Hereditary Risk Assessment for BRCA Breast and/or Ovarian Cancer
Article Information
María Teresa Martínez1*, Marta Tapia1*, Lina Candia1, Begoña Bermejo1,2, Iris Garrido-Cano3, Anna Adam-Artigues3, Pilar Eroles2,3, Ana Lluch1,2 Inmaculada de Juan 5, Estela Contel1, Cristina Hernando1, Sara S Oltra2,4, Juan M. Cejalvo1,2 and Isabel Chirivella1
1Medical Oncology Department. INCLIVA Biomedical Research Institute, Hospital Clínico of Valencia, University of Valencia, 46010 Valencia, Spain
2Center for Biomedical Network Research on Cancer (CIBERONC). 28029 Madrid, Spain
3INCLIVA Biomedical Research Institute, 46010, Valencia, Spain
4Center for Biomedical Network Research on Cancer (CIBERONC). 28029 Madrid, Spain
5Molecular Biology Unit, Service of Clinical Analysis. La Fe University and Polytechnic Hospital, 46026 Valencia, Spain
*These authors contributed equally to this work.
*Corresponding Authors: María Teresa Martínez, Medical Oncology Department. INCLIVA Biomedical Research Institute, Hospital Clínico of Valencia, University of Valencia, 46010 Valencia, Spain
Isabel Chirivella Gonzalez, Medical Oncology Department. INCLIVA Biomedical Research Institute, Hospital Clínico of Valencia, University of Valencia, 46010 Valencia, Spain
Received: 02 July 2022; Accepted: 12 July 2022; Published: 21 July 2022
Citation: María Teresa Martínez, Marta Tapia, Lina Candia, Begoña Bermejo, Iris Garrido-Cano, Anna Adam-Artigues, Pilar Eroles, Ana Lluch, Inmaculada de Juan, Estela Contel, Cristina Hernando, Sara S Oltra, Juan M. Cejalvo, Isabel Chirivella. Hereditary Risk Assessment for BRCA Breast and/or Ovarian Cancer. Journal of Cancer Science and Clinical Therapeutics 6 (2022): 251-261.
View / Download Pdf Share at FacebookAbstract
Germline BRCA1 and BRCA2 (BRCA1/2) mutations are most frequently associated with hereditary breast/ovarian cancer. The study of genetic discriminators and dysregulated pathways involved in hereditary breast/ovarian syndromes has been key in the development of molecular diagnostic strategies, targeted therapies (such as PARP inhibitors), and prevention approaches. The recent development and implementation of next generation sequencing technologies has improved patient selection processes to offer such prevention and surveillance strategies. This review summarizes current knowledge on management and follow-up of BRCA mutation patients and carriers, and also reviews current research lines on the subject that could help improve future management of BRCA germline mutant patients.
Keywords
<p>BRCA; Hereditary; Breast cancer</p>
BRCA articles BRCA Research articles BRCA review articles BRCA PubMed articles BRCA PubMed Central articles BRCA 2023 articles BRCA 2024 articles BRCA Scopus articles BRCA impact factor journals BRCA Scopus journals BRCA PubMed journals BRCA medical journals BRCA free journals BRCA best journals BRCA top journals BRCA free medical journals BRCA famous journals BRCA Google Scholar indexed journals Hereditary articles Hereditary Research articles Hereditary review articles Hereditary PubMed articles Hereditary PubMed Central articles Hereditary 2023 articles Hereditary 2024 articles Hereditary Scopus articles Hereditary impact factor journals Hereditary Scopus journals Hereditary PubMed journals Hereditary medical journals Hereditary free journals Hereditary best journals Hereditary top journals Hereditary free medical journals Hereditary famous journals Hereditary Google Scholar indexed journals Breast cancer articles Breast cancer Research articles Breast cancer review articles Breast cancer PubMed articles Breast cancer PubMed Central articles Breast cancer 2023 articles Breast cancer 2024 articles Breast cancer Scopus articles Breast cancer impact factor journals Breast cancer Scopus journals Breast cancer PubMed journals Breast cancer medical journals Breast cancer free journals Breast cancer best journals Breast cancer top journals Breast cancer free medical journals Breast cancer famous journals Breast cancer Google Scholar indexed journals ovarian cancer articles ovarian cancer Research articles ovarian cancer review articles ovarian cancer PubMed articles ovarian cancer PubMed Central articles ovarian cancer 2023 articles ovarian cancer 2024 articles ovarian cancer Scopus articles ovarian cancer impact factor journals ovarian cancer Scopus journals ovarian cancer PubMed journals ovarian cancer medical journals ovarian cancer free journals ovarian cancer best journals ovarian cancer top journals ovarian cancer free medical journals ovarian cancer famous journals ovarian cancer Google Scholar indexed journals ovarian syndromes articles ovarian syndromes Research articles ovarian syndromes review articles ovarian syndromes PubMed articles ovarian syndromes PubMed Central articles ovarian syndromes 2023 articles ovarian syndromes 2024 articles ovarian syndromes Scopus articles ovarian syndromes impact factor journals ovarian syndromes Scopus journals ovarian syndromes PubMed journals ovarian syndromes medical journals ovarian syndromes free journals ovarian syndromes best journals ovarian syndromes top journals ovarian syndromes free medical journals ovarian syndromes famous journals ovarian syndromes Google Scholar indexed journals molecular diagnostic strategies articles molecular diagnostic strategies Research articles molecular diagnostic strategies review articles molecular diagnostic strategies PubMed articles molecular diagnostic strategies PubMed Central articles molecular diagnostic strategies 2023 articles molecular diagnostic strategies 2024 articles molecular diagnostic strategies Scopus articles molecular diagnostic strategies impact factor journals molecular diagnostic strategies Scopus journals molecular diagnostic strategies PubMed journals molecular diagnostic strategies medical journals molecular diagnostic strategies free journals molecular diagnostic strategies best journals molecular diagnostic strategies top journals molecular diagnostic strategies free medical journals molecular diagnostic strategies famous journals molecular diagnostic strategies Google Scholar indexed journals surveillance strategies articles surveillance strategies Research articles surveillance strategies review articles surveillance strategies PubMed articles surveillance strategies PubMed Central articles surveillance strategies 2023 articles surveillance strategies 2024 articles surveillance strategies Scopus articles surveillance strategies impact factor journals surveillance strategies Scopus journals surveillance strategies PubMed journals surveillance strategies medical journals surveillance strategies free journals surveillance strategies best journals surveillance strategies top journals surveillance strategies free medical journals surveillance strategies famous journals surveillance strategies Google Scholar indexed journals protein kinase ataxia-telangiectasia mutated articles protein kinase ataxia-telangiectasia mutated Research articles protein kinase ataxia-telangiectasia mutated review articles protein kinase ataxia-telangiectasia mutated PubMed articles protein kinase ataxia-telangiectasia mutated PubMed Central articles protein kinase ataxia-telangiectasia mutated 2023 articles protein kinase ataxia-telangiectasia mutated 2024 articles protein kinase ataxia-telangiectasia mutated Scopus articles protein kinase ataxia-telangiectasia mutated impact factor journals protein kinase ataxia-telangiectasia mutated Scopus journals protein kinase ataxia-telangiectasia mutated PubMed journals protein kinase ataxia-telangiectasia mutated medical journals protein kinase ataxia-telangiectasia mutated free journals protein kinase ataxia-telangiectasia mutated best journals protein kinase ataxia-telangiectasia mutated top journals protein kinase ataxia-telangiectasia mutated free medical journals protein kinase ataxia-telangiectasia mutated famous journals protein kinase ataxia-telangiectasia mutated Google Scholar indexed journals Breast-conserving surgery articles Breast-conserving surgery Research articles Breast-conserving surgery review articles Breast-conserving surgery PubMed articles Breast-conserving surgery PubMed Central articles Breast-conserving surgery 2023 articles Breast-conserving surgery 2024 articles Breast-conserving surgery Scopus articles Breast-conserving surgery impact factor journals Breast-conserving surgery Scopus journals Breast-conserving surgery PubMed journals Breast-conserving surgery medical journals Breast-conserving surgery free journals Breast-conserving surgery best journals Breast-conserving surgery top journals Breast-conserving surgery free medical journals Breast-conserving surgery famous journals Breast-conserving surgery Google Scholar indexed journals contralateral breast cancer articles contralateral breast cancer Research articles contralateral breast cancer review articles contralateral breast cancer PubMed articles contralateral breast cancer PubMed Central articles contralateral breast cancer 2023 articles contralateral breast cancer 2024 articles contralateral breast cancer Scopus articles contralateral breast cancer impact factor journals contralateral breast cancer Scopus journals contralateral breast cancer PubMed journals contralateral breast cancer medical journals contralateral breast cancer free journals contralateral breast cancer best journals contralateral breast cancer top journals contralateral breast cancer free medical journals contralateral breast cancer famous journals contralateral breast cancer Google Scholar indexed journals
Article Details
Graphical Abstract
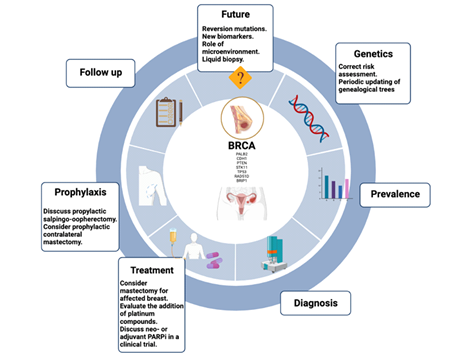
Highlights
- Germline mutations in BRCA1/2 genes are associated with an increased risk of breast cancer (BC).
- All patients carrying mutations in BRCA1/2 genes should be advised on prevention and risk reduction issues.
- Patients with BC and BRCA1/2 gene mutations should receive individualized surgical and medical treatments for their hereditary breast carcinoma condition.
Abbreviations: ATM- protein kinase ataxia-telangiectasia mutated; BC- Breast cancer; BCS- Breast-conserving surgery; BER- base excision repair; BRCT- BRCA1 C Terminus; CA 125- cancer antigen 125; CBC- contralateral breast cancer; CDIS- ductal carcinoma in situ; DNA- deoxyribonucleic acid; dsDNA- double strand deoxyribonucleic acid; ER- estrogen receptors; EUSOMA- European Society of Breast Cancer Specialists; FP- fork protection; HRR- Homologous recombination repair; HRT- hormone replacement therapy; LGRs- large genomic rearrangements; mOS- median overall survival; MRI- magnetic resonance imaging; NBN- Nibrine; NCCN- National Comprehensive Cancer Network; NER- nucleotide excision repair; OC- Ovarian cancer; OS- overall survival; PARP- Poly (ADP-ribose) polymerase; PARPi- Parp inhibitor; PBS- population- based screening; pCR- pathologic complete response; PD-L1- Programmed Death-ligand 1; PR- progesterone receptors; PV- Pathogenic variants; RING- Really Interesting New Gene; RRM- risk-reducing mastectomy; RRSO- risk-reducing bilateral salpingo-oophorectomy; SABCS- San Antonio Breast Cancer Simposyum; SERM- estrogen receptor modulator; SPM- second primary malignancy; ssDNA- single strand deoxyribonucleic acid; TNBC- Triple negative breast cancer
1. Introduction
BC is the most common cancer and the leading cause of cancer death in women worldwide [1]. Family history is among the most important risk factors associated with BC, given that up to 5-10 % are hereditary. The National Cancer Institute defines hereditary breast and ovary syndrome as “an inherited disorder in which the risk of BC (especially before the age of 50) and ovarian cancer is higher than normal” [2]. Kuchenbaecker et al. conducted a prospective study in 3886 carriers of the BRCA mutation, reporting a cumulative incidence of BC up to age 70 of 66% for BRCA1 and 61% for BRCA2 mutation carriers [3]. For female carriers of germline mutation in the BRCA1 gene the risk of BC increases substantially between the ages of 30 and 50, while for women with BRCA2 mutation, the risk increases between the ages of 40 and 60 [4]. Once diagnosed with invasive BC, they have an increased risk of developing a second ipsilateral or contralateral BC, and also have a significantly higher risk of ovarian cancer [5].
Early identification of these high-risk gene families allows us to treat them more specifically, to obtain a more individualized assessment of the risk of developing cancer and to implement prevention strategies, treatment and monitoring according to these risks. This review provides an overview of the literature on hereditary BC and genes that increase cancer risk such as BRCA1/2, and their penetrance. This work focuses particularly on management of BC-associated BRCA pathogenic variants (PVs), highlighting prevention options and available treatments. We review the key findings of publications to date and discuss advances in prevention, diagnosis, and treatment in this high-risk population.
2. Diagnosis and Genetic Testing Criteria
High-risk hereditary breast/ovarian cancer families are defined as multiple cases of BC or ovarian cancer (OC) diagnosed within a family. Accurate risk assessment requires a complete family history going back at least three generations (transmission through both maternal and paternal routes), indicating all cases of cancer, documentation confirming diagnosis of any neoplasm and associated diseases (pathological reports if available), age of diagnosis and death, bilateral or multifocal involvement, and periodically updated genealogical trees. Some differences can be found among the available guidelines. National Comprehensive Cancer Network (NCCN) guidelines are the most inclusive, identifying nearly twice the number of women as high risk for hereditary breast and ovarian cancer than other guidelines [4, 6].
BRCA1/2 testing criteria have shown high sensitivity, with 94.2% of BRCA PVs meeting classical testing criteria, such as BC diagnosed under the age of 50, and multiple primary and triple-negative BCs. However, there is special interest in a patient subset with PVs in BRCA1/2 who do not meet testing criteria for these genes (5.8%), suggesting a need to review testing criteria to identify at-risk patients more precisely [7].
Some authors suggest a high benefit of broad population genetic testing, which could detect individuals not usually eligible according to conventional genetic testing criteria in different guidelines worldwide. Given these benefits, population-based screening (PBS) for a broad number of genes related to increased cancer susceptibility in an extended population should be implemented in daily clinical practice over the next ten years [8]. However, this strategy is not exempt from criticism from other authors regarding drawbacks such as cost-effectiveness, over or under treatment, lack of data for results interpretation, and low experience among healthcare providers, as well as the psychological impact on carriers and patients.
In summary, the most readily available and complete information is obtained by multi-gene panel testing, but its use presents some challenges [9,10] Figure 1.
3. BRCA
3.1. BRCA mutation
The BRCA1 gene was first identified by Hall et al. in 1990. Early-onset family-related BC was linked to chromosome 17q21 during studies in families with BC using genetic polymorphism [11], and BRCA2 was described in 1995 by Wooster et al. [12] Figure 1.
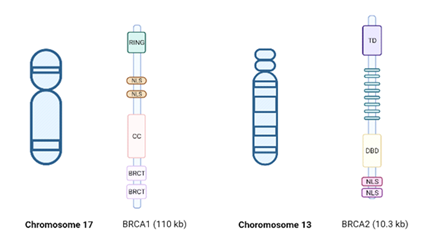
Figure 1: BRCA1/2 genes. BRCA 1 (chromosome 17), including the Really Interesting New Gene (RING) domain, coiled-coil domain, and the two BRCA1 C-terminus (BRCT) domains. BRCA2 (chromosome 13) includes 8 repeated motifs, denoted BRCs, each of which directly bind RAD51 by BioRender, April 2020.
Although DNA is constantly damaged by internal and external factors, several repair mechanisms exist, such as single-strand DNA (ssDNA) break repair, double-strand DNA (dsDNA) break repair and base mismatch repair (MMR), as illustrated in Figure 3. Depending on the type of damage, DNA is repaired by base excision repair (BER) or nucleotide excision repair (NER) [13].
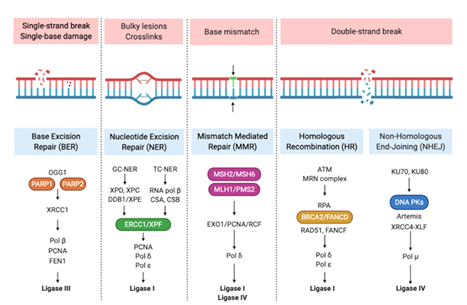
Figure 2: DNA Repair Mechanisms. Cells have developed a series of pathways comprising a network of proteins that detect, signal, and/or repair DNA damage. Several hundred proteins are involved in this response as represented in the figure. Reprinted from "DNA Repair Mechanisms", by BioRender, April 2020.
Hormonal stimulation acts as a trigger signal, activating breast cell mechanisms of transcription and DNA replication. To counteract genome instability, cells hold checkpoint pathways as a protective mechanism, targeting the repair of accumulated DNA breaks [14], as shown in Figure 4. BRCA1/2 genes are involved in repairing DNA double-strand breaks via homologous recombination repair (HRR) to maintain genomic stability [15]. In the context of inherited deficits, genome maintenance pathways become dysfunctional, which can drive tumor development through genomic instability [16]. Beyond the more restricted function of BRCA2 in RAD51-dependent DNA repair mechanism and maintenance of genomic stability, BRCA1 plays a broad role in a range of diverse cellular processes, including DNA-damage response [17].
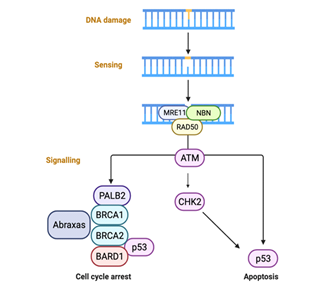
Figure 3: DNA damage checkpoint control. The MRN complex (protein complex consisting of Mre11, Rad50 and Nbs1)is thought to recruit ATM via NBN, thus initiating kinase cascades, which suppress cell cycle progression in G1, S and G2 phases. by BioRender, April 2020.
PVs, the most common BRCA1/2 genes, comprise small deletions, insertions or changes of a nucleotide which affect coding regions, exons, and nucleotide changes in the intron-exon junction regions, usually causing premature termination of BRCA1/2 protein synthesis. In addition to point mutations (substitutions and small insertions/deletions), large genomic rearrangements (LGRs) have also been found to affect a substantial part of the BRCA1/2 gene sequence. LGRs represent between 4-27% of cases, and their systematic study in high-risk breast-ovarian cancer syndrome families has been recommended for population groups in which LGRs represent ≥10% of BRCA1/2 gene mutations. Most germline mutations are concentrated in the amino-terminal RING domain and the BRCA1 carboxyl-terminal (BRCT) domain which underlies the homologous recombination (HR) pathway [18].
A hormonal-carcinogenic environment is also needed in this process, yet the reason why BRCA1 mutational carriers develop breast and ovary cancer still remains unclear. In this context, Sasanuma et al. demonstrated that physiological concentration of estrogens efficiently induces TOP2β-dependent double-strand breaks in the absence of BRCA1 in vitro [2].
3.2. BRCA mutation prevalence
As described above, BRCA1/2 PVs are estimated to occur at a frequency of one in 400-500 in the general population [19]. The reported mutation frequency of the first three variants (c.68_69delAG of BRCA1, c.5266dupC, and c.5946delT of BRCA2) is 1 in 40 in Ashkenazi Jews (Paluch-Shimon et al., 2016). Founder mutations have also been described in various locations (Northern, Western and Eastern Europe) [3].
3.3. Cancer risk estimation
Penetrance, defined as the probability that a BRCA1/2 mutation carrier will develop cancer over a lifetime, is usually expressed as the cumulative risk of cancer at age 70. Penetrance depends on the degree of familial cancer aggregation. In 2017, Kuchenbaecker et al, calculated that cumulative BC risk up to age 80 was 72% (95% CI, 65%-79%) for BRCA1 and 69% (95% CI, 61%-77%) for BRCA2 carriers among a prospective cohort study of 6036 BRCA1 and 3820 BRCA2 female carriers [3]. A recent population-based study performed by Hu et al. found that PVs in BRCA1 or BRCA2 yielded a lifetime risk of approximately 50% [4]. Furthermore, the risk of BC in men is higher in BRCA 1/2 mutation carriers than in the general population. BRCA1 PVs are associated with risk of male BC (RR 4.30) and BRCA2 PVs correlate with even higher risk (RR 44.0) [20]. Risk of OC increases to 44% in patients with BRCA1 mutations and 17% in BRCA2 mutation carriers [3].
Association with risks for other cancers has also been suggested for BRCA1/2; for example, with colorectal, liver, stomach cancer, bone, brain, blood, gallbladder and malignant melanoma. Specifically, cervix, uterine, kidney and testicular cancer have been linked to BRCA1. However, these associations are based on studies with small sample sizes, resulting in imprecise estimates of cancer risk.
In a recent study, Shuai Li et al. evaluated the risks associated with having a BRCA1/2 mutation for 22 primary cancers BC and OC, drawing a spectrum of BRCA1/2 mutation-associated cancer. They concluded that BRCA1/2 mutations are associated with increased risk of BC in men, pancreatic and stomach cancers in both sexes, and that only BRCA2 carriers are at elevated prostate cancer risk. Nonetheless, BRCA1/2 were not associated with risk of any other previously suggested cancers [21].
4. Recommendations in Healthy BRCA1/2 Mutation Carriers
Genetic counselling after detection of a BRCA1/2 mutation includes discussing preventive measures with the patient and the possibility of follow-up tailored to their genetic diagnosis.
4.1. Management in BRCA1/2 mutation carriers
According to NCCN and JCO guidelines: [22,23]
- Instruction and education in monthly postmenstrual breast self-examination, recommended from 18 years onwards.
- Exploration of breast and lymph node drainage territories, to be carried out by an expert. Most programs perform breast examinations every 6 months. Despite its low sensitivity (7-25%) and limited data on its efficacy, this test can improve screening sensitivity in high-risk women and has unquestionable psychological benefit. Recommended to start from age 25, with a periodicity of 6 months.
- Periodic mammograms are unfortunately relatively insensitive (approximately 40%) in this group of women, due partly to high breast density in younger ages, and also to rapid tumor growth rates with expansive margins. Concern has been expressed about the cumulative effects of radiation from repeated mammograms in a population particularly sensitive to DNA damage from ionizing radiation, with the consequent theoretical increased risk of radiation-induced BC. Studies differ regarding the risk of radiation in these women, an effect seemingly influenced by the age at which exposure begins and the total dose. Mammograms are recommended once annually from 30 years old (or 10 years before the youngest diagnosis in the family).
- Breast magnetic resonance imaging (MRI) holds advantages over mammography as a test. It is not accompanied by risk of irradiation, is a valid tool in dense breasts, and although less specific, its sensitivity is higher than mammography, especially in younger breasts. Its specificity is especially low for typifying immediate postsurgical lesions (indeed, the procedure is not recommended until 6 months after breast surgery). Six prospectively published series carried out in high-risk women included BRCA mutation carriers. These non-randomized prospective studies congruently confirmed a much greater sensitivity (71-100%) for breast MRI than mammography and breast ultrasound for detection of hereditary BC. Based on the evidence, the American Cancer Society, National Comprehensive Cancer Network (NCCN) and European Society of BC Specialists (EUSOMA) recommend performing annual breast MRI (in conjunction with mammography) for screening women at high risk (lifetime risk greater than 20-25%), BRCA mutation carriers, and untested relatives of BRCA In conclusion, the systematic annual breast MRI is recommended as part of the follow-up program for all women with a BRCA mutation from 25 years onward. These should be done between days 7-15 of the menstrual cycle in premenopausal women. Mammography and breast MRI can be done at the same time annually, or alternately every 6 months.
- Screening for OC consists of gynecological examination with transvaginal ultrasound (preferably on day 1-10 of the cycle in premenopausal women) and serum determination of CA 125 (preferably after day 5 of the cycle in premenopausal women) with a biannual periodicity for women with BRCA mutation from the age of 30 should only be recommended in women not undergoing risk reduction surgery, or until the moment of performing it, due to the clear inferiority of the former compared with the latter.
4.2. Risk-reducing surgery
4.2.1. Bilateral mastectomy: Bilateral risk-reducing mastectomy (RRM) is the most effective approach to reduce risk of BC in patients with BRCA PVs [24-26]. Current NCCN guidelines support “discussing the risk-reducing mastectomy option for women on a case-by-case basis".
In a published meta-analysis, bilateral mastectomy was associated with a significant reduction in BC incidence, but the impact on all-cause mortality was below statistical significance. Compared to BRCA mutation carriers with two intact breasts, those with who underwent prophylactic surgery had a significantly reduced risk of developing BC. The relative risk was 0.11 (95% CI: 0.04-0.32) and was based on data from six non-overlapping studies with a total of 2,555 patients. The level of risk reduction was similar in carriers of a BRCA1 (RR=0.13; 95% CI 0.02-0.94) and BRCA2 (RR=0.18; CI 95% 0.07-0.47) (Li et al., 2016, p. 1).
Heemskerk-Gerritsen et al. evaluated the impact of mastectomy on BC-specific mortality, and concluded that there was no association between mastectomy and outcome (HR=0.29; 95% CI 0.03-2.61), based on 212 women who underwent preventive surgery but with a relatively short follow-up period (median, 6.13 years) [27]. Women considering preventive surgery have the option of choosing between bilateral skin-sparing surgery where the nipple-areola complex is removed, or a bilateral mastectomy with preservation of the nipple-areola complex [28].
There is some concern about the risk of cancer with mastectomy with nipple preservation, due to the remaining glandular tissue, although data exists suggesting no increased risk with this type of surgery [29]. After prophylactic bilateral mastectomy, breast reconstruction is usually performed in the same surgical intervention (immediate reconstruction), since it enables the use of the same incision as the skin-sparing mastectomy, thus preserving the skin wrapping of the breast, minimizing scarring and improving its contour and symmetry.
Strategies to facilitate decision-making after mutation detection, as well as waitlist prioritization for surgery should be applied in this population to reduce the number of women developing an interval cancer before surgery, as has been shown recently by Macadam et al. [30]. Some authors suggest that age has a role in the benefit of RRM. At age 25, the probability of being alive at age 80 by having a mastectomy increases by 8.7% but this expected benefit declines rapidly with rising age at surgery, being increased by only 2.7% if surgery is performed at age 50 [31].
Since age-specific BC incidence varies by BRCA mutation type, the timing of preventive surgery should be discussed among women considering this preventive option. For example, for women with a BRCA1 mutation, the risks are highest between ages 30 and 50, while for BRCA2 mutation women the rates peak between ages 40-60 [32]. Giannakeas and Narod have shown that preventive mastectomy should be considered before age 50 to maximize the mortality benefit up to age 80 [31]. BRCA1/2 mutation carriers who have not had bilateral mastectomy should undergo high-risk breast screening of remaining breast tissue with annual mammogram and MRI [33].
4.2.2. Risk-reducing bilateral salpingo-oophorectomy (RRSO): Currently, surgery with preventive intent is an effective strategy to reduce the risk of BC, achieving a risk reduction of 40%-50% of BC for BRCA mutation carriers [34]. NCCN guidelines recommend that BRCA1/2 PVs carriers undergo RRSO if between 35-40 years old in women with BRCA1.Women with BRCA2 mutation are recommended the same procedure at a later age, preferably between 40 and 45.
Families with OC cases at younger ages might also be eligible for RRSO strategies. For women who opt against RRSO, surveillance should be performed with a combination of transvaginal ultrasound and CA125 [23]. This procedure should include tube removal due to the increased risk of tubal cancers in BRCA mutation carriers.
Nonetheless, the efficacy of RRSO is not absolute, as a marginal risk of 5-10% of primary peritoneal carcinoma persists [35]. A meta-analysis of the breast/ovarian cancer risk-reducing effect of RRSO showed that OC risk was reduced by 79% and BC risk by 51%; OR 0.49 (0.37-0.6) [36].
The protection conferred by RRSO against breast and gynecologic cancers may differ between carriers of BRCA1 and BRCA2 mutations, as was shown in a multicenter prospective study. Some studies suggested a greater BC risk reduction in carriers of BRCA2 mutations than BRCA1 carriers [14]. Further studies evaluating the efficacy of risk-reduction strategies in BRCA mutation carriers should stratify by the specific mutated gene [37].
In a prospective analysis of 5,783 women carrying the BRCA mutation and with a mean follow-up of 5.6 years, Finch et al. [38] estimated that RRSO in a woman with no personal history of cancer was associated with a significant 77% reduction in all-cause mortality (95% CI: 0.13-0.39; p < 0.001). The reduction in all-cause mortality was present in women both with (HR=0.32; 95% CI 0.26-0.39; p < 0.001) and without a previous BC diagnosis (HR=0.23; 95% CI 0.13-0.39; p < 0.001). Although RRSO is unlikely to have an impact on BC incidence, this substantial protective effect on survival confirms the important role of preventive ovarian surgery in this high-risk population.
4.2.3. Chemoprevention: NCCN guidelines indicate chemoprevention for women at high risk for BC, including those with a 5-year risk of ≥1.7% or personal history of atypical hyperplasia or lobular carcinoma in situ. Chemoprevention may include a selective estrogen receptor modulator (SERM) such as tamoxifen or an aromatase inhibitor such as exemestane (postmenopausal women). Tamoxifen is a SERM used as adjuvant hormonal therapy to treat women with BC that are positive for estrogen receptors (ER) and/or progesterone receptors (PR) [39].
In a meta-analysis, tamoxifen was associated with an overall risk reduction of 33% (HR=0.67, 95% CI: 0.59-0.76), which was maintained for 5-10 years thereafter [39]. This protective effect was limited to positive ER (HR=0.56, 95% CI: 0.47-0.67) and ductal carcinoma in situ (DCIS) (HR=0.72, 0.72; 95% CI: 0.57-0.92). Although tamoxifen is not validated as chemoprevention for primary BC in BRCA mutation carriers, it has been shown to prevent contralateral BC by up to 50% [40,41].
In 2015, Xu et al. published a meta-analysis wherein the use of tamoxifen for first-line treatment of BC resulted in a significant 44% reduction in the risk of a second BC in both BRCA1 and BRCA2 mutation-positive patients (HR=0.56 95% CI: 0.41-0.76) [42]. The corresponding risk estimates were 0.47 (95% CI 0.37-0.60) for BRCA1 mutation carriers and 0.39 (95% CI 0.28-0.54) for BRCA2 mutation carriers. The side effects of tamoxifen, which include increased risk of endometrial cancer and venous thromboembolism, must be taken into account; nonetheless, these risks appear higher among postmenopausal than premenopausal women [43].
Few women with a BRCA mutation choose to take tamoxifen in the preventive setting (~6%); these rates have not changed substantially since 2009 [26]. There is also evidence to support the use of aromatase inhibitors such as exemestane and anastrozole for BC prevention in high-risk women; however, there have been no trials conducted among women with a BRCA mutation [44].
|
BC risk reduction |
|
Lifestyle modifications |
|
Breastfeeding should be encouraged. |
|
Regular exercise, maintaining healthy body weight and limiting alcohol consumption should be encouraged and HRT (hormone replacement therapy) should be avoided. |
|
Screening |
|
Clinical breast examination every 6-12 months is recommended from age 25, or 10 years before the youngest BC diagnosis in the family, whichever is earlier. |
|
All carriers should be encouraged to be ‘breast aware’ and to seek immediate medical attention if they perceive any changes in the breast or lumps in the axilla. |
|
MRI is recommended within the follow-up program for all women with a BRCA mutation from 25 years of age, to be conducted between day 7-15 of the menstrual cycle in premenopausal women. Mammography and breast MRI can be done at the same time annually or alternately every 6 months. |
|
Breast ultrasonography can be considered if MRI is unavailable and may also be used as an adjunct to mammography. |
|
Risk-reducing surgery |
|
Immediate breast reconstruction should be offered. |
|
Contralateral mastectomy can be considered in patients with a previous BC diagnosis. |
Table 1: BC risk reduction [45].
5. Recommendations for BRCA1/2 BC Patient Treatment
BRCA1/2 mutations play a significant role in determining clinical prognosis and survival curves in BC patients. In Zhu et al.’s 2016 meta-analysis BRCA mutation conferred lower overall survival than in non-BRCA-mutated BC [46]. These patients also develop tumors with a specific phenotype. Those with germline mutations in BRCA1 generally develop invasive ductal carcinomas and mostly triple-negative BC, characterized by a lack of ER, PR and ERBB2/HER2 expression. The pathology of BRCA2-mutated cancers is more heterogeneous, showing a trend to ER positivity, HER2 negativity, and low/intermediate histological grade in most of these carcinomas [47]. For these reasons, specialized multidisciplinary care is necessary to attend and advise patients with an inherited BRCA mutation and to select the best treatment option.
The deficiency in HRR present in breast tumors makes them especially sensitive to chemotherapy (although treatment specifically with platinum currently remains a matter of debate) and PARP inhibitor (PARPi) therapies. Currently, numerous PARPi are being tested in phase III trials for various indications, as will be described below. Given their immunogenic profile, the addition of immunotherapy as a treatment for BC patients harboring BRCA mutations could be a promising therapeutic option.
Despite the challenges posed by BRCA1/2 BCs, substantial progress has been made over the past few decades in developing effective therapies. The conventional treatment paradigm for BC comprising immediate interventional procedures and comprehensive cytotoxic chemotherapies has shifted towards mechanistic models, including treatment targeted to tumor subtypes and tissue-specific methods as an adjunct to surgeries and treatment. Hormone-targeted therapies and the development of more specific inhibitors have shown efficacy in treating hormone receptor-positive BCs, as well as those showing HER2/neu amplification. These treatment options are less effective in treating BRCA1/2 BCs, as they are usually triple-negative BC [48].
5.1. Surgery
Breast-conserving surgery (BCS), unilateral mastectomy or unilateral therapeutic mastectomy with concomitant contralateral prophylactic mastectomy could be offered as local management for BRCA1/2 BC patients. In 2010, Pierce et al. followed 655 women with BRCA1/2 mutations diagnosed with BC, treating 302 women with BCS and 353 with mastectomy. No differences in systemic recurrences were found, but local failure was higher at 15-year cumulative risk in those treated with BCS (23.5%) than in those who underwent mastectomy (5.5%), mainly because of second ipsilateral primary tumors [49]. In a 2014 systematic review with 526 BRCA1/2 patients and 2,340 sporadic BC patients, BCS conferred no increased risk for ipsilateral BC comparing carriers with non-carriers, although a higher risk was observed in studies with longer follow-up [50].
The effect of contralateral RRM in BRCA-mutation women with diagnosed BC is not clear. The survival benefit is not well defined in the literature, as most studies are biased by including young and healthy women [51]. Meanwhile, the benefit of contralateral RRM in reducing contralateral breast cancer (CBC) is important to consider in patients with BRCA PVs, particularly when diagnosed at young age [52]. Regarding the risk of CBC in women with BC and BRCA1/2 mutations treated with unilateral mastectomy, contralateral RRM and RSSO should be offered, depending on patient risk level, prognosis and functional status [27].
5.2. Chemotherapy
Triple negative breast cancer (TNBC) tumors are classically associated with high resistance to chemotherapy due to their great heterogeneity. However, BRCA1/2-deficient TNBC are more sensitive to standard chemotherapy than TNBCs with functional BRCA1/2 proteins [53]. Platinum salts are alkylating agents that result in DNA adducts and intra- and inter-strand crosslinks causing ssDNA and dsDNA breaks These drugs induce DNA damage, leading to accumulation and cell death. A retrospective study in young women with BRCA1-mutated BC (n=102) showed a higher rate of pathologic complete response (pCR) after treatment with cisplatin than with other types of neoadjuvant chemotherapy [54]. BRCA-deficient cancers have conventionally been considered hypersensitive to cisplatin due to their inability to repair cisplatin-induced dsDNA breaks by HRR [55].
Recently, Panzarino et al. showed that ssDNA replication gaps, rather than defects in HRR or fork protection (FP), underlie the hypersensitivity of BRCA-deficient cancers to cisplatin. Hence, ssDNA may underlie the BRCA cancer phenotype ("BRCAness") fundamental to the mechanism of action of genotoxic chemotherapies. In support of this concept, when gaps persist, they show that HRR or FP proficient cells can nevertheless be hypersensitive to genotoxins [56].
In a phase II clinical trial aiming to demonstrate the efficacy of platinum in monotherapy in metastatic TNBC patients (n=86), platinum efficacy was observed in a patient subgroup [57]. Despite this finding, it is also known that patients with BRCA-mutated BC previously sensitive to platinum treatment may become resistant to treatment due to reversal of BRCA mutations that restore BRCA1/2 protein function, a point to take into account in the course of treatment [58]. Following on from these findings, four major studies have evaluated the impact on pCR rate of adding platinum agents to standard neoadjuvant chemotherapy, with heterogeneous results [54,59,60].
The BrighTNess study, comparing a taxane alone versus carboplatin plus taxane and also evaluating the PARPi veliparib in combination with carboplatin plus taxane, showed that patients treated with carboplatin plus taxane had a significantly higher pCR 57.5% compared to 31% in patients treated with only taxanes. The neoadjuvant GeparSixto study in BRCA patients on neoadjuvant treatment determined that adding carboplatin improved the pCR rate of BRCA wild type (WT) tumors. BRCA-mutated TNBC patients did not have superior response rates with the addition of carboplatin.
Given these controversial results, their extrapolation to clinical practice in the neoadjuvant context in BC has generated intense debate. Platinum salts are not currently considered standard treatment in clinical practice guidelines. However, considering the strong biological rationale and the more consistent results in pCR, the detection of a BRCA mutation is an incentive for many oncologists to add platinum to standard treatment in the neoadjuvant setting. Due to the aforementioned results, these agents are currently also being studied in the adjuvant context [61].
5.3. Radiotherapy
Radiation causes DNA damage, either directly by ionization or indirectly causing DNA damage (DNA base changes, DNA and protein crosslinking, and DNA single- or double-strand breaks). Given the repair function of BRCA and the increased risk of carcinogenesis in BRCA-mutated patients, it is reasonable to suppose that radiation in these patients would increase carcinogenesis risk compared to the wild type population. However, second primary malignancy (SPM) rates after radiotherapy in BRCA mutation carriers have rarely been reported. If the high risk of SPM were confirmed, it would affect the safety of breast conservation for early BC or prophylactic radiation as a method of prevention. In 2020, in the largest cohort to date of women treated with radiation therapy for BRCA-associated BC, Shlosser [62] found no signs of an increased risk of radiation-induced second primary malignancies compared with general BC.
Considering radiotherapy as adjuvant therapy, the question also arises as to whether tumor cells in hereditary BC have the same sensitivity to radiation as tumor cells in sporadic BC [63]. One theory is that cells associated with the BRCA1/2 mutation have high sensitivity to irradiation [64]. The curative potential of ionizing radiation results in the accumulation of DNA damage and cell death [65]. Preclinical studies have shown that BRCA1 deficiency can cause radiosensitivity. In spite of this, clinical studies are contradictory, showing that there is no increased sensitivity to radiotherapy when compared with sporadic BC patients [66]. Regarding toxicity secondary to radiotherapy, several studies show no increase in complications in BRCA mutated patients [49,67]. Classifying second events as recurrences or new primary cancers after radiotherapy treatment is debatable. Strategies to prevent new BC events are important, especially in patients carrying BRCA mutations.
In conclusion, complete follow-up data, improved future designs and future studies are needed to elucidate the role of BRCA mutations in tumor radioresponse.
5.4. New agents in BRCA cancer treatment
5.4.1. PARP inhibitors: BRCA1 and BRCA2 genes are responsible for genomic stability due to their role in dsDNA damage repair through HRR. PARP protein, in turn, is responsible for repairing ssDNA damage. Since 2005, in vitro and in vivo studies have demonstrated that PARPi utilizes the principle of synthetic lethality [68]. PARP protein inhibition leads to increased accumulation of cellular damage, inducing the phenomenon known as synthetic cell lethality or death by apoptosis in patients with BRCA mutations and homologous recombination deficit. There are differences in efficacy and safety between PARP inhibitors that could be related to their mechanism of action and cytotoxic specificity. On this biological basis, several studies have achieved approval of these drugs (talazoparib and olaparib) in patients with germline BRCA-mutated metastatic BC.
In 2017 Robson et al. published the OlympiAD study which reported olaparib monotherapy as providing a significant benefit over standard therapy in metastatic BC. Furthermore, progression-free survival was 2.8 months longer and the risk of disease progression or death 42% lower with olaparib monotherapy than with standard therapy [69]. Extended, exploratory follow-up analysis at San Antonio Breast Cancer Simposyum (SABCS) 2019 showed a median overall survival (mOS) of 19.3 months with olaparib vs 17.1 months with chemotherapy (HR: 0.84; 95% CI: 0.63-1.12); 4-yr overall survival (OS) rates were 18.9% vs 14.2%, respectively [70].
In 2018 Litton et al. published the findings of the EMBRACA study. These results showed that survival was significantly longer in the talazoparib group than in the standard therapy group (8.6 months vs. 5.6 months; hazard ratio for disease progression or death, 0.54; % confidence interval [CI], 0.41-0.71; P<0.001) [71]. However, no overall survival advantage for talazoparib was reported. As a result of these two previous studies, Talazoparib and Olaparib are currently approved drugs in patients with metastatic BC and BRCA germline mutation.
In the context of neoadjuvant treatment, there is no current evidence that justifies the use of PARPi in these patients. A study published in 2018, the BrighTNess trial, showed no benefit in terms of pCR from adding veliparib to carboplatin and paclitaxel chemotherapy [59]. Nevertheless, in ASCO 2021, preliminary results of a Phase II study with talazoparib in neoadjuvant therapy showed an increase of 45.8% in the pCR rate, requiring more powerful data which could lead to approval in the neoadjuvant context.
A study recently published in the New England Journal of Medicine whose results were presented at ASCO 2021 shows encouraging data reporting for the use of PARPi in adjuvant therapy. The OlympiA trial [72] reported that olaparib following completion of local treatment and neoadjuvant or adjuvant chemotherapy was associated with significantly longer survival free of invasive or distant disease in comparison with placebo in BC high-risk patients with BRCA mutation. To date, the drug has not been approved for this indication by the drug regulatory agencies, but these are encouraging data with an impact on the patient's clinical benefit.
Currently ongoing phase II/III studies include PARP inhibitors combined with immune checkpoint inhibitors for treatment of TNBC [NCT04837209, NCT03544125].
5.4.2. Immunotherapy: BRCA-mutated tumors appear immunogenic due to their higher levels of tumor-infiltrating lymphocytes, expression of immune checkpoint inhibitory molecules and higher mutational tumor burden compared to wild-type BRCA BC [73].
Statistically significant longer progression-free survival and overall survival has been demonstrated in the phase III trial IMPASSION 130, which combined Nab-paclitaxel plus atezolizumab as a first-line treatment in patients with PD-L1(programmed death-ligand 1) immune cell-positive locally advanced or metastatic TNBC. This combination has shown benefit in patients with PD-L1-positive tumors; however, this study does not report the percentage of BRCA-positive patients included [74]. In a recent study [75], the combination of olaparib and durvalumab showed promising antitumor activity and a good safety profile, with the caveat that it is a phase I/II study that requires further research in a randomized setting.
6. Future Directions
6.1. Reversion mutations in BRCA genes
BRCA1/2 mutations are highly sensitive to platinum drugs and PARPi. Reversion due to secondary mutations which restore BRCA protein expression have been described in the literature as a mechanism of resistance to this treatment. Tobalina et al. analyzed published sequencing data of BRCA genes mainly of germline origin (from tumor or circulating tumor DNA) in 327 patients, the majority with ovarian cancer plus only 27 BC patients. They reported that most amino acid sequences encoded by exon 11 in BRCA1 and BRCA2 are dispensable in generating resistance to platinum or PARPi.
It is worth noting that different BRCA protein domains may confer resistance, as in BRCA1 mutations in exon 11 are involved in resistance, while in BRCA2 reversions in exon 11 have been shown to encode BRC repeats, which are the binding domains for the RAD51 recombinase, essential for BRCA2 function [76].
6.2. Role of tumor microenvironment
A recently published review [77] examined the central role of BRCA1/2 mutations in the BC microenvironment, and the different mechanisms that arise resulting in more aggressive tumor cells. Nowadays the importance of the tumor microenvironment in cancer development and progression is well known. Therefore, it is crucial to understand how the presence of a germline mutation will affect all the cells of the tumor microenvironment and thereby characterize these tumors, promoting the pathogenesis of hereditary BC.
Different authors conclude that the patient’s microenvironment can contribute significantly to BC development by creating a pro-tumorigenic niche, also describing how other cellular components of the microenvironment could induce metastatic changes in the tumor itself [78]. Future research focused on these aberrant microenvironment mechanisms could lead to improved treatment strategies for BC patients carrying germline mutations.
6.3. Role of liquid biopsy
At present, precision oncology aims to understand intra- and inter-tumor heterogeneity to offer the most adequate and effective treatment to each patient. Liquid biopsy is emerging as a minimally invasive diagnostic and prognostic tool that allows detection of prognostic and predictive biomarkers. In a recently published article [79] Piombino et al. report that exosomal miRNAs can act as new biomarkers in BC as their expression profile correlates with tumor progression and carcinogenesis. The authors propose anticipating radiological diagnosis in patients with high-risk TNBC (carriers of BRCA mutations) using a serial panel of multiple specific exosomal miRNAs in serum or plasma.
These strategies could reduce patient mortality and morbidity by overcoming the limits of current screening programs, and warrant further studies in larger clinical trials.
7. Conclusions
BRCA1/2 are the most important genes involved in genetic BC. It is therefore essential that all patients who meet the criteria for a genetic study receive appropriate genetic counseling, and families with BRCA1/2 PVs genes should be informed about prevention and risk reduction methods. It is imperative that women with BC and mutations in BRCA1/2 genes receive individualized surgical and medical treatment, and beyond this, new approaches and greater understanding of personalized medicine are still needed in this field. PARPi have been shown to be useful in patients with BC and BRCA germline mutations, opening a big opportunity for these patients. But it is still needed to determine more clinical scenarios in which this therapy may be applied and to develop other strategies in progression or resistance setting.
Conflict of Interest
The authors declare no conflict of interest for this article.
Funding
This research did not receive any specific funding from academic, commercial or nonprofit agencies.
Acknowledgements
We wish to thank grant from Asociación Española Contra el Cáncer (AECC, POSTD20028OLTR).
References
- Baretta Z, Mocellin S, Goldin E, et al. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine (Baltimore) 95 (2016): e4975.
- Yoshida R. Hereditary breast and ovarian cancer (HBOC): review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer (2020).
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 317 (2017): 2402.
- Hu C, Hart SN, Gnanaolivu R, et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N Engl J Med 384 (2021): 440-451.
- Lubinski J, Huzarski T, Gronwald J, et al. Age-specific risks of incident, contralateral and ipsilateral breast cancer among 1776 Polish BRCA1 mutation carriers. Breast Cancer Res Treat 174 (2019): 769-774.
- Kotnik U, Peterlin B, Lovrecic L. Identification of women at risk for hereditary breast and ovarian cancer in a sample of 1000 Slovenian women: a comparison of guidelines. BMC Cancer 21 (2021): 665.
- Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol 28 (2010): 893-901.
- LaDuca H, Polley EC, Yussuf A, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med 22 (2020): 407-415.
- De Simone LM, Arjunan A, Vogel Postula KJ, et al. Genetic counselors’ perspectives on population-based screening for BRCA- related hereditary breast and ovarian cancer and Lynch syndrome. J Genet Couns 30 (2021): 158-169.
- McAlarnen L, Stearns K, Uyar D. Challenges of Genomic Testing for Hereditary Breast and Ovarian Cancers. Appl Clin Genet 14 (2021): 1-9.
- Hall JM, Lee MK, Newman B, et al. Linkage of Early-Onset Familial Breast Cancer to Chromosome 17q21. Science. 1990 250 (4988): 1684-1689.
- Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 (6559): 789-792.
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature 481 (2012): 287-294.
- Nielsen FC, van Overeem Hansen T, Sørensen CS. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer 16 (2016): 599-612.
- Venkitaraman AR. Cancer Suppression by the Chromosome Custodians, BRCA1 and BRCA2. Science 343 (2014): 1470-1475.
- Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer 4 (2004): 814-9.
- Christou C, Kyriacou K. BRCA1 and Its Network of Interacting Partners. Biology 2 (2013): 40-63.
- Sasanuma H, Tsuda M, Morimoto S, et al. BRCA1 ensures genome integrity by eliminating estrogen-induced pathological topoisomerase II-DNA complexes. Proc Natl Acad Sci 115 (2018).
- Tennen RI, Laskey SB, Koelsch BL, et al. Identifying Ashkenazi Jewish BRCA1/2 founder variants in individuals who do not self-report Jewish ancestry. Sci Rep 10 (2020): 7669.
- Pujol P, Barberis M, Beer P, et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer 146 (2021): 30-47.
- Li S, Silvestri V, Leslie G, et al. Cancer Risks Associated With BRCA1 and BRCA2 Pathogenic Variants. J Clin Oncol. Jan JCO.21.02112 (2022).
- Daly MB, Karlan BY, Pal T, et al. NCCN Guidelines Index Table of Contents Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Discussion. Risk Assess 119 (2020).
- Tung NM, Boughey JC, Pierce LJ, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol 38 (2020): 2080-2106.
- Carbine NE, Lostumbo L, Wallace J, et al. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Breast Cancer Group, editor. Cochrane Database Syst Rev (2019).
- Hartmann LC, Lindor NM. The Role of Risk-Reducing Surgery in Hereditary Breast and Ovarian Cancer. Longo DL, editor. N Engl J Med 374 (2016): 454-468.
- Wuttke M, Phillips KA. Clinical management of women at high risk of breast cancer. Curr Opin Obstet Gynecol 27 (2015): 6-13.
- Heemskerk-Gerritsen BAM, Menke-Pluijmers MBE, Jager A, et al. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol 24 (2013): 2029-2035.
- Galimberti V, Vicini E, Corso G, et al. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. The Breast 34 (2017): S82-S84.
- Jakub JW, Peled AW, Gray RJ, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg 153 (2018): 123.
- Macadam SA, Slater K, Cheifetz RE, et al. Prophylactic Surgery in the BRCA+ Patient: Do Women Develop Breast Cancer While Waiting? Curr Oncol 28 (2021): 702-715.
- Giannakeas V, Narod SA. The expected benefit of preventive mastectomy on breast cancer incidence and mortality in BRCA mutation carriers, by age at mastectomy. Breast Cancer Res Treat 167 (2018): 263-267.
- Kotsopoulos J. BRCA Mutations and Breast Cancer Prevention. Cancers 10 (2018): 524.
- Warner E. Screening BRCA1 and BRCA2 Mutation Carriers for Breast Cancer. Cancers 10 (2018): 477.
- Li X, You R, Wang X, et al. Effectiveness of Prophylactic Surgeries in BRCA1 or BRCA2 Mutation Carriers: A Meta-analysis and Systematic Review. Clin Cancer Res 22 (2016): 3971-3981.
- Xiao YL, Wang K, Liu Q, et al. Risk Reduction and Survival Benefit of Risk-Reducing Salpingo-oophorectomy in Hereditary Breast Cancer: Meta-analysis and Systematic Review. Clin Breast Cancer 19 (2019): e48-65.
- Sekine M, Enomoto T, Arai M, et al. Correlation between the risk of ovarian cancer and BRCA recurrent pathogenic variants in Japan. J Hum Genet (2022).
- Sekine M, Nishino K, Enomoto T. BRCA Genetic Test and Risk-Reducing Salpingo-Oophorectomy for Hereditary Breast and Ovarian Cancer: State-of-the-Art. Cancers 13 (2021): 2562.
- Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 Gynecol Oncol 100 (2006): 58-64.
- Cuzick J. Preventive therapy for cancer. Lancet Oncol 18 (2017): e472-e482.
- Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer inBRCA1 andBRCA2 carriers: An update. Int J Cancer 118 (2006): 2281-2284.
- Phillips KA, Milne RL, Rookus MA, et al. Tamoxifen and Risk of Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. J Clin Oncol 31 (2013): 3091-3099.
- Xu L, Zhao Y, Chen Z, et al. Tamoxifen and risk of contralateral breast cancer among women with inherited mutations in BRCA1 and BRCA2: a meta-analysis. Breast Cancer 22 (2015): 327-334.
- Chalas E, Costantino JP, Wickerham DL, et al. Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol 192 (2005): 1230-1237.
- Metcalfe K, Lynch HT, Foulkes WD, et al. Oestrogen receptor status and survival in women with BRCA2-associated breast cancer. Br J Cancer 120 (2019): 398-403.
- Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primer 5 (2019): 66.
- Zhu Y, Wu J, Zhang C, Sun S, Zhang J, Liu W, et al. BRCA mutations and survival in breast cancer: an updated systematic review and meta-analysis. Oncotarget 7 (2016): 70113-70127.
- Larsen MJ, Kruse TA, Tan Q, et al. Classifications within Molecular Subtypes Enables Identification of BRCA1/BRCA2 Mutation Carriers by RNA Tumor Profiling. Prokunina-Olsson L, editor. PLoS ONE 8 (2013): e64268.
- Lee A, Moon BI, Kim TH. BRCA1/BRCA2 Pathogenic Variant Breast Cancer: Treatment and Prevention Strategies. Ann Lab Med 40 (2020): 114-121.
- Pierce LJ, Phillips KA, Griffith KA, et al. Local therapy in BRCA1 and BRCA2 mutation carriers with operable breast cancer: comparison of breast conservation and mastectomy. Breast Cancer Res Treat 121 (2010): 389-398.
- Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat 144 (2014): 443-455.
- Kaas R, Verhoef S, Wesseling J, et al. Prophylactic Mastectomy in BRCA1 and BRCA2 Mutation Carriers: Very Low Risk for Subsequent Breast Cancer. Ann Surg 251 (2010): 488-492.
- Riis ML. Management of patients with BRCA mutation from the point of view of a breast surgeon. Ann Med Surg 65 (2021): 102311.
- Jiang T, Shi W, Natowicz R, et al. Statistical measures of transcriptional diversity capture genomic heterogeneity of cancer. BMC Genomics 15 (2014): 876.
- Byrski T, Gronwald J, Huzarski T, et al. Pathologic Complete Response Rates in Young Women With BRCA1 -Positive Breast Cancers After Neoadjuvant Chemotherapy. J Clin Oncol 28 (2010): 375-379.
- Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res 18 (2008): 99-113.
- Panzarino NJ, Krais JJ, Cong K, et al. Replication Gaps Underlie BRCA Deficiency and Therapy Response. Cancer Res 81 (2021): 1388-1397.
- Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 33 (2015): 1902-1909.
- Kennedy RD, Quinn JE, Mullan PB, et al. The Role of BRCA1 in the Cellular Response to Chemotherapy. JNCI J Natl Cancer Inst 96 (2004): 1659-1668.
- Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 19 (2018): 497-509.
- Telli ML, Jensen KC, Vinayak S, et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib As Neoadjuvant Therapy for Triple-Negative and BRCA1 / 2 Mutation-Associated Breast Cancer With Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. J Clin Oncol 33 (2015): 1895-1901.
- t’Kint de Roodenbeke MD, Pondé N, Buisseret L, Piccart M. Management of early breast cancer in patients bearing germline BRCA Semin Oncol 47 (2020): 243-248.
- Schlosser S, Rabinovitch R, Shatz Z, et al. Radiation-Associated Secondary Malignancies in BRCA Mutation Carriers Treated for Breast Cancer. Int J Radiat Oncol 107 (2020): 353-359.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol 23 (2018): 45-51.
- Sadeghi F, Asgari M, Matloubi M, et al. Molecular contribution of BRCA1 and BRCA2 to genome instability in breast cancer patients: review of radiosensitivity assays. Biol Proced Online 22 (2020): 23.
- Lomax ME, Folkes LK, O’Neill P. Biological Consequences of Radiation-induced DNA Damage: Relevance to Radiotherapy. Clin Oncol 25 (2013): 578-585.
- Kan C, Zhang J. BRCA1 Mutation: A Predictive Marker for Radiation Therapy? Int J Radiat Oncol 93 (2015): 281-293.
- Pierce LJ, Strawderman M, Narod SA, et al. Effect of Radiotherapy After Breast-Conserving Treatment in Women With Breast Cancer and Germline BRCA1/2 J Clin Oncol 18 (2000): 3360-3369.
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly (ADP-ribose) polymerase. Nature 434 (2005): 913-917.
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA N Engl J Med 377 (2017): 523-533.
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 30 (2019): 558-566.
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA N Engl J Med 379 (2018): 753-763.
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1 - or BRCA2 -Mutated Breast Cancer. N Engl J Med. NEJMoa2105215 (2021).
- Buisseret L, Garaud S, de Wind A, et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. OncoImmunology 6 (2017): e1257452.
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 379 (2018): 2108-2121.
- Domchek SM, Postel-Vinay S, Im SA, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol 21 (2020): 1155-1164.
- Tobalina L, Armenia J, Irving E, et al. A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance. Ann Oncol 32 (2021): 103-112.
- Miklikova S, Trnkova L, Plava J, et al. The Role of BRCA1/2-Mutated Tumor Microenvironment in Breast Cancer. Cancers 13 (2021): 575.
- Kucerova L, Kovacovicova M, Polak S, et al. Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma. 58 (2011): 361-370.
- Piombino C, Mastrolia I, Omarini C, et al. The Role of Exosomes in Breast Cancer Diagnosis. Biomedicines 9 (2021): 312.
