High Seroprevalence of Dengue Virus Ns1 in HIV-1 Infected Children Suggests Impaired Immune Response to Pediatric Dengue Virus Infection
Article Information
Jules Colince Tchadji1,2†, Loveline Ngu Ndengkoh1,3†, Nji Nadesh1, Hervé Fotso Ouambo1, Inès Nyebe1,2, Apeh Alfred Ngoh1, Salomon Bonsi Tchuandom5, Georgia Ambada1,2, Carole S Sake Ngahane6, Mireille Chouegouong Tuedom7, Bertrand Sagnia1, Rachel Kamgaing Simo8, Martin Samuel Sosso8, Sylvie Moudourou8, Park Chae Gyu9, Charles O. Esimone10, Okeke I Malachy11, René Kamgang2,12, Alain Bopda Waffo13,Godwin W Nchinda1,4,*
1Laboratory of vaccinology/biobanking, chantal biya international reference center for research on the prevention and management of HIV/AIDS (CIRCB), P.O. Box 3077 Messa-Yaoundé, Cameroon
2Department of animal biology and physiology, faculty of sciences, P.O Box 812, University of Yaounde I, Cameroon
3Department of biochemistry, faculty of sciences, P.O Box 812, University of Yaounde I, Yaounde, Cameroon
4African network of excellence for clinical and translational sciences (ACECTS), Yaounde Cameroon
5 Public school of medical laboratory technicians, Yaoundé, Cameroon
6 Department of microbiology, faculty of sciences, P.O Box 812, University of Yaounde I, Yaounde, Cameroon.
7Institute of agricultural research for development (IRAD), P.O. Box 2067 Yaoundé, Cameroon
8The Chantal biya international reference center for research on the prevention and management of HIV/AIDS (CIRCB), P.O. Box 3077 Messa-Yaoundé, Cameroon
9Laboratory of immunology, brain Korea 21 PLUS Project for medical science, severance biomedical science institute, Yonsei University College of Medicine, Seoul 03722, Republic of Korea.
10Department of pharmaceutical microbiology and biotechnology, Nnamdi Azikiwe University, Awka, Nigeria
11Department of natural and environmental sciences, biomedical science concentration, School of Arts and Sciences, American University of Nigeria, 98 Lamido Zubairu Way, PMB 2250 Yola, Nigeria
12Institute of medical research and medicinal plants studies (IMPM), P.O. Box 6163 Yaoundé, Cameroon
13Biochemistry and molecular biology, indiana university school of medicine, 635 Barnhill Drive, MS1017Q Lab MS1015, Indianapolis, IN, 46202, USA.
†These authors contributed equally to this work.
*Corresponding author: Godwin W. Nchinda, Laboratory of vaccinology/biobanking, chantal biya international reference center for research on the prevention and management of HIV/AIDS (CIRCB), P.O. Box 3077 Messa-Yaoundé, Cameroon.
Received: 19 July 2023; Accepted: 31 July 2023; Published: 22 August 2023.
Citation: Jules Colince Tchadji, Loveline Ngu Ndengkoh, Hervé Fotso Ouambo, Ndah Teddy, Inès Nyebe, Apeh Alfred Ngoh, Bertrand Sagnia, Salomon Bonsi Tchuandom, Georgia Ambada, Carole S Sake Ngahane, Nji Nadesh, Mireille Chouegouong Tuedom, Rachel Kamgaing Simo, Martin Samuel Sosso, Sylvie Moudourou, Park Chae Gyu, Charles O. Esimone, Okeke I Malachy, René Kamgang, Alain Bopda Waffo,Godwin W Nchinda. High Seroprevalence of Dengue Virus Ns1 in Hiv- 1 Infected Children Suggests Impaired Immune Response to Pediatric Dengue Virus Infection. Archives of Clinical and Biomedical Research. 7 (2023): 502-509.
View / Download Pdf Share at FacebookAbstract
The DENV non-structural protein 1 (NSI) is not only a clinical biomarker for early detection of DENV infection but is equally reported to play pathogenic roles during dengue fever. Thus in a cohort of 600 children vertically infected with HIV-1 and 176 age matched negative counterparts, we have assessed the prevalence of serological markers of DENV infection. The children were recruited from HIV care services within the Centre region of Cameroon. Dengue infection was diagnosed using the Onsite Duo Dengue Ag+IgM/IgG rapid test (CTK Biotech, Inc. San Diego, USA). The plasma level of the DENV NS1 protein was determined using the Platelia™ NS1 Ag enzyme immunoassay (Bio-Rad Laboratories, France). Children infected vertically with HIV-1 showed significantly (p<0.0001) higher level (58.33%) of the DENV NS1 protein vis-à-vis their negative counterparts (27.77%). Plasma levels of DENV NS1 protein were also significantly higher in HIV- 1 positive children (163.00 ± 96.17 ng/mL for HIV-1 positive and 39.95 ± 43.86 ng/mL for HIV-1 negative). In contrast age matched HIV-1 negative children showed a significantly higher level (72.22%) of DENV specific IgG (p<0.0001). Thus vertical HIV-1 infection impairs the development of DENV specific immunity in children and probably exacerbates plasma accumulation of DENV NS1 protein.
Keywords
Dengue fever; HIV-1; Elisa; Seroprevalence; DENV NS1 protein; Biomarkers
Article Details
1. Introduction
In Cameroon like in most sub Saharan African countries Dengue fever, a mosquito-borne viral disease with similar symptoms to malaria is endemic [1,2]. In addition two common mosquito vectors responsible for Dengue virus (DENV) transmission including, Aedes aegypti and Aedes albopictus have been reported in Cameroon [3]. Therefore children especially those vertically infected with the human immunodeficiency type one virus (HIV-1) as a consequence of HIV-1 mediated immunosuppression are highly vulnerable not only to malaria but equally to DENV infection. DENV is a flavivirus and a member of the falviviridae family which consists of four genetically distinct serotypes including DENV-1, DENV-2, DENV-3 and DENV-4. DENV is an enveloped virus with a single positive-stranded RNA genome coding for three structural proteins and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The three structural proteins are the capsid, pre-membrane and envelope proteins respectively [4,5]. The NS1 protein is secreted into the plasma early during DENV infection and as such is used as a diagnostic biomarker for acute DENV infection [6]. Dengue NS1 can also be associated with the cell membrane or bound upon the cell surface where it has been reported to play a role in disease severity by causing hemorrhage and vascular leakage [7]. DENV infection by anyone of the four serotypes results in a wide variety of symptoms ranging from subclinical disease to severe flu-like symptoms overlapping strongly with malaria symptoms. The critical role of NS1 during acute Dengue and disease severity makes it a suitable candidate for monitoring the impact of vertically transmitted HIV-1 on pediatric DENV infection in disease endemic regions. Given the intensity of malaria in children within the sub-Saharan African region there is need to carefully differentiate dengue fever from clinical malaria especially with regards to the long term management of pediatric HIV-1. Wrong administration of malaria drugs to children suffering from Dengue fever can exacerbate the clinical outcome of their infections. In 2021 sub-Saharan Africa accounted for approximately 88 per cent of children and adolescents living with HIV worldwide, with an estimated 52,000 (39,000 – 62,000) cases in Cameroon [8]. HIV infection remains a huge public health problem in most sub Saharan African countries with comorbidities like dengue and malaria being strong compounding factors. It has been demonstrated that the interaction of HIV with other tropical pathogens such as Plasmodium spp, is associated with accelerated HIV mediated disease progression [9], but little is known about the interaction of HIV with DENV especially in children less than 18 years old. In this study we have investigated the plasma levels of DENV NS1 protein as serological markers of impaired immunity against DENV in children infected vertically with HIV-1 infection.
2. Materials and methods
2.1 Recruitment of participants
A cross-sectional study was carried out from January 2017 to December 2019 in the Center region of Cameroon. Children less than 18 years old were recruited during medical visit, in HIV care units or pediatric units located in various hospitals of the town of Yaoundé (Capital city of Cameroon) and Bikop (at about 60 km from the city of Yaoundé). Stratified random samplings were conducted, and the selection was consecutive to the physician’s examination. Children were recruited irrespective of clinical characteristics, HIV or dengue virus infection status. Once the parental authorization and assent was obtained, children were enrolled in the study, and using pretested questionnaires, information on the participant were collected. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Cameroon National Ethics Committee (ref 00612011/CE/CIRCB).
2.2 Blood collection
1 to 3 mL of blood was collected in EDTA tubes, by well-trained health personals affiliated to the project. Within 4 hours of sample collection all samples were processed. After centrifugation at 1,500 rpm for 10 min at 4oC, plasma fraction was harvested sterile under the hood, aliquoted in small, single-use cryotubes and stored at -20°C.
2.3 Laboratory diagnosis
2.3.1 HIV diagnosis: Children vertically infected with HIV-1 are diagnosed in the early infant diagnosis (EID) program of the ministry of public health of the republic of Cameroon. Briefly for children less than 18 months, a PCR screening using a Roche amplicor DNA PCR version 1.5, was carried out following the manufacturer's instructions. Children infected with HIV-2 or both HIV-1 and 2 were excluded from the study. Additionally HIV was also diagnosed using rapid tests according to the Cameroon national algorithm for HIV screening in children aged over 18 months (the Alere DetermineTM HIV-1/2 rapid test for the first test and the SD Bioline HIV-1 rapid test /2 3.0 for the second test on all samples).
2.3.2 DENV NS1 Ag/IgM/IgG detection and plasma DENV NS1Ag quantification:
Dengue infection was diagnosed using the OnSite Duo Dengue Ag+IgM/IgG rapid test according to the manufacturer's instructions (CTK Biotech, Inc. San Diego, USA). Circulating plasma concentration of DENV NS1 protein was estimated using the Platelia™ Dengue NS1 Ag-ELISA kit (Bio-Rad Laboratories, Marnes-La-Coquette, France) as indicated by the manufacturer. To obtain real plasma values of DENV NS1 two separate standard curves were generated using recombinant DENV-1 and DENV-2. Briefly a standard curve was constructed using two-fold serial dilutions of either DENV-1 or DENV-2 recombinant NS1 proteins purchased from Bio-Rad (Bio-Rad Laboratories, France). All ELISA assays were as described by the manufacturer.
2.3.4 Dengue virus serotype determination:
This was done using an internal indirect IgM/IgG Dengue ELISA test optimized and described previously by our research group at the Laboratory of Vaccinology and Biobank of the CIRCB [10].
2.4 Statistical analysis
Participant’s variable was presented as median, IQR, percentages, means and standard deviation. Using the Chi square test and the Student t test, variables were compared and the difference was considered as signficant for p < 0.05. Figures were generated using the Graph Pad Prism version 6.0 software.
2.5 Ethical considerations
This study received ethical clearance from the Cameroon national ethic committee (ref 00612011/CE/CIRCB) as well as an administrative authorization from the Cameroon Ministry of Public Health (number 631-111). Informed written consent/assent was obtained from study participants parents or legal guardians. Collected data were confidential and anonymized during processing. Dengue virus positive participants were referred to clinicians for subsequent follow-up.
2.6 Data availability
The datasets used and/or analysed during the current study is available from the corresponding author on reasonable request.
3. Results
3.1 Study population
A total of 600 HIV-1 positive and 176 HIV-1 negative children were recruited from pediatric HIV care units in the Centre Region of Cameroon, with 56.57% being females. The median age was 7 (2-11) years in HIV-1 infected children and 16 (10-17) in HIV-1 negative children (Table 1). Plasma samples from all dengue-suspected participants were tested using the OnSite Duo Dengue Ag+IgM/IgG point of care assay (CTK Biotech, Inc. San Diego, USA). Whereas 12.00% of HIV-1 positive children were co-infected by the dengue virus, 10.23% of HIV-1 negative children were DENV+ (Table 1).
Table1: Study population characteristics
|
Gender |
Total (n=776) |
Females (n=439) |
Males (n=337) |
|||
|
HIV-1 statut |
HIV-1+ 600 (77.32) |
HIV-1- |
HIV-1+ 327 (74.49) |
HIV-1- 112 (25.51) |
HIV-1+ 273 (81.01) |
HIV-1- 64 (18.99) |
|
Number (%) |
176 (22.68) |
|||||
|
Median age, (IQR) |
7 (2-11) |
16 (10-17) |
7 (2-11) |
16 (13-17) |
7 (3-11) |
15 (5-17) |
|
DENV positive cases n (%) |
72 (12.00) |
18 (10.23) |
39 (11.93) |
9 (8.04) |
33 (12.09) |
9 (14.06) |
|
DENV negative cases n (%) |
528 (88.00) |
158 (89.77) |
288 (88.07) |
103 (91.96) |
240 (87.91) |
55 (85.94) |
n: number of participants; %: Percentage; IQR: interquartile range; DENV: Dengue virus,
HIV-1: Human Immunodeficiency viruses-1, HIV-1+: HIV-1 infected, HIV-1-: HIV-1 uninfected
3.2 Profile of Dengue virus infection biomarker in HIV-1 infected and uninfected children.
IgG: Immunoglobulin G; IgM: Immunoglobulin M; Ag: Antigen.
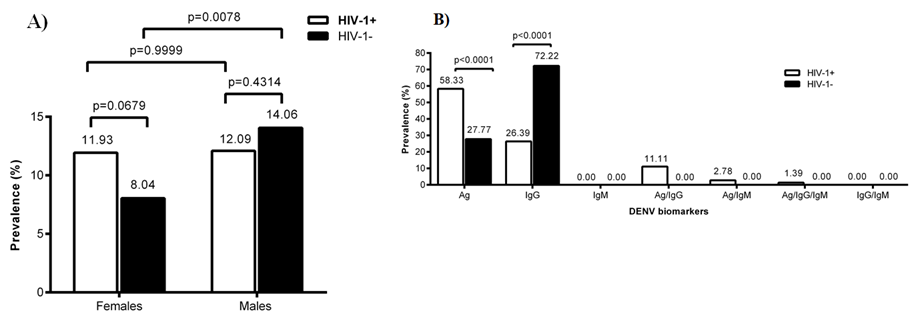
The seroprevalence of DENV was determined in HIV-1 infected and uninfected children. The data obtained were grouped according to gender. Simultaneously, DENV infection biomarkers (Ag, IgG, IgM) was determined in the plasma of children. We targeted here the NS1 antigen of DENV as well as IgG and IgM antibody response specific to NS1. Data obtained was compared between HIV-1 infected and uninfected children. In figure 1A data is shown for the global presence of DENV serological markers in HIV-1 positive and negative children according to sex. In figure 1B data is shown for the profile of DENV serological markers in both HIV-1 positive and negative children.
The global level of DENV serological markers in HIV-1 positive and negative children was similar between females or males irrespective of HIV-1 as shown in figure 1A. However male HIV negative children seen to show significantly higher level (P<0.0078) of DENV serological markers than their female counterparts (figure 1A). Furthermore, in HIV-1 infected children the biomarkers level included Ag (58.33%), IgG (26.39%), IgM (0%), Ag/IgG (11.11%), Ag/IgM (2.78%), Ag/IgG/IgM (1.39%), IgG/IgM (0%) respectively (figure 1B). The overall analysis of the DENV serological markers level’s, according to HIV-1 co-infection showed that HIV-1 positive children displayed a significantly higher (P<0.0001) level of NS1 protein across all combinations of serological markers where it was detected. In contrast HIV-1 negative children displayed a significantly higher level (P<0.0001) of IgG vis-à-vis their positive counterparts (figure 1B).
3.3 Seroprevalence of Dengue virus infection biomarker profile’s in children with respect to the sex.
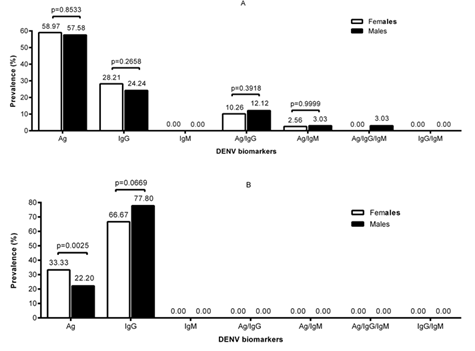
In figure 2A data is shown for the level of serological markers in HIV-1 infected children whilst in figure 2B is the level in HIV-1 negative children
When the level of DENV serological markers was analyzed according to sex, there was no significant difference between males and females HIV-1 positive children. As shown in figure 2A the level of all combinations of biomarkers was similar between male and female HIV positive children (figure 2A). As expected from the analysis above, the level of DENV NS1 was practically two times the IgG level in HIV-1 infected children. In addition the DENV serological markers of HIV-1 infected children were also highly heterogeneous with several combinations of markers in both females and males. In HIV-1 negative children as shown in figure 2B the level of DENV NS1 was significantly higher in females (P<0.0025). In the negative children one other prominent serological marker whose level was similar in females and males was IgG. The level of DENV serological markers in HIV-1 negative children as shown in Figure 2B was more homogeneous with only two peaks (figure 2B). The heterogeneous nature of the level of biomarkers in HIV-1 infected children is probably due to varying degrees of immune-depletion in these children since there were not of the same age.
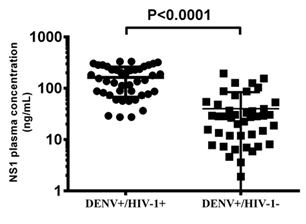
3.4 DENV Non-structural protein 1 (NSI) Antigenemia
In figure 3 data is shown for circulating NSI levels in HIV-1 positive and negative children (163.00 ± 96.17 ng/mL for HIV-1 positive and 39.95 ± 43.86 ng/mL for HIV-1 negative). DENV infection of children vertically infected with HIV-1 was associated with significantly higher (P<0.0001) plasma levels of DENV NS1 protein.
3.5 Dengue virus serotypes in HIV-1 infected and uninfected children
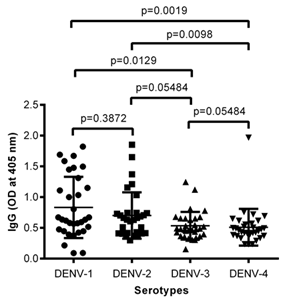
In Figure 4 data is shown for the reactivity of plasma from HIV-1 infected children to envelopes of the four DENV serotypes including DENV-1, 2, 3 and 4 respectively.
ELISA plates was coated with envelopes of the four DENV serotypes and probed with plasma from HIV-1 infected children. IgG antibodies specific to envelopes of DENV serotype 1& 2 (DENV-1 and DENV-2) were significantly higher (figure 4). There were some peaks also with DENV-3 but it is not clear whether these are cross reactive envelope specific antibodies. We previously showed that the predominant DENV serotypes circulating in Cameroon were DENV-1 and DENV-2. However DENV-3 has also been reported in Cameroon. Till now DENV-4 has been reported in Cameroon. Thus it’s most likely these children like their negative counterparts were mainly infected with DENV-1 or 2
4. Discussion
In Africa, misdiagnoses of dengue fever as malaria is a common scenario, although this specific infection is neither systematically investigated nor generally considered by clinicians [11]. The DENV non-structural protein 1 (NSI) is a viral virulence factor which is known to activate immune cells [12], alter the complement system [13,14], and could also weaken the endothelial barrier [15,16]. Since DENV NSI circulates in serum of patients during the acute phase of dengue fever we have evaluated its utility as a biomarker of disease severity during dual DENV-HIV-1 infection in children. Children because of their fragile immune system are generally highly vulnerable to infectious diseases. This vulnerability is futher exacerbated during HIV-1 infection which depletes the immune system and vector borne diseases which keep the immune system in perpetual inflamation. Sub Saharan Africa still disproportionately bears the brunt of pediatric HIV-1 infection, globally accounting for 88 per cent of children and adolescents living with HIV-1. Given that most children acquire HIV from HIV-infected mothers during pregnancy, birth or breastfeeding [17], their immune system is often affected early on in life, making them highly susceptible to mosquito borne diseases. In Cameroon therefore pediatric HIV-1 occurs alongside many other tropical infections, including dengue virus infection. Using the the OnSite Duo Dengue Ag+IgM/IgG rapid test (CTK Biotech, Inc. San Diego, USA), detecting DENV NSI (Ag) DENV specific IgG or IgM antibodies we profiled DENV plasma biomarkers in HIV-1 infected and uninfected children. Plasma DENV biomarkers including Ag, IgG and IgM were detected as single, in pairs or in triples especially in HIV-1 infected children. Although all the children in this study were vertically infected with HIV-1 our data show that the overall presence of DENV infection was similar between HIV-1 infected and negative children (Fig.1A). This suggests that there was no preferential exposure of any subgroup of the children to DENV infection. However HIV-1 infected children showed a significantly lower level (P<0.0001) of DENV specific IgG antibodies when compared to their negative counterparts thereby probably demonstrating an impaired ability to mount commensurate DENV specific IgG antibodies (Fig.1B). This low level of DENV specific IgG antibodies coincided with a significantly high level of DENV NSI (P<0.001) in HIV-1 infected children when compared to their uninfected counterparts. In addition the DENV biomarker profile in HIV-1 infected children was also highly heterogenous consisting of mixtures of DENV specific IgG or IgM mixed with NSI (Ag) with the NSI dominating. This was in contrast with the DENV plasma biomarker profile of HIV-1 negative children which was mainly DENV specific IgG antibodies dominating. High level of NSI in plasma from HIV-1 infected children is an indication of impaired ability to clear the DENV by the depleted immune system of these children. The heterogenous nature of the DENV plasma biomarker profile of the HIV-1 infected children is probably a reflection of the varying degree of immune depletion in these children. Since these children were not infected during the same time and were also of varying ages; they probably also represented different degrees of HIV-1 mediated immune depletion. When the biomarker profile was analyzed according to sex in HIV-1 infected children no significant difference was observed between the female and male children (Fig.2A). On the contrary in the HIV-1 negative children a significantly higher level (P<0.0025) of DENV NS1 was observed in females relative to their males counter parts. Given the population size of the HIV-1 negative participants no conclusion can be drawn from this.
The plasma quantity of viral components such as secreted DENV NS1 was also significantly higher (P<0.0001) in HIV-1 infected children when compared to their negative counter parts. The DENV NS1 is known marker of dengue disease severity [18] and has been incriminated in driving the pathogenesis of the virus [19].
HIV-1 infection is associated with several impaired immune functions. In the context of children where the immune system is still fragile and further depleted by vertical HIV-1 infection it is still to be known what role the DENV NS1 protein will play. Never the less the persistent of NSI in the HIV-1 infected children is also a marker of persistent DENV viraemia. DENV-1 was the most predominant serotype detected in this study followed DENV-2, then DENV-3 and DENV-4 among HIV-1 infected children. This distribution of all DENV serotypes reflects the intense transmission of the disease within this population. Our group had previously reported the circulation of DENV in children [10,20] in Cameroon and also in blood donors in blood banks in Cameroon [21]. In Cameroon both vectors of the DENV in including Aedes aegypti and Aedes Albopictus have been reported. In Cameroon intense malaria transmission overlap with areas of DENV endemicity therefore there is a need for a differential diagnosis of the two diseases in children to ensure adequate management. Otherwise the treatment of malaria in place of DENV infection in HIV-1 infected children could further compound their suffering. Thus the depletion of the immune system after HIV-1 infection could exacerbate DENV pathogenesis in Cameroon.
5. Conclusion:
Children vertically infected with HIV-1 living in Cameroon like in most Sub Saharan Africa are highly vulnerable to Arboviruses like DENV. During dual HIV-1-DENV infection in children prominence of circulating viral components such as secreted DENV NS1 are vital biomarkers of viral pathogenesis and impaired immune mediated viral clearance.
Acknowledgement
This work would have not been possible without the willingness of the participants, whom we deeply thank. We also thank the personnel of the Chantal Biya Foundation, the Akonolinga District Hospital, the Bikop Catholic Health Center and School, and the Buea Regional Hospital for their support in collecting the blood samples.
Competing interests
The authors declare that they have no competing interests.
Author contributions
Conceived and designed the experiments: GWN, JCT, RK and HFO
Performed the experiments: JCT, LNN, SBT, GA, CSSN, NN and IN
Technical assistance: AAN, MCT, RKS, ABW, PCG, COE, OIM, MSS, BS and SM
Analyzed the data: JCT, GWN, HFO and RK
Wrote the paper: JCT, GWN, HFO and RK
Funding statement
This project was funded by grant EDCTP (grant #TA.2010.40200.016); Canada grand challenge (#0121-01); TWAS (12059RG/BIO/AF/AC_G); AMFAR (Grant ID: 109848-65-RGRL, PTC Grant:# 121628) to Godwin W Nchinda; This study was also funded by the Cameroonian government through CIRCB.
References:
- Nkenfou CN, Fainguem N, Dongmo-Nguefack F, et al. Enhanced passive surveillance dengue infection among febrile children: Prevalence, co-infections and associated factors in Cameroon. PLoS Negl Trop Dis 15 (2021): 1-10.
- Tewelde T Gebremariam, Henk DFH Schallig, Zeleke Mekonnen, et al. Concurrent malaria and dengue fever in (sub-Saharan) Africa: a systematic review and meta-analysis (2022): 1-27.
- Kamgang B, Yougang AP, Tchoupo M, et al. Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. . s.l. : Parasites Vectors 10 (2017): 469.
- Iglesias NG, Byk LA, Gamarnik AV. Dengue and Dengue Hemorrhagic Fever. CAB International; 11 (2013): 334-365.
- Alcalá AC, Palomares LA, Ludert JE. Secretion of Nonstructural Protein 1 of Dengue Virus from Infected Mosquito Cells: Facts and Speculations. J Virol 92 (2018): e00275-18.
- Casenghi M, Kosack C, Li R, et al. NS1 antigen detecting assays for diagnosing acute dengue infection in people living in or returning from endemic countries. Cochrane Database Syst Rev 2018 (2018): CD011155.
- Chen HR, Lai YC, Yeh TM. Dengue virus non-structural protein 1: a pathogenic factor, therapeutic target, and vaccine candidate. J Biomed Sci 25 (2018): 58.
- Estimates, UNAIDS (2022).
- Alemu A, Shiferaw Y, Addis Z, et al. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors 6 (2013): 18.
- Tchuandom SB, Tchadji JC, Tchouangueu TF, et al. A cross-sectional study of acute dengue infection in Paediatric clinics in Cameroon. BMC Public Health 19 (2019): 958.
- Gainor EM, Harris E, LaBeaud AD. Uncovering the burden of dengue in Africa: considerations on magnitude, misdiagnosis, and ancestry. Viruses 14 (2022): 233.
- Libraty DH, Endy TP, Houng HSH, et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis 185 (2002): 1213-1221.
- Allonso D, Meneses MDF, Fernandes CA, et al. Assessing positivity and circulating levels of NS1 in samples from a 2012 dengue outbreak in Rio de Janeiro, Brazil. Plos one 9 (2012): e113634.
- Cabezas S, Bracho G, Aloia AL, et al. Dengue Virus Induces Increased Activity of the Complement Alternative Pathway in Infected Cells. J Virol 92 (2018): e00633-18.
- Avirutnan P. Vascular leakage in severe dengue virus infections: A potential role for the nonstructVascular leakage in severe dengue virus infections: A potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis 193 (2006): 1078-1088.
- Puerta-Guardo H, Glasner DR, Espinosa DA, et al. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep 26 (2019): 1598-1613.
- WHO, Treatment of children living with HIV. WHO (2012).
- Paranavitane SA. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect. Dis 14 (2014): 570.
- Thomas SJ. NS1: A corner piece in the dengue pathogenesis puzzle? Sci. Transl. Med 7 (2015): 304fs37.
- Tchuandom SB, Tchouangueu TF, Nkondjio CA, et al. Seroprevalence of dengue virus among children presenting with febrile illness in some public health facilities in Cameroon. Pan African Medical Journal 31 (2018): 177.
- Tchuandom SB, Lissom A, Ateba GHM, et al. Dengue virus serological markers among potential blood donors: an evidence of asymptomatic dengue virus transmission in Cameroon. Pan Afr Med J 36 (2020): 185.