and Other Receptor Tyrosine Kinases (c-MET/erbB-2) by Down-Regulation of Multiple Signal Transduction Pathways. Archives of Clinical and Biomedical Research 5 (2021): 262-284.
View / Download Pdf Share at FacebookAbstract
Background: Clinical significance of anti-cancer effects of Huaier (Trametes robiniophila murr) has been emphasized recently [1-3]. We have already reported the successful Huaier therapy based on the individual genomic potential and miRNA-mediated transcription control [4-7]. In prostate cancer, Huaier also proved to have a significant efficacy on the prevention of cancer progression [6].
Objective: We have been initiating clinical research for thorough understanding of molecular basis of Huaier effects, which contributes to define the responsible molecules and biosystems not only for recovery, but also for prevention of cancer progression and tumorigenesis.
Methods: The peripheral blood samples from the volunteer patients were analyzed by total RNA and non-coding small RNA sequencing on BGISEQ-500 Platform [8, 9]. KEGG pathway classification was hired for the analysis of the obtained information in the present study (https://www.genome.jp/kegg/) [10].
Results: In the present study, we focused on the patient and her family correlated with the mutated EGFR [11] and other receptor tyrosine kinases (c-MET [12-14]/erbB-2 [15, 16]). At first, high ratio of SNP variants as A-G transitions (241,896 in total, 37.0%) and A-C transversions (43,213 in total, 6.5 %), with skipped-exon (51%), which influenced significant changes in transcriptional factors and corresponding gene expression in multiple signal transduction pathways. The ratio of alteration ranged from 10% to 30% of total number of transcripts, detected as a drastic down-regulation by Huaier administration. Although the patient was diagnosed as benign meningioma, there were no genomic alteration in RNA polymerase II and its subunits [17], but only quantitative down-regulation in the attached transcriptional fac
Keywords
Huaier (Trametes robiniophila murr); Cancer therapy; EGFR: Epidermal growth factor receptor (ErbB-1, HER1 in humans); c-MET: Tyrosine-protein kinase Met or hepatocyte growth factor receptor (HGFR); HER2/neu: Receptor tyrosine-protein kinase erbB-2, former CD340)
Huaier (Trametes robiniophila murr) articles, Cancer therapy articles; EGFR: Epidermal growth factor receptor (ErbB-1, HER1 in humans); c-MET: Tyrosine-protein kinase Met or hepatocyte growth factor receptor (HGFR) articles; HER2/neu: Receptor tyrosine-protein kinase erbB-2, former CD340) articles
Article Details
1. Introduction
Recent proceedings of Huaier effects proved significant anti-cancer efficacy even on the advanced severe cases including pancreas adenocarcinoma with multiple metastasis, and more importantly, on the prevention of prostate cancer progression [7]. Since the prominent potential on the recovery from hepatocellular carcinoma and breast cancer [1-3], it is not surprising to observe Huaier efficacy on many other cancers.
After we succeeded to prove the molecular basis of anti-cancer effects of Huaier by a simple method using Drosophila mutants [4], we initiated clinical research total RNA and small non-coding RNA analysis to confirm the reality of significance of Huaier treatment on cancer [5-7]. The results provided the massive alteration and modification of the transcripts by up-and down-regulation, based on the miRNA-mediated transcriptional control [5-7].
We have identified significant high-responders to Huaier treatment, and the present study specifically focused on the patient diagnosed benign meningioma, one of the top high-responders. We did not identify the apparent reason why benign tumor required as such quantitative alterations to recover (patient No. 10 in the previous report [5]). By systematic search for the possible changes to cause the changes up to 30% of all the transcripts, we identified alterations in EGFR (erbB1) [11], c-MET [12-14], HER2/neu (erbB2) [15, 16], and also in neurotransmitter SLC6A4 solute carrier family 6 member 4 (Htt) [17], and PRNP (prion protein); CD230 [18]. Although dysfunctions in EGFR and receptor tyrosine kinases have been reported as the cause of many severe health problems including cancers [11-16], there has not yet effective therapeutics without severe toxicity.
In addition, these alterations were identified among the patient family, who have no health problems for over 70 years of their lives (the parents). It is speculated that only multiple genetic alterations were not the cause of pathogenicity, something more matters to maintain healthy conditions among family and, for more tumorigenesis in the patient.
Huaier treatment on meningioma patient was first intended to promote repair potential after surgical dissection. Together with meningioma, this patient had a long history of many sufferings irrelevant each other, such as migraine headache, symptoms from hormonal discoordination, mild depression, continuous skin rashes (occasionally appeared in different places), symptoms from accumulated stresses such as hepatic dysfunction, sleep disorders. The memory loss was often observed, which leads her to a lack of conformity to social expectations. About 4 years ago, cranial CT and MRI analysis according to the symptoms (headache, and visual field defects) detected tumor mass (1.5x2.0 cm) in the olfactory groove, and diagnosis as benign meningioma was confirmed by pathological examination of dissected tissues. Huaier administration started just after the tumor detection, from 1 month before the endoscopic dissection. Until present time, Huaier treatment (20g per day for 2 years) resulted in 1) swift recovery of the injury by surgical operation, and 2) the prevention of the recurrence, frequently observed in similar cases in Japan.
Recently, more cases of c-MET mutation related non-small cell adenocarcinoma in lung were reported [12-14], but even with the advent of novel therapeutic agents, it is still difficult to overcome the severity of diseases.
The data obtained here Huaier provides a clue to rescue and compensate the defected functions by those multiple mutations controlling cell growth, proliferation, metabolism, and many other processes regulating a wide variety of cell communications. The effects of Huaier influences multiple signal transfer pathways, without correction or normalization of mutated genes. In addition, the differential pathogenicity of those hereditary mutations in family member might be linked to the epigenetic post-transcriptional regulation.
2. Materials and Methods
2.1 Project Design and patients’ profile
The present study specifically focussed on Huaier effects to the patient with the mutated EGFR [11] and other receptor tyrosine kinases (c-MET [12-14]/erbB-[15-16]). This patient appeared as the patient No.10 in the former paper [5], with her parents joined as normal healthy controls (the Patient No. 21/25[5-7] and No.34 [7].
Huaier compounds were provided by the manufacturer for this purpose with a strict control on transfer to Japan, good condition for maintenance, and provision to the patient volunteers, just as the same as the previous reports [5-7].
The present study was strictly conducted according to the guidelines of the Declaration of Helsinki and the principles of good clinical practice. Written informed consent was obtained from the patients. This clinical research was applied according to the Consolidated Standards of Clinical Research Trials guidelines and was applied to the Japanese Medical Association on 9th February 2018, and approved on 5th March, 2018 (ID: JMA-IIA00335). The project has been strictly conducted with a monthly review by the ethics committee consisted by the experts on Medicine, Nursing, Laws, Pharmaceutics and Business Community (first committee held on 9th February, 2018).
We used Huaier compounds as complementary therapy, without any chemotherapy and radiotherapy which disrupt the molecular systems. Only surgical operation was allowed if applicable, even in the period of during Huaier therapy. We thus planned and initiated an open-style, before-after controlled study, using peripheral blood as sampling materials to understand the almost all molecular events in each Huaier taking patient. The sampling materials were total blood, the same as reported previously [5-7]. To compare with the other sampling, RNA extraction using nuclear cell components in peripheral blood rapidly reflects the biophysiological changes, and that more sensitive to monitor the course of any treatment than any other samples such as dissected organs.
2.2 Total RNA and small RNA analysis
RNA extraction, miRNA library construction, and Total RNA- and small non-coding RNA-sequencing on the MGISEQ-2000 and BGISEQ-500 platform were processed in BGI, Shenzhen, China, as descrived previously [5-7]. The subseuent bioinformatics work was also processed in BGI, Shenzhen, China. The detailed protocols were provided and demonstrated at BGI website: http://www.bgitechsolutions.com/.
The identified DEGs (differentially expressed genes) and DESs (differentially expressed small RNAs) were analyzed between samples and do clustering analysis and functional annotations.
With quantitative analysis of DEGs, we performed Gene Ontology (GO) classification by three categories of molecular biological function, cellular component and biological process, with a consideration of time course of Huaier administration. We also analysed every signal transduction pathway by KEGG pathway classification [10] (https://www.genome.jp/kegg/). Furthermore, we applied the enrichment analysis of DEG in KEGG database.
The obtained novel transcripts and small nuclear non-coding RNA have been deposited to The NCBI GEO (GSE157086).
3. Results
3.1 Molecular Characterization of the patient
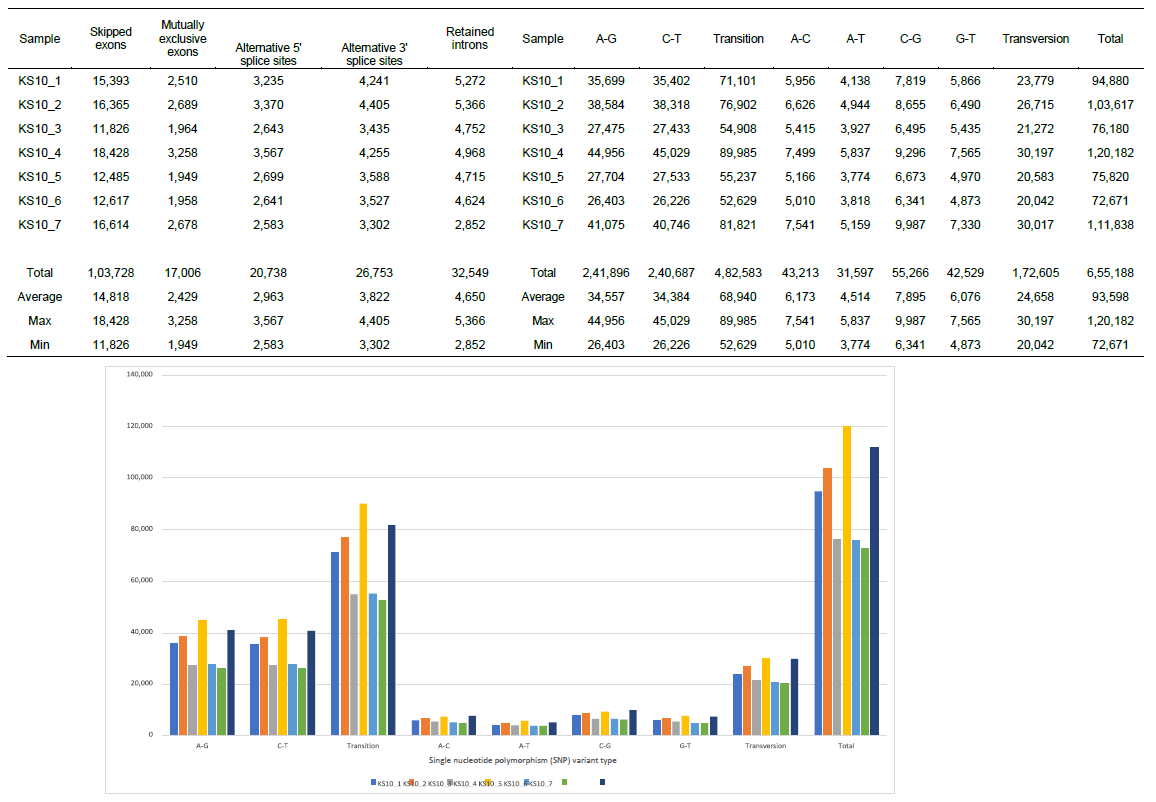
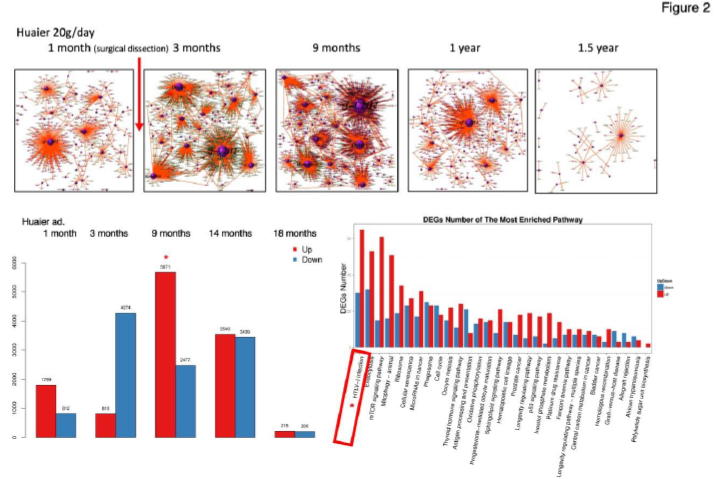
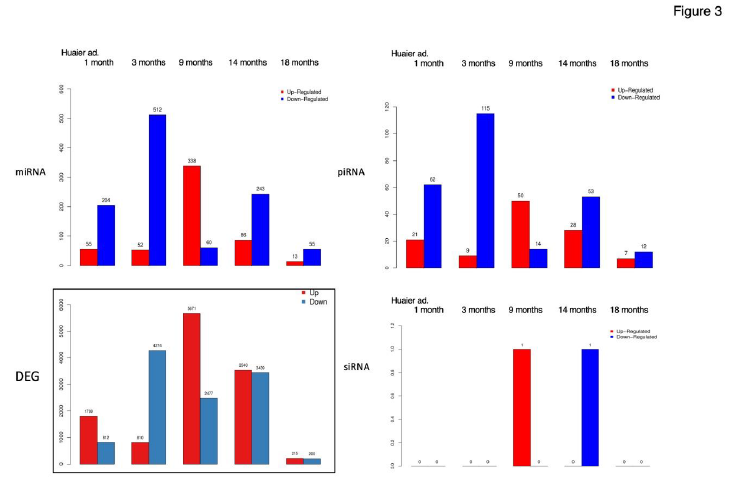
Figure 1-3 summarized the information for general understanding of molecular characteristics of the patient focused in the present study. Figure 1 demonstrates SNP and the splice type variants, Figure 2 demonstrates comparison of Transcription factor (TF)-Differentially expressed genes (DEG) network with the quantitative alterations in the transcriptomes, and Figure 3 summarized miRNA- and piRNA contribution to the up- and down-regulation of the transcriptomes according to the time course of Huaier administration. There was only one siRNA molecule up-regulated throughout the observation period.
We identified total 655,188 SNP variant types in 7 samples from the patient, and 93,598 SNP per sample in mean number (ranging from 72,671 to 120,182), whereas 22,688 in total among normal healthy individuals [19]. As for the SNP variations, A-G transitions were the most common mutations (241,896 in total, 37.0%), and the other C-G changes was A-C transversions (43,213 in total, 6.6 %), which showed no significant differences among the data in the other cancer patients [3] and the data obtained in oesophageal squamous carcinoma cells [19, 20] (Figure 1).
The summary of splicing variation comparison is also shown in Figure 1. DSGs are regulated by alternative splicing (AS), which allows the production of a variety of different isoforms from one gene only. We detected total 200,774 splicing events in 7 samples, and among five types, skipped exon (SE) was most prominent (51.7%), compared with the other four events; alternative 5’ splicing site (A5SS), alternative 3’ splicing site (A3SS), mutually exclusive exons (MXE), and retained intron (RI). Just like SNP variants, the ratio of splicing events showed no significant differences among the other cancer patients [5].
The process of the recovery was clearly shown by the TF-DEG network (functional lineage map) in Figure 2, upper column. Lower right in Figure 2 demonstrates the quantitative changes in Differentially Expression Genes (DEGs) by the time course of Huaier administration. The surgical operation often caused massive increase of up-regulated transcriptomes [5], which might reflect the repair function and tissue reproduction by Huaier, however, massive increase of down-regulation DEGs were observed here (about 15.6 % of the total transcripts). In contrast, the opportunistic virus infection caused the utmost increase of up-regulated DEGs (20.6 %), highlighted by asterisks in lower left of Figure 2.
Figure 2: Genome-scope profile of the patient. Upper column: Transcription Factor (TF) - Deferentially Expressed Genes (DEGs) network by the time course of Huaier administration as previously demonstrated [5]. The number of altered DEGs were shown in lower column (left), and the KEGG Gene Ontology analysis [10] revealed the opportunistic infection in lower right (*).
Figure 3 well demonstrated miRNA-mediating gene silencing effects after 1 month of Huaier administration. The significant changes in up- and down-regulated DEGs were in inverse proportion to those observed in DESs in miRNA and piRNA
(smaller in number). The increase of down-regulated miRNAs resulted in massive increase of down-regulated DEGs (4,274; 15.6% of the total). Interestingly, after one month of Huaier treatment, the changes in DEGs and DESs became proportionally bigger in number. Then the opportunistic virus infection influenced the drastic up-regulation of both DEGs and DESs, except siRNA. There was no significant correlation of DESs in siRNA detected throughout the treatment period with Huaier.
Huaier could not prevent virus infection, however, the infection remained asymptomatic and totally cured within reasonable period of time (one month in her father) [5-7].
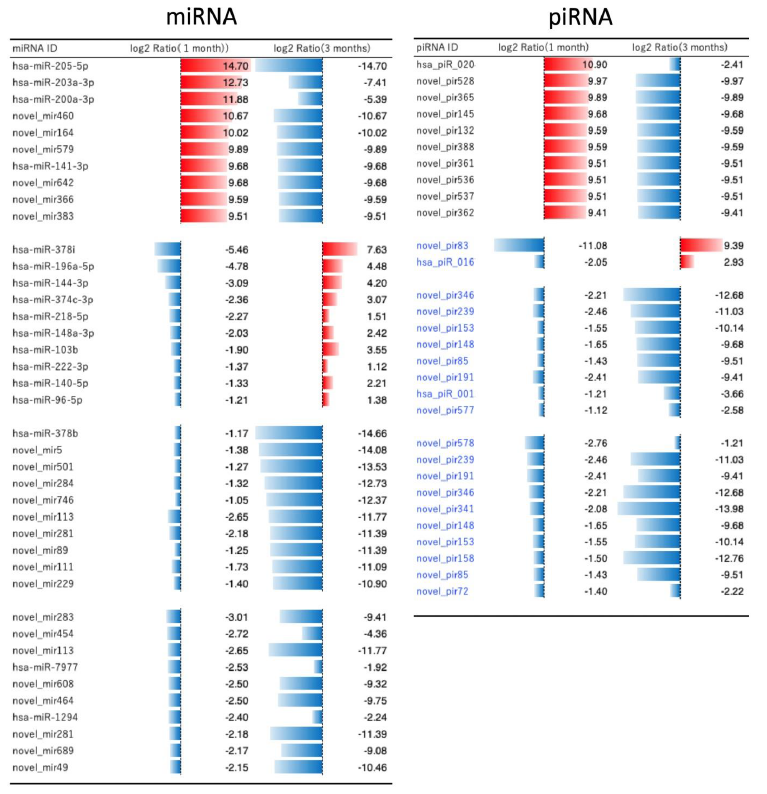
Figure 4 demonstrates the further detailed analysis on the changes in miRNA and piRNA. The names of top ten up- and down-regulated molecules were noted, and compared the numbers between 1 month and 3 months after Huaier treatment. There were enough numbers of miRNAs which showed reverse regulation (from red to blue) within 3 months after treatment, whereas only
two piRNA molecules found similar changes. The piRNA changes were almost similar to those in miRNAs, with much less in quantity, and mainly with the novel piRNAs. Massive increase of down-regulated miRNAs was observed until 3 months of treatment, 2 months after surgical dissection. These down-regulations were reversed to be striking up-regulation at the time of virus infection (9 months after Huaier treatment), together with her father [5].
Figure 4: MiRNA and piRNA-mediated transcriptional control by a comparison according to the time course of Huaier administration; 1 month (before surgical dissection) and 3 months (recovery period after the surgical dissection). The quantitative changes were indicated by log2 transformed fold change calculation by the red bars (up-regulated molecules) and blue bars (down-regulated ones).
3.2 Multiple mutation detected in oncogenes and tumour suppressor genes, and consequent alterations in major signalling pathways
Tables 1 and 2 demonstrated and summalized the periodical changes of the altered and modified in transcripion processes, and their functional lineage in signaling pathways using KEGG pathway characterizaion [10]. Table 1 showed the list of oncogenes and tumor suppressor genes with altered expression, with up-regulation in red color and down-regulation in blue color, black letters mean no quantitative change in expression. It is surprising to see this mach alteration in major molecules responsible for health maintenance. Then we investigated detailed functional lineage of these altered molecules in signaling pathways by KEGG pathway characterizaion [10]. Table 2 demonstrates the dynamics of signal transfer in many signaling pathways organized by the molecules shown in Table 1.
Table 1: The major oncogenes and tumor suppressor genes with the alteration of expression. Red highlights: up-regulated altered genes, and blue highlights: down-regulated altered genes. Black letters indicate genes with no quantitative changes.
Table 2: The signal transfer pathways with genetic alterations described in Table 1. The pathways were in two categories; down-regulated and up-regulated. The typical related functions of each pathway were indicated and highlighted by bold arrows. The changes of the regulation were written by the time course of Huaier administration.
We have already indicated Huaier effects on the rescue of Hippo signalling pathway function using Drosophila mutants [4], and predicted that more functional lineage should exist to explain a broad spectrum of Huaier efficacy. The present study, together with the previous results [5-7], provides the systematic and quantitative background. Huaier influenced a broad spectrum of biophysiological systems including; cell growth and differentiation; proliferation; survival; mortality; angiogenesis; for the control of carcer progression and tumorigenesis, together with apoptosis; chemoresistance; cytokine-cytokine receptor interaction; for cancer treatment, mismatch repair; nucleotide excision repair systems; for prevention of carcinogenesis and stem cell control, together with neural transmitter and signal transfer intra/inter neurons.
DYRK1A (dual-specificity tyrosine-regulated kinase 1A) [21], a kinase with multiple implications for embryonic development, especially in the nervous system, has been well known to link all these functions written above, and the results obtained from the present study indicated the similar potential of Huaier to link multipe signaling pathways. By the way, no alterations in DYRK1A was found in this patient and family.
3.3 The additional important genetic alterations identified correlated with meningioma, the other symptoms and disorders.
Meningioma is known as benign, typically a slow-growing tumor, and molecular basis still remains unclear with few exceptions [22]. The case study of meningioma has reported the somatic mutations in POLR2A define a distinct subset of meningiomas [23].
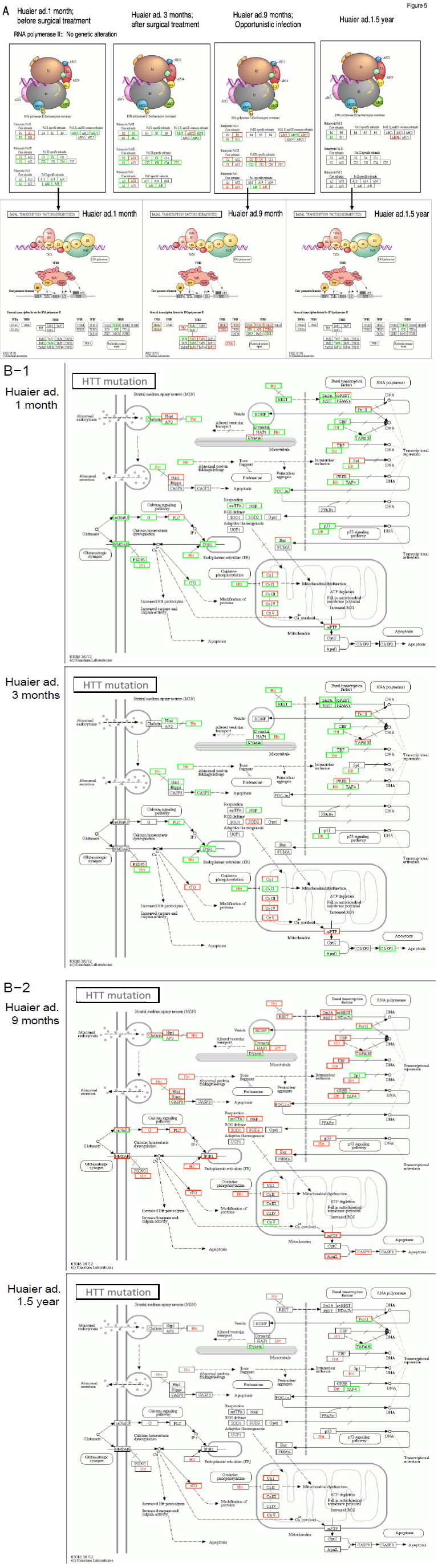
Thus we identified possible mutations and changes related to the reported meningioma-linked genes. Figure 5 showed, 1) no mutations in RNA polymerase I, II and its subunits, and III genes (Panel A, upper column), 2) transciptional factors attached to RNA polymerase II drastically down-regulated (Panel A, lower column), 3) in Panel B, signal transfer intra/inter neurons were strongly down-regulated by neurotransmitter mutation (SLC6A4 solute carrier family 6 member 4, also known as:HTT; 5HTT;
OCD1; SERT; 5-HTT; SERT1; hSERT; 5-HTTLPR) [17], and 4) mutations in all the isoforms of prion protein; the normal PrPC and protease-resistant forms designated PrPRes such as the disease-causing PrPSc(scrapie) [18].
Figure 5: Massive down-regulation for the compensation for the defected functions in Panel A; RNA polymerases and the transcription factors attached to RNA polymerase II, in Panel B; Neural transmission signaling cascade by SLC6A4 solute carrier family 6 member 4 (Also known as:HTT; 5HTT; OCD1; SERT; 5-HTT; SERT1; hSERT; 5-HTTLPR) [17], in Panel C; PRNP (prion protein) regulating cascade [18] , at 90 days after Huaier administration by KEGG pathway classification [10].
These transciptional mutations might explain the continuous and sequential disorders in the patient, however, the family member is totally asymptomatic, in a good health condition, even with the same alterations. The results shown in Figure 5 indicated more than the rescue of transcription control dysfunction were required to interpret the mechanisms, together with massive modification in signal transfer in multiple signalling pathways.
4. Discussion
It is a general understanding that, when signaling pathways interact with one another they form networks, they allow cellular responses to be coordinated, often by combinatorial signaling events [24]. At the molecular level, such responses include changes in the transcription or translation of genes, and post-translational and conformational changes in proteins, as well as changes in their location. These molecular events are the basic mechanisms controlling cell growth, proliferation, metabolism and many other processes [25]. In multicellular organisms, signal transduction pathways regulate cell communication in a wide variety of ways.
Based on those general understandings, it is beyond our knowledge to identify as such multiple mutations in key molecules identified in the patient focused in the present study. First, the patient had mutations in all the members of the epidermal growth factor receptor (EGFR [11]; ErbB-1; HER1 in humans), among ErbB family of receptors, a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). It is well known that mutations affecting EGFR expression or activity could result in cancer. It is noteworthy that the family member easily had virus infection as suggested [26], HIV virus infections in the patient, and papilloma virus infection in her father.
In addition, EGFR plays a critical role in TGF 1 dependent fibroblast to myofibroblast differentiation, the mutations often resulted in impaired tissue or skin function, and delayed the recovery from the injury including surgical treatment [11]. Mutations, amplifications or misregulations of EGFR or family members are implicated in about 30% of all epithelial cancers [26]. The identification of EGFR as an oncogene has led to the series of development of highly toxic anticancer therapeutics directed against EGFR (called "EGFR inhibitors", EGFRi), including recent development of Osimertinib, a third-generation tyrosine kinase inhibitor [27]. Huaier treatment was simultaneously proved its significant efficacy on breast cancer, chiefly by the rescue of defected function in the transcriptional control of Hippo signaling pathway [4, 28, 29].
As for HER2/neu, amplification or over-expression of this oncogene has been reported to play an important role in the development and progression of certain aggressive types of breast cancer [28]. In recent years the protein has become an important biomarker and target of therapy of breast cancer patients.
Signaling pathways activated by HER2 include the following signaling pathways, which appeared in
Table 2.
- mitogen-activated protein kinase (MAPK)
- phosphoinositide 3-kinase (PI3K/Akt)
- protein kinase C (PKC)
- Signal transducer and activator of transcription (STAT)
- phospholipase C γ
These signaling through the ErbB family of receptors promotes cell proliferation and opposes apoptosis, and therefore must be tightly regulated to prevent uncontrolled cell growth from occurring [11-16]. Over-expression, and other modifications found in the present study is also known to correlate with ovarian, stomach, and lung cancer and, more importantly to the patient in the present study, aggressive forms of uterine cancer.
Another notable mutation was identified in c-Met [12-14], also called tyrosine-protein kinase Met or hepatocyte growth factor receptor (HGFR), essential for embryonic development, organogenesis and wound healing. Abnormal MET activation in cancer correlates with poor prognosis, where aberrantly active MET triggers tumor growth, formation of new blood vessels (angiogenesis) that supply the tumor with nutrients, and cancer spread to other organs (metastasis). MET is deregulated in many types of human malignancies, including cancers of lung, kidney, liver, stomach, breast, and brain. Cancer stem cells are thought to hijack the ability of normal stem cells to express MET, and thus become the cause of cancer persistence and spread to other sites in the body. MET pathway plays an important role in the development of cancer through activation of key oncogenic pathways (RAS, PI3K, STAT3, beta-catenin).
Coordinated down-regulation of both MET and its downstream effector extracellular signal-regulated kinase 2 (ERK2) by miRNA (miR-199a) may be effective in inhibiting not only cell proliferation but also motility and invasive capabilities of tumor cells We observed significant up-regulation of miR-199a at 1 month after Huaier administration, but changed to down-regulation after 3 months.
According to the consideration of the outcomes by the mutated EGFR and other receptor tyrosine kinases (c-MET/erbB-2), we had to conclude that Huaier prevented the patient from possible carcinogenesis and tumorigenesis, by the compensation of defected signaling pathways through the massive alterations in numerous molecules.
We also performed detailed analysis on PNA polymerases and their subunits, with information of the attached transcription factors [23]. As shown in Figure 5, no transcriptional alterations were found in all the RNA polymerases and their subunits, but quantitative inhibition in the attached transcription factors were identified. These changes in transcriptional factors might be the main compensation for RNA polymerase functions and consequent production of proteins. On the contrary, oncogenic PI3K mutations together with AKT1 were identified as reported [31, 32]. Again, the alterations in signaling pathways overlapped in the patient’ mutations shown in Tables 1 and 2, and the same targets required for compensations on the course of recovery by Huaier administration.
In addition, we had to screen the molecular characterization in signal transfer network in neurons, by consideration of symptoms and disorders observed in the patient. SLC6A4 solute carrier family 6 member 4, also known as:HTT; 5HTT; OCD1; SERT; 5-HTT; SERT1; hSERT; 5-HTTLPR encodes an integral membrane protein that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons [18]. The encoded protein terminates the action of serotonin and recycles it in a sodium-dependent manner. This protein is a target of psychomotor stimulants, such as amphetamines and cocaine, and is a member of the sodium: neurotransmitter symporter family. A repeat length polymorphism in the promoter of this gene has been shown to affect the rate of serotonin uptake. There have been conflicting results in the literature about the possible effect, if any, that this polymorphism may play in behavior and depression observed in the patient.
PRNP (prion protein, also known as CD230) most predominant protein in the nervous system but occurs in many other tissues throughout the body [18]. The protein can exist in multiple isoforms, the normal PrPC and protease—resistant forms designated PrPRes such as the disease-causing PrPSc(scrapie), and an isoform located in mitochondria. The obtained data indicated the transcriptional mutation in all these forms of PRNP.
A wide range of studies has been conducted investigating the role in cell proliferation, differentiation, death, and survival [18]. Engagement of PrP has been linked to activation of signal transduction, PrP has been proposed as dynamic surface protein functioning in signaling pathways. Specific sites along the protein bind other proteins, biomolecules, and metals. These interfaces allow specific sets of cells to communicate based on level of expression and the surrounding microenvironment The modification of PRNP might have an important role for the alteration in many signaling pathways required in the patient and family. Consequently, the protein could serve as either a copper homeostasis mechanism, a calcium modulator, or a sensor for copper or oxidative stress. Loss of PrP function has been linked to the defects in long-term memory. This effect, together with synaptic transmission, was reported to indicate PrP importance related to the memory function [34, 35].
These mutations written above seemed to be hereditary events and including Htt and all the isoforms of PNRP prion protein were observed equally among family members. However, no serious carcinogenesis or tumorigenesis were observed at all, except in the parient with benign meningioma. They all had Huaier administration 20g per day, and no significant alterations in signaling pathways were detected [5-7].
The post-transcriptional control, epigenetic processes seemed to matter the differences in pathogenicity among family members, from the observation of the altered levels of DNA repair enzymes such as MGMT, MLH1-3, and p53. In addition, miRNA genes are also drastically altered which associated with CpG islands, that may be repressed by epigenetic methylation [36, 37].
At the same time, alteration of the expression level of WASP [38, 39] and SUMO (Small Ubiquitin-like Modifier) [40] were detected to support the compensation of defected functions in signaling pathways such as; protein stability, nuclear-cytosolic transport, and transcriptional regulation. The SUMO-1 modification of RanGAP1 (the first identified SUMO substrate) was also involved for compensation of Ubiquitin mediated proteolysis by trafficking from cytosol to nuclear pore complex.
5. Conclusion
The present study provides striking ability of Huaier to compensate for defected signal transfer in multiple signalling pathways correlated especially with EGFR and other receptor tyrosine kinases (c-MET [12-14] /erbB-2 [15, 16]. These mutations and alterations were hereditary process, and post-transcriptional modifications seemed to decide the pathogenic progression. Huaier showed significant inhibitory effects on carcinogenesis and tumorigenesis among the family members. However, even after 2 years of Huaier treatment, the mutations and alterations in transcription in these molecules were stably existed, only the regulation of transcription changed.
The other family members were asymptomatic without any serious health problems except virus infections, and it is hard to estimate the ratio of these mutations in asymptomatic, healthy families in total population in Japan.
Thus, Huaier provides practical compensation of defected and impaired function in signal transfer in multiple signalling pathways successfully, which also contributes to reduce a risk of carcinogenesis correlated with hereditary gene mutations.
6. Acknowledgements
The authors wish to thank cancer patient volunteers and many healthy volunteers kindly collaborated with the present study. The present study was grant-in-aid from QiDong Gaitianli Medicines Co., Ltd. And Japan Kampo New Medicine, Co., Ltd.
7. Author contributions
T.T., M.T., designed the study from the clinical observation of the cancer patients with Huaier treatment (as a complementally therapy), and managed the sampling and clinical assessment of the patient volunteers, statistically analyzed the data, and drafted the manuscript. F.T., H.L., N. L., Z. L., managed total RNA and small nuclear RNA sequencing and conducted systematic analysis of the data. D.W., Z.L., contributed to the provision of Huaier granules and clinical evaluation of the data, especially focused on Immunological evaluation.
Conflict of interest
The authors have no competing interest to declare.
Author information
The authors have no competing interest to declare. Readers are welcome to comment on the paper. Correspondence should be addressed to T.T. (ttanaka@bradeion.com) and M.T. (manami-tanaka@bradeion.com), and those researchers contributed equally to this work. Requests for Huaier extract and commercially-available granules should be addressed to D.W. (teii@newkampo.co.jp) and M.T. (manami-tanaka@bradeion.com)
References
- Song X, Li Y, Zhang H, Yang, Q. The anticancer effect of Huaier (Review). Oncol Rep 34 (2015): 12-21.
- Wang X, Wang N, Cheung F, Lao L, Li C, et al. Chinese medicines for prevention and treatmentofhumanhepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med 13 (2015): 142-164.
- Chen Q, Shu, C, Laurence, AD, Chen Y, Peng BG, et al. Effect of Huaier granule on recurrence after curative resection of HCC: A multicentre, randomised clinical trial. Gut 67 (2018): 2006-2016.
- Tanaka T, Suzuki T, Nakamura J, Kawamura Y, Sadahiro S, et al. Huaier Regulates Cell Fate by the Rescue of Disrupted Transcription Control in the Hippo Signaling Pathway. Arch Clin Biomed Res 1 (2017): 179-199.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Huaier Induces Cancer Recovery by Rescuing Impaired Function of Transcription Control Based on the Individual Genomic Potential. Arch Clin Biomed Res 4 (2020): 817-855.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Anti-cancer effects of Huaier on prostate cancer; miRNA-mediated transcription control induced both inhibition of active progression and prevention of relapse. J Alternative Compl Integr Med 7 (2021): 146-155.
- Tanaka M, Tanaka T, Teng F, Lin H, Li N, et al. Complete remission of the severe advanced stage cancer by miRNA-mediated transcriptional control of Bcl-xL with Huaier therapy compared to the conventional chemotherapy with platinum (II) complex. Arch Clin Biomed Res 5 (2021): 230-261.
- Kong L, Zhang Y, Ye Z, Liu X, Zhao S, et al. CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res 35 (2007): 345-349.
- DePristo MA, Banks E, Poplin R, Garimella KV Maguire JR, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43 (2011): 491-498.
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res 36 (2008): 480-484.
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Rad Oncol Biol Physics 59 (2 Suppl) (2004): 21-6.
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 251(1991): 802-4.
- Cooper CS. The met oncogene: from detection by transfection to transmembrane receptor for hepatocyte growth factor. Oncogene 7 (1992): 3-7.
- Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer (2018);18:341-358.
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, et al. ErbB receptors: from oncogenes to targeted cancer treatment. J Clin Invest 117 (2007): 2051-8.
- Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230 (1985): 1132-9.
- Coleman JA, Gfreen EM, Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature 532 (2016): 334-339.
- Liao YC, Lebo RV, Clawson GA, Smuckler EA. Human prion protein cDNA: molecular cloning, chromosomal mapping, and biological implications. Science 233 (1986): 364-367.
- Peng Z, Cheng Y, Tan BC, Kang L, Tian Z, et al. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat Biotechnol 30 (2012): 253-260.
- Song Y, Li L, Ou Y, Gao Z, Li E, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509 (2014): 91-95.
- Fernández-Martínez P, Zahonero C, Sánchez-Gómez P. DYRK1A:the double-edged kinase as a protagonist in cell growth and tumorinenesis. Mol Cell Oncol (2015) 30; 2(1): e970048.
- ClarkVE, Erson-Omay EG, Serin A, Yin J, Cotney J, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and Science 339 (2013): 1077-80.
- Clark VE, Harmanci AS, Bai H, Youngblood MW, Lee TI et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat Genet 48 (2016); 1253-1259.
- Papin JA, Hunter T, Palsson BO, Subramaniam S. Reconstruction of cellular signalling networks and analysis of their properties. Nature Rev Mol Cell Biol 6 (2005): 99-111.
- Krauss G. in Biochemistry of Signal Transduction and Regulation. (2008) Wiley-VCH. p. 15. ISBN 978-3527313976.
- Walker F, Abramowitz L, Benabderrahmane D, Duval X, Descatoire V, et al. Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Human Pathol 40 (2009): 1517-27.
- Greig SL Osimertinib: First Global Approval. Drugs 76 (2016): 263-73.
- Keshamouni VG, Mattingly RR, Reddy KB. Mechanism of 17-beta-estradiol-induced Erk1/2 activation in breast cancer cells. A role for Her2 and PKC-delta. J Biol Chem 277 (2002): 22558-22565.
- Mo JS, Park JW, Guan, KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 25 (2014): 642-65.
- Kim S, Lee UJ, Kim MN, Lee EJ, Kim JY, et MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2). J Biol Chem 283 (2008): 18158- 18166.
- Abedalthagafi M, Bi Wl, Alzer AA, Merrill PH, Brewster R, et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol 18 (2016): 649-655.
- Brastianos PK, Horowits PM, Santagata S, Jones RT, Mckenna, A, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 Nat Genet 45 (2013) :285-289.
- Linden R, Martins VR, Prado MA, Cammarota M, Izquierdo I, et al. Physiology of the prion protein. Physiol Rev 88 (2008): 673-728.
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron 44 (2004): 49-57.
- Barco A, Bailey CH, Kandel Common molecular mechanisms in explicit and implicit memory. J Neurochem 97 (2006): 1520-33.
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433 (2005): 769-73.
- Lee D, Shin C. MicroRNA-target interactions: new insights from genome-wide Annals NY Acad Sci 1271 (2012): 118-28.
- Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391 (1998): 93-96.
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88 (1997): 97-107.
- Hay RT. SUMO: a history of modification. Molecular Cell 18 (2005): 1-12.