Hepatitis E Virus (HEV) Seroprevalences in Pigs and among pork Butchers in two regions of Northern Togo
Article Information
Komi V-M. Setondji*, 1, 2 †, Kuan A. Traoré1, 3 †, Jean B. Ouoba1, Essodolom Taale4, Bruno L. Ouoba1, Bissah K. Nyakou2, Judith Akpandja2, Gnimdou Kpanegue5, Pierre Roques6, 7, 8, 9, Simplice D. Karou4 and Nicolas Barro1
1Laboratoire de Biologie Moléculaire, d’Épidémiologie et de Surveillance des Bactéries et Virus Transmissibles par les Aliments (LaBESTA), Université Joseph KI-ZERBO, Ouagadougou BP 7021, Burkina Faso
2Laboratoire d’analyse biomédicale du centre régional de transfusion sanguine, Sokodé, Togo
3Laboratoire de Sciences de la Vie et de la Terre (LaSVT), Université Norbert ZONGO, Koudougou BP 376, Burkina Faso
4Laboratoire de microbiologie et de contrôle de qualité des denrées alimentaires, Université de Lomé, Lomé, Togo
5Laboratoire d’analyse biomédicale de la Clinique TOUT EST GRACE, Kara, Togo
6IDMIT Département/IBFJ|CEA, 92265 Fontenay-aux-Roses, France
7Immunology of Viral Infections and Autoimmune Diseases (IMVA-HB), U1184, INSERM, 92265 Fontenay-aux-Roses, France
8UMR1184, IMVA-HB, Université Paris-Saclay, 91400 Orsay, France
9Virology Unit, Institut Pasteur de Guinée, Conakry BP 4416
*Corresponding author: Komi V-M. Setondji. Laboratoire de Biologie Moléculaire, d’Épidémiologie et de Surveillance des Bactéries et Virus Transmissibles par les Aliments (LaBESTA), Université Joseph KI-ZERBO, Ouagadougou BP 7021, Burkina Faso.
†These authors contribute equally to this work
Received: 24 April 2023; Accepted: 05 May 2023; Published: 12 May 2023
Citation: Komi V-M. Setondji, Kuan A. Traoré, Jean B. Ouoba, Essodolom Taale, Bruno L. Ouoba, Bissah K. Nyakou, Judith Akpandja, Nicolas Kpanegue, Pierre Roques, Simplice D. Karou and Nicolas Barro. Hepatitis E Virus (HEV) Seroprevalences in Pigs and among pork Butchers in two regions of Northern Togo. Fortune Journal of Health Sciences. 6 (2023): 191-199.
View / Download Pdf Share at FacebookAbstract
In the last decade in West Africa, the number of autochthonous cases of hepatitis E has significantly increased. Some hepatitis E virus (HEV) infections have been attributable to zoonotic transmission. This study was carried out to assess the seroprevalence of HEV among the exposed human and local pig populations in the cities of Kara and Sokodé (Togo), and the surrounding localities. A total of 89 breeders-butchers (5 women and 84 men), were recruited from November 2021 to February 2022 and their HEV serological status and socio-demographic status were assessed. In addition, 176 serum samples from slaughtered pig belonging to these breeders-butchers were collected. All human and swine sera were tested for the presence of HEV antibodies. We used the serological survey data from the general population cohort to compare with the current butcher/breeder cohort. The association between anti-HEV status and potential risk factors was evaluated. HEV IgM and IgG antibodies were detected in 20.22% (95% CI: 19.33 - 21.10%) and 5.6% (95% CI: 5.09 - 6.10) of the butcher serum samples, respectively. No specific behavior of the butchers was associated with seropositivity in butchers (p ≥ 0.05). Total anti-HEV antibody seropositivity was 80.11% (95% CI: 79.66 - 80.55) in pigs. These results from asymptomatic population suggest 1) circulation of HEV in the pig butcher population and 2) pigs as the virus reservoir with probable zoonotic transmission in these areas. These data could provide evidence to understand the epidemiology and clues to control transmission in Kara, Sokodé and their surrounding localities in Togo.
Keywords
Hepatitis E Virus (HEV), seroprevalence, pork butchers, swine, Togo
Hepatitis E Virus (HEV) articles, seroprevalence articles, pork butchers articles, swine articles, Togo articles
Article Details
1. Introduction
Hepatitis E Virus (HEV) is one of the leading causes of acute hepatitis in the world. HEV is a small icosahedral virus of the family Hepeviridae, which includes two genera Orthohepevirus and Piscihepevirus. The genus Orthohepevirus contains four species designated as Orthohepevirus A through D. Within Orthohepevirus A, 8 distinct mammalian genotypes (gt) have been identified to date (HEV 1-8) [1]. The gt 1 and 2 infect only humans and cause epidemics in developing countries in Africa and Asia, as well as in Mexico. They are primarily transmitted via the fecal-oral route due to fecal contamination of drinking water. Thus, it is related to the absence or failure of a safe water supply [2-3]. In contrast, gt 3 and gt 4 viruses have proven zoonotic potential and infect humans and several other animal species, such as pigs (Sus scrofa domesticus), wild boar (Sus scrofa scrofa), and deer (Cervidae) [4]. Zoonotic transmission of gt 3 or gt 4 occurs through consumption of undercooked meat or contact with infected animals [5]. Gt 5 and gt 6 infect wild boar, while gt 7 infects dromedaries (Camelus dromedarius). A recent study showed zoonotic transmission of genotype 7 related to consumption of camel meat. Gt 8 infects the Bactrian camel (Camelus bactrianus) [6]. HEV Infection is generally self-limiting in healthy human patients. Fecal-oral transmission is its primary route of transmission [7]. But recent work has reported that transmission of the virus is becoming increasingly zoonotic with different animal species (rats, deer, monkeys, and suids) as source of HEV [8]. The animal reservoir, primarily swine, plays a major role in this transmission. Food transmission by ingestion of raw or undercooked meat has been demonstrated by strain homology between the incriminating food and the infected subject and by case-control studies [2]. Occupational exposure is a risk factor; veterinarians, slaughterers/butchers, and forestry workers have shown a prevalence of HEV antibodies compared to individuals in other occupations [9-10]. Transmission of HEV within a herd occurs naturally through direct contact with HEV-infected animals or the feces of diseased animals, or through contaminated food or water sources [11]. Animals can acquire HEV virus at different times during their growing season. Most studies show that pigs become infected between 8 and 15 weeks of age, and some remain positive at slaughter [12]. In addition, 2-15% of pigs have been shown to be infected with HEV at slaughter [10]. Pork butchers may also be a confounding factor; it is likely that butchers live in less than optimal hygienic conditions, making them frequently exposed to HEV [13]. These data raise many concerns about the health of populations.
In Togo, to date, there is no information about HEV seroprevalence among pork butchers and in pigs. The objective of this study was to determine the seroprevalence of HEV in the pork butcher population of two regions of Northern Togo, Central and Kara, where pork production and consumption by the population are important, to identify risk factors associated with the infection that would contribute to its persistence in the population, and to determine the seroprevalence in slaughtered pigs intended for human consumption. This study provided the first mapping of HEV prevalence in the pork butchers and pig population in Togo.
2. Material and Methods
2.1 Study design and study site
From November 2021 to February 2022, a cross-sectional study was conducted and included 89 asymptomatic individuals in contact with pigs or pork meat (5 women and 84 men) and 289 individuals in general population (not in contact with pig) from Sokodé and 176 pigs slaughtered for consumption. The data from the general population (not in contact with pig) cohort analyzed in this study come from a previous serological survey conducted in Sokodé, Togo [14]. The study was approved by the Bioethics Committee for Health Research under No: 040/2019/CBRS, and furthermore the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as evidenced by the approval of the Ministry of Health and Public Hygiene (Togo) under No: 027/2020/MSHP/CAB/SG/DGAS/DPML/CBRS. An administrative authorization was also obtained from the Polyclinic and the Regional Blood Transfusion Center of Sokodé.
2.2 Study site, data collection and sampling
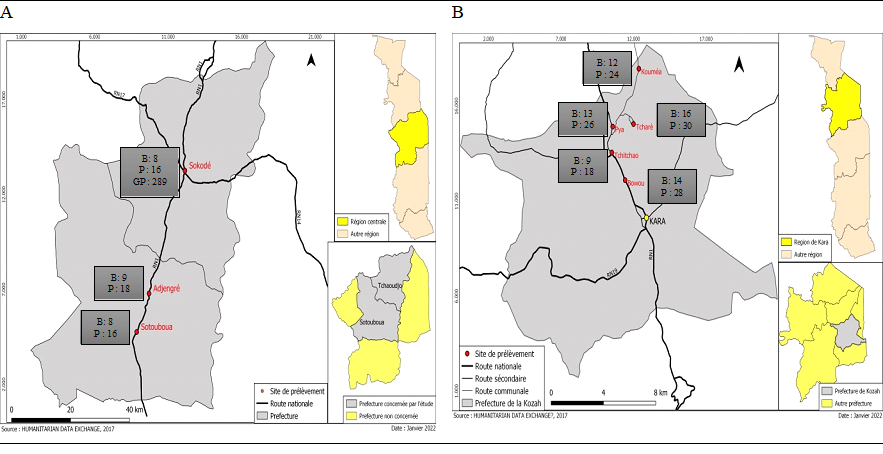
The central region with the capital city of Sokodé has a population of approximately 240 360 (INSEED, 2023) and is located in the center of Togo. The Kara region, located just above the central region, has the city of Kara as its capital, with a population of about 283 738 (INSEED, 2023). Located 320 km and 423 km from Lomé, respectively, the central region and Kara experience a single rainy season from April to October per year with a peak between July and September with a tropical climate. Participants (butchers) included in the research were face to face interviewed and their sera collected on site. Figure 1 shows the number of butchers and pigs tested by sample site. Demographic and background characteristics included were age, gender, area of residence, number of years in occupation, religion. We collected data regarding the practice of animal husbandry, wearing of work gloves, source of water use, times of hand washing, knowledge of hepatitis, having ever been screened or vaccinated against hepatitis (Table I). Five milliliters of blood were collected in a non-heparinized tube from each participant. The blood samples were processed according to the requirements of the Blood Bank Management Committee of the Regional Blood Transfusion Center (RBTC). The sample was centrifuged at 3000×g for 10 minutes at 25°C. Sera were collected in cryotubes and stored at -20 ± 5°C until serological analysis.
Table I: Sociodemographic characteristics of butchers
|
Population: |
Breeder-Butcher |
|
N=89(%) |
|
|
Age |
|
|
≤25 |
35 (39.3) |
|
26-35 |
24 (27.0) |
|
36≥ |
30 (33.7) |
|
Gender |
|
|
Female: |
5 (15.6) |
|
Male: |
84 (94.4 |
|
Number of years in activity |
|
|
[0 - 5] |
46 (51.7) |
|
[5 - 10] |
10 (11.2) |
|
[10 - 15] |
11 (12.4) |
|
[15 - 20] |
8 (9.0) |
|
[20 - 25] |
7 (7.8) |
|
≥ 25 |
7 (7.8) |
|
Level of study |
|
|
Primary |
27(30.3) |
|
Secondary |
46 (51.7) |
|
University |
16 (18.0) |
|
Area of residence |
|
|
Urban: |
15 (16.1) |
|
Rural: |
74 (83.1) |
|
Profession |
|
|
Breeder-Butcher: |
89(100) |
|
Butcher No Breeder |
0(0) |
|
Other: |
0(0) |
|
Religion |
|
|
Christian: |
29 (32.6) |
|
Muslim: |
0 (0) |
|
animist: |
60 (67.4) |
|
Source of water consumed |
|
|
Well: |
32 (36.0) |
|
Tap: |
57 (64.0) |
|
Mineral: |
0(0) |
|
Water treatment before consumption (chlorine or slurry) |
|
|
No: |
89(100) |
|
Yes: |
0(0) |
|
Cooking method of the meat before consumption |
|
|
Cooked to medium rare: |
89(100) |
|
No medium rare: |
0(0) |
|
Consume pork meat |
|
|
No: |
0(0) |
|
Yes: |
89(100) |
|
Hands washing with detergent |
|
|
No: |
0 (0) |
|
Yes: |
89 (100) |
|
Have knowledge of hepatitis A, B and C |
|
|
No: |
|
|
Yes: |
46 (51.7) |
|
43 (48.3) |
|
|
Already screened for hepatitis B |
|
|
No: |
60 (67.4) |
|
Yes: |
29 (32.6) |
|
Already vaccinated against hepatitis B |
|
|
No: |
73 (82.0) |
|
Yes: |
16 (18.0) |
2.3 Serological analyses
A total of 89 serum samples collected from butchers were tested for the presence of HEV antibodies (IgM and IgG) with enzyme-linked immunosorbent assays (ELISA): HEV ELISA kit for HEV immunoglobulin G (IgG) and HEV IgM ELISA 3.0 kit for HEV immunoglobulin M (IgM) (MP Biomedicals Asia Pacific Pte Ltd, Singapore; formerly Genelabs Diagnostics Pte). 176 swine serum samples were tested with the enzyme-linked immunosorbent assay (ELISA): VHE ELISA 4.0 kit (For veterinary use only) for total HEV antibodies (IgG, IgM, and IgA) (MP Biomedicals Asia Pacific Pte Ltd,). The tests were performed according to the manufacturer's instructions. MP Diagnostic HEV ELISA 4.0 uses a proprietary recombinant antigen, which is highly conserved between different strains (12-14) of HEV, to detect the presence of specific antibodies, including IgG, IgM and IgA, to HEV. The MPD HEV IgM ELISA 3.0 and MPD HEV IgM ELISA 3.0 are indirect immunoassays that utilize a highly conserved conformational epitope encoded by the hepatitis E virus open reading frame 2 (ORF 2). The MPD HEV IgM ELISA 3.0 and MPD HEV ELISA have a sensitivity of 98% and a specificity of 96.7%. All three ELISAs calculate a result based on the signal-to-cut ratio (CO). Specimens with absorbance values less than the CO value are considered non-reactive, and those with absorbance values greater than or equal to the CO value are considered initially reactive. The cut-off value (CO) is the average absorbance value of the three negative controls multiplied by 0.20 for the MP Diagnostic HEV ELISA 4.0, by 0.40 for the MPD HEV IgM ELISA 3.0 and by 0.50 for the MPD HEV IgM ELISA respectively. Human samples were tested with the Rapid Diagnostic Test BiolineTM Malaria Ag Plasmodium falciparum Histidine 2 Rich (HRP2) which has a sensitivity of 99.7% and a specificity of 99.5%, to identify false positives or cross-reactions with HEV antigens.
2.4 Statistical analysis
Data analysis was performed with SPSS version 21 software. Descriptive statistical analysis was used to calculate the mean, median, percentages of sociodemographic variables, percentage of seropositivity to anti-IgM and anti-IgG antibodies at the 95% confidence interval (95%CI). Statistical analysis was performed using the Chi-square test (x2). The test was considered significant at a p value < 0.05.
3. Results
3.2 Socio-demographic characteristics of study participants
A total of 89 butchers participated in this study, of which 84 (94.4%) were men and 5 (5.6%) were women. The mean age of the butchers was (32.3 ± 9.9SD). For men, it was (32.3 ± 9.9SD) with an age range of 16 to 65 years and for women, it was (33.4 ± 8.7SD) with an age range of 18 to 51 years. As for the general population of the 289 individuals who participated in the previous study [14], 118 (40.8%) were female and 171 (59.2%) were male. The age range was 17 to 51 years, with a mean age of 26.16 years (± 6.53SD) and a median of 24 years. 46 (51.7%) of the butchers had less than 5 years of experience and 46 (51.7%) had a high school level compared to 163 (56.4%) in the general population. 272 (94.1%) of the general population lived in urban areas compared to 74 (83.1%) of butchers who lived in rural areas. 3 (1%) butchers (not in contact with pork), were observed in the general population. Indeed, before starting their activity, the future butchers underwent a certain number of tests, including those for viral hepatitis, in order to practice freely. Of the butchers surveyed, only 29 (32.6%) had been tested for hepatitis B and/or C and only 16 (18%) had received the hepatitis B vaccine. Other types of hepatitis viruses were unknown or ignored. A total of 176 pigs were included in this study, all of which were of slaughter age (3 months and older). Table I presents the socio-demographic characteristics of the butchers.
3.1 HEV Seropositivity
The table II shows the seroprevalence of the different study populations. A total of 18 samples (20.22%) (95% CI: 19.33-21.10) of the 89 pork butchers were positive for IgM antibodies, indicating a recent HEV infection, and 5 samples (5.6%) (95% CI: 5.09-6.10) were positive for IgG antibodies, indicating a recovering or old infection. There were 2 double positive IgG+IgM+. Because the risk of IgG false positive associated to Malaria fever was found in much serological analysis. We also evaluate the Malaria status of the human population thus five samples (27.7%) (95% CI: 5.09-6.10) of the anti-IgM HEV positive were found to be positive for the Rapid Diagnostic Test Plasmodium falciparum versus none in the anti-IgG HEV positive lot.
Regarding detection in pigs 141/178 (80.1%) (95% CI: 79.66-80.55) were positive for total HEV antibodies.
Table II: Results of HEV total and M immunoglobulin detection in butchers / pigs
|
Serological characteristics |
% (n/N) |
IC 95% |
|
Total antibodies positives (IgM+ or IgG+) |
||
|
Human: |
23.6 (21/89) |
22.7-24.5 |
|
Anti-IgM positives (human) |
20.2 (18/89) |
19.3-21.1 |
|
Anti-IgG positives (human) |
5.6 (5/89) |
5.1-6.1 |
|
Anti-IgM positives and IgG negative |
||
|
Human: |
17.9 (16/89) |
17.1-18.8 |
|
Anti-IgM negative and IgG positive |
||
|
Human: |
3.3 (3/89) |
3.0-3.8 |
|
Anti-IgM positives and Ag Malaria Pf Positive Human: |
27.7 (5/18) |
46.4-8.9 |
|
Anti-IgG positives and Ag Malaria P f Positive Human: |
0 (0/5) |
- |
|
Total antibodies positives (IgM+IgG) Pigs: |
80.1(141/178) |
79.7-80.5 |
IC: Confidence interval, n: size, (-): Not calculated
Whatever was the geographical origin of the pigs, the HEV seroprevalence is the same (Table III), indicating that circulation of HEV is probably an old story but with some silence period as the youngest animals have already the very same prevalence as the oldest one. In Bohou there was a tendency to a prevalence increase with age that may signed a continuous virus circulation.
Table III: Seroprevalence of pigs (P) tested per sample site
|
Sampling site |
Seroprevalence |
Seroprevalence |
||
|
% (n/N) |
(Age in months) |
|||
|
[3-6] |
[6-12] |
≥12 |
||
|
Sotouboua |
81(13/16) |
100(4/4) |
71(5/7) |
80(4/5) |
|
Adjengré |
77(14/18) |
67(4/6) |
100(5/5) |
71(5/7) |
|
Sokodé |
75(12/16) |
80(4/5) |
86(6/7) |
50(2/4) |
|
Bohou |
86(24/28) |
75(6/8) |
80(8/10) |
100(10/10) |
|
Tchitchao |
72(13/18) |
67(4/6) |
60(3/5) |
86(6/7) |
|
Pya |
85(22/26) |
100(7/7) |
80(8/10) |
78(7/9) |
|
Tcharè |
77(23/30) |
75(6/8) |
75(9/12) |
80(8/10) |
|
Kouméa |
83(20/24) |
86(6/7) |
78(7/9) |
88(7/8) |
|
Total |
80(141/176) |
80(41/51) |
78(51/65) |
82(49/60) |
3.3 Risk factors associated with HEV infection
Observation of the risk factor analysis shows that no factor was significantly associated with anti-HEV IgM in butchers (p ≥ 0.05). To avoid confounding factors with risk factors associated with infection, the 5 individuals positive for both anti-IgM HEV and plasmodium in butchers were excluded from the data. In the general population, 1% (3/289) of butchers who did not touch pigs or handle pig carcasses were observed and 37.0% (107/289) were involved in raising poultry, goats, cattle, dogs and cats. The ratio of anti-IgM VHE of butchers and the general population was (OR: 2) with p = 0.202. Tables IVa and IVb present successively the risk factors and behavioral risk factors associated with anti-IgM HEV infection in both the general population and butchers and those associated with anti-IgG HEV infection in butchers.
Table IVa: Anti-HEV immunoglobulins associated with risk factors
Table IVb: Anti-HEV immunoglobulins associated with comportemental risk factors
*Significant (p<0.005), n: size, (-): Not calculated
a: Comparison of butchers’ exposure to Anti-IgM VHE+ and the risk factor
b: Comparison of butchers’ exposure to Anti-IgG VHE+ and the risk factor
c: Comparison of general population exposure to Anti-IgM VHE+ and the risk factor
4. Discussion
In previous studies in Togo, it was established evidence of the diffusion of HEV in the general human [14]. Although contamination of drinking water is the main source of virus spread, numerous studies have shown that zoonotic transmission is also one of the sources of HEV spread. Pork meat, which is highly appreciated by consumers because of its taste, is increasingly becoming part of the eating habits of most Togolese, especially those living in the northern part of the country. Pork butchers find it a great opportunity to increase their income. Unfortunately, the activity is unstructured and poorly organized, so that pigs are slaughtered in makeshift slaughterhouses without any health regulations. We observed that the pork butchery attracts mostly males, 84 (94.4%) who reside in the outskirts of towns 74 (83.1%) with a secondary education level 46 (51.7%). This result was similar to that reported in Benin where 92.8% were male [15] and in Chad where 77% were educated [16]. These data suggest that, like any activity requiring a great deal of physical effort, men were the most solicited and the essential link in this economic activity.
In the previous study, the anti-IgM and IgG seroprevalence of HEV in the general population in 2023 was 11.7% and 5.6% respectively. In the present study, the anti-IgM HEV seroprevalence was 20.22% with ratio (OR: 2; p= 0.202), and that of IgG was 5.6%. These data suggested that HEV circulates more actively in this population group of swine butchers and they had twice the chance of contracting the virus. These results could be explained by the lack of hygiene around this activity, handling meat, organs and feces of pigs without protection. The high IgM to IgG ratio in these studies may suggest that there are several foci of transmission with a higher frequency of new infections or that those tested had associated autoimmune diseases or had malaria at the time of testing with a possible interaction. The difference could be due to the high sensitivity of the anti IgM kits, the versions of the tests to detect low concentrations of IgG, but also to differences in the type of IgG antibodies present in the sera [17]. Five (5; 27.7%) were positive for antibodies to Plasmodium falciparum out of 18 positives for HEV anti-IgM. As some studies have shown, antibodies to Plasmodium Ag may interact nonspecifically with antigens of other agents such as HEV that could test positive, resulting in false positives to anti HEV. Thus, this false positive rate could abnormally increase the seroprevalence of HEV immunoglobulin. Certainly, a study with a higher number of samples could help to understand this difference. In Burkina Traoré et al. (2015) reported a similar seroprevalence which was 1% for anti-IgM and 76% for total anti-HEV antibodies in the population of pig butchers. This reflects that HEV is endemic and circulating in this population. The seropositivity for gender was 20.2% (95% CI: 19.4 - 21.0) in men and 20.0% (95% CI: 6.0 - 34.0) in women, this result translates that they were exposed to the same source of contamination and were unaware of the hygiene rules. Many studies suggest that zoonotic transmission of HEV may occur through frequent contact with biological samples (feces, blood) and organs of HEV-infected animals [18]. In agreement with many similar studies in the world, we have observed high seroprevalences in pork butchers who have direct contact with pigs or pork meat [19-21]. Comparing this study to other studies conducted in other countries of the sub-region, such as Burkina Faso [9], the seroprevalence observed among butchers was higher than that observed in the general population. The data from these studies suggested that zoonotic contamination could be one of the main sources of HEV infection; however, our data suggest that in Togo, in addition to the zoonotic source of contamination, there are other reservoirs of contamination that are totally unknown and that fuel the spread of infection. Although in this study no risk factors such as gender, age, education, area of residence, number of years in business, husbandry practices and hygiene were associated with anti-IgM and IgG HEV seroprevalences, studies have reported that they may constitute significant risks in the contamination and spread of HEV [22]. In addition, the seroprevalence of total anti-HEV antibodies observed in pigs at threshing age was 80.11%. This result suggests that HEV is endemic in pig populations in Togo. This result was similar to those observed in Ghana 62.4% [23] and Burkina 80% [9], which could be a source of contamination and spread of HEV for professionals in the sector [10,22]. In addition, the study revealed similar seroprevalences according to the age of the pigs which were 80%, 78% and 82% respectively in 3-6 months, 6-12 months and over 12 months of age. This suggests that, pigs at slaughter age could be at the same risk of HEV contamination.
Limitations of this study
This study has limitations. The study did not allow the detection of HEV RNA, and the characterization of HEV genotypes circulating in the pig and pork butcher population was not performed. The genotypic characterization of HEV to be determined in pigs and humans will provide a more accurate picture of the zoonotic transmission of this virus.
Conclusion
This study showed the presence of HEV antibodies in the serum samples from butchers and pigs slaughtered for consumption, and could reflect that they were in contact with HEV. The relatively low IgG seroprevalence in the butchers reflects a very low endemic status of the infection that is not different from the general population which is in contrast to the observed situation in Burkina. We did not know if this was might be due to a lack of sensitivity or the current test compared to the old test used in Burkina by our group. Whatever the case, however, the high seroprevalence of IgM might reflects a major risk of HEV transmission in this population and a largely changing status. In addition, the high seroprevalence of total antibodies observed in pigs confirms their HEV reservoir characteristics and their potential role as a source of contamination and virus propagation. Further studies are needed to characterize the circulating genotypes in pork butchers and pigs in order to establish the link between them and to identify other factors fueling the HEV status in the human population.
Consent to Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Funding
This study was financed by the Directorate of Scholarships and Training of the Togolese State.
Author Contributions
KVMS, KAT, JBO, ET, PR, SDK, and NB participated in the study design. KVMV, BKN, JA, GK and KAT performed serological analyses, KVM, KAT, BLO, and PR performed the statistical analysis, and all authors participated in drafting and revising the manuscript. All authors read and approved the final version.
Acknowledgements
Not applicable.
Ethical Approval
The study was approved by the Bioethics Committee for Health Research of the Ministry of Health and Public Hygiene of Togo under No.: 040/2019/CBRS, and furthermore the study protocol complies with the ethical guidelines of the 1975 Declaration of Helsinki, as evidenced by the approval of the Ministry of Health and Public Hygiene (Togo) under No.: 027/2020/MSHP/CAB/SG/DGAS/DPML/CBRS. The administrative authorization was also obtained from the Polyclinic and the Regional Blood Transfusion Center of Sokodé. Informed consent was obtained from all study participants after they were informed of the project.
Availability of Data and Materials
Not applicable.
References
- Purdy MA, Harrison TJ, Jameel S, Meng X-J, Okamoto H, Van der Poel WHM, et al. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol 98 (2017): 2645–2646.
- Roque-Afonso Anne-Marie. Hépatite E. CNR des virus des hépatites à transmission entérique, Virologie, INSERM U1193, Hôpital Paul Brousse, 12 avenue Paul Vaillant-Couturier (2016): 94804.
- Kamar N, Izopet J, Pavio N, Aggarwal R, Labrique A, Wedemeyer H, et al. Hepatitis E virus infection. Nat Rev Dis. Primer 3 (2017): 17086.
- Anheyer-Behmenburg HE, Szabo K, Schotte U, Binder A, Klein G, Johne R. Hepatitis E Virus in Wild Boars and Spillover Infection in Red and Roe Deer, Germany, 2013–2015. Emerg. Infect Dis 23 (2017): 130–133.
- Drobeniuc J, Greene-Montfort T, Le N-T, Mixson-Hayden TR, Ganova-Raeva L, Dong C, et al. Laboratory-based Surveillance for Hepatitis E Virus Infection, United States, 2005–2012. Emerg Infect Dis 19 (2013): 218–222.
- Woo PCY, Lau SKP, Teng JLL, Cao K-Y, Wernery U, Schountz T, et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis 22 (2016): 2219–2221.
- Wu J, Si F, Jiang C, Li T, Jin M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes 50 (2015): 410-417.
- Spahr C, Knauf-Witzens T, Vahlenkamp T, Ulrich RG and Johne R. Review article Hepatitis E virus and related viruses in wild, domestic and zoo animals. Zoonoses Public Health 65 (2017): 11–29.
- Traoré KA, Ouoba JB, Huot N, Rogée S, Dumarest M, Traoré AS, et al. Hepatitis E Virus Exposure is Increased in Pork Butchers from Burkina Faso Am. J. Trop. Med. Hyg 93 (2015): 1356–1359.
- Crossan C, Grierson S, Thomson J, Ward A, Nunez-Garcia J, Banks M, et al. Prevalence of hepatitis E virus in slaughter-age pigs in Scotland. Epidemiol. Infect 143 (2015): 2237–2240.
- Bouwknegt M, Frankena K, Rutjes SA, Wellenberg GJ, de Husman AMR, van der Poel WHM, et al. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet Res 39 (2008):1.
- Krog JS, Larsen LE, Breum SØ. Tracing Hepatitis E Virus in Pigs from Birth to Slaughter. Front Vet Sci (2019): 6.
- Osundare FA, Klink P, Majer C, Akanbi OA, Wang B, Faber M, et al. Hepatitis E Virus Seroprevalence and Associated Risk Factors in Apparently Healthy Individuals from Osun State, Nigeria. Pathogens 9 (2020): 392.
- Setondji Komi Victor-Mari, Traoré Kuan Abdoulaye, Ouoba Jean-Bienvenue, Taale Essodolom, Ouoba Bruno Lalidia, Nyakou Bissah Kokou, et al. Seroprevalence of hepatitis E virus (HEV) antibodies in human populations of Sokodé, Togo. Archives of Clinical and Biomedical Research 7 (2023): 36-44.
- Djimènou D, Adoligbé CM, Mensah SEP, Aboh AB, Edénnakpo AK, Atchadé TGS, et al. Transformation et commercialisation de la viande de porcs au Sud du Bénin: Caractéristiques socioéconomiques et contraintes. Journal of Animal & Plant Sciences 48 (2021): 8618-8636
- Mopate YL, Koussou MO, Kabore ZCY, Gouro A. Commerce et consommation de viande porcine dans la zone de N’Djaména (Tchad). Revue sénégalaise des recherches agricoles et agroalimentaires 1 (2006): 39-48.
- Avellon A, Morago L, Garcia-Galera M del C, Munoz M, and Echevarr J-M. Comparative Sensitivity of Commercial Tests for Hepatitis E Genotype 3 Virus Antibody Detection. Journal of Medical Virology 87 (2015): 1934–1939.
- Temmam S, Besnard L, Andriamandimby SF, Foray C, Rasamoelina-Andriamanivo H, Heraud JM, et al. High prevalence of hepatitis E in humans and pigs and evidence of genotype-3 virus in swine, Madagascar. Am J Trop Med Hyg 88 (2013): 329–338.
- Krumbholz A, Mohn U, Lange J, Motz M, Wenzel JJ, Jilg W, et al. Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to pigs. Med Microbiol Immunol (Berl) 201 (2012): 239–244.
- Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res 41 (2010): 46
- Adjei AA, Aviyase JT, Tettey Y, Adu-Gyamfi C, Mingle JA, Ayeh-Kumi PF. Hepatitis E virus infection among pig handlers in Accra, Ghana. East Afr Med J 86 (2009): 359–363.
- Takova k, Koynarski T, Minkov I, Ivanova Z, Tonéva V, Zahmanova G. Augmentation de la séroprévalence du virus de l’hépatite E chez les porcs domestiques et les sangliers en Bulgarie. J. animaux 10 (2020).
- Bagulo H, Majekodunmi AO, Welburn SC, Bimi L. Hepatitis E seroprevalence andrisk factors inhumans andpig inGhana. BMC Infectious Diseases 22 (2022): 132.
- Institut National de la Statistique et des Etudes Economiques et Démographiques (INSEED) (2023).