Heart Shock Protein Beta-1 Promotes Doxorubicin-Induced Hl-1 Cardiomyocyte Pyroptosis By Inhibiting Apoptosis Antagonizing Transcription Factor
Article Information
Yao Tang1, Yaxiu Liu1, Hui Fu2, Shuang Fu1, Xinbin Zheng1, and Deling Yin1,3,4*
1Xiangya School of Pharmacy, Central South University, Changsha, Hunan 410013, China.
2 Science and Technology Center, Fenyang College, Shanxi Medical University, Fenyang, Shanxi 032200, China.
3 Department of Cardiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009, China.
4 Cardiovascular Key Laboratory of Zhejiang Province, Hangzhou, Zhejiang 310009, China.
*Corresponding author: Deling Yin. Xiangya School of Pharmacy, Central South University, Changsha, Hunan 410013, China, Department of Cardiology. The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009, China. Cardiovascular Key Laboratory of Zhejiang Province, Hangzhou, Zhejiang 310009, China.
Received: 13 October 2023; Accepted: 21 October 2023; Published: 09 January 2024.
Citation: Yao Tang, Yaxiu Liu, Hui Fu, Shuang Fu, Xinbin Zheng and Deling Yin. Heart Shock Protein Beta-1 Promotes Doxorubicin-Induced Hl-1 Cardiomyocyte Pyroptosis By Inhibiting Apoptosis Antagonizing Transcription Factor. Cardiology and Cardiovascular Medicine. 8 (2024): 15-24.
View / Download Pdf Share at FacebookAbstract
Doxorubicin (DOX) is a treatment for many types of cancer that works well. Long-term use of DOX is limited because of possible risks, such as cardiotoxicity. A lot of research has been done on the ways in which DOX causes cardiotoxicity, but more research is still needed. The role of heat shock proteins (HSPs) in apoptosis and pyroptosis in cells has just been discovered. Doxorubicin makes cardiomyocytes go through pyroptosis, but it is not known if heat shock protein beta-1 (HSPB1), a member of the HSPs family, plays a role in this process. We looked at the effects of apoptosis antagonizing transcription factor (AATF) and heat shock protein beta-1 (HSPB1) on HL-1 cardiomyocyte pyroptosis caused by 5 μM DOX for 9 hours. When cardiomyocytes are treated with DOX, HSPB1 levels go up a lot while AATF levels go down a lot. When HSPB1 was turned off or AATF was turned up, it inhibited DOX from causing pyroptosis in cardiomyocytes. There is a chance that stopping HSPB1, raising AATF, or both could make DOX less harmful to the heart. Our results show that the HSPB1-AATF-caspase-3-GSDME pathway controls cardiomyocyte pyroptosis in response to DOX treatment in a way that wasn't known before. This suggests that HSPB1-AATF-dependent pyroptosis may be a good way to treat DOX-induced cardiotoxicity.
Keywords
Heart Shock Protein Beta-1; Apoptosis Antagonizing Transcription Factor; Pyroptosis; Doxorubicin; Cardiomyocyte
Heart Shock Protein Beta-1 articles; Apoptosis Antagonizing Transcription Factor articles; Pyroptosis articles; Doxorubicin articles; Cardiomyocyte articles
Article Details
1. Introduction
The significant cardiotoxicity, encephalotoxicity, and nephrotoxicity of the anthracycline cancer drug doxorubicin (DOX) put limits on how well it can be used in the clinic [1, 2]. When DOX is used to treat cancer, it can cause problems with the ECG, irreversible degenerative cardiomyopathy, irregular heartbeats, and congestive heart failure [3, 4]. Doxorubicin causes cardiotoxicity through multiple pathogenic mechanisms, such as reactive oxygen species (ROS), ROS-associated autophagy, peroxynitrite-associated necrosis, lipid peroxidation-associated ferroptosis, DNA damage, cytokine release or regulation, mitochondrial dysfunction, activation of apoptosis/necroptosis/pyroptotic signaling cascades, and inflammatory responses [4, 5]. More research is needed to figure out what causes DOX to be harmful to the heart. Pyroptosis is a rare type of programmed cell death that is marked by enlarged cells, bubbles of plasma, and the death of the cells [6, 7]. In addition to cytokines and other molecules that send signals, pyroptosis may also release intracellular compounds and substances that cause inflammation. Pyroptosis may depend on the executioner protein gasdermin D (GSDMD), which is cut by caspases 11, 4, and 5 in response to LPS and inflammatory mediators [8]. Also, pyroptosis may be caused by the activation of caspase-3 (CASP3) and the subsequent cutting of Gasdermin E (GSDME) [9]. CASP3 activates GSDME-mediated pyroptosis, contributing to the toxicity of chemotherapy in mice [10]. We don't know how DOX causes pyroptosis through the GSDME-mediated pyroptosis pathway, but we recently showed that 5 μM DOX increases pyroptosis in HL-1 cardiomyocytes through the Bnip3/CASP3/GSDME pathway [11]. The exact way that DOX makes cardiomyocytes go through pyroptosis is not known, though. Heat shock protein beta-1 (HSPB1) is a member of the mammalian small heat shock protein (sHSP) family. It is also called HSP25 in rodents and HSP27 in people [12]. Skeletal, smooth, and cardiac muscle have the most HSPB1 out of all the normal human tissues 13]. Heat shock, oxidative stress, cytokines, growth factors [12, 14], myocardial infarction [15], and heart failure [16] have all been shown to activate HSPB1, and studies have shown that this protein helps with both anti-apoptosis and autophagy. The work of Guo et al. [17] suggests, on the other hand, that CASP3 may help HSPB1 act as a pro-apoptotic mediator. So, no one knows if HSPB1 helps cells die or hurts them. Combinations of anticancer drugs that include HSPB1 inhibitors have been shown to make tumors much less sensitive to the drugs [18]. This suggests that HSPB1 may not always protect cells from dying because of chemotherapy. But it is still not clear if HSPB1 has anything to do with the pyroptosis that DOX makes happen in cardiomyocytes.
AATF, which stands for "apoptosis-antagonizing transcription factor," is a protein that is found in all eukaryotes and has many different functions [19]. RNA polymerase II's partner, Che-1, is another name for this protein. By stopping the cell cycle, causing autophagy, repairing DNA, and stopping apoptosis, AATF may promote cell growth or stop apoptosis to keep more cells alive [19, 20]. Wang et al. [21] found that AATF might help control how quickly heart cells die after myocardial ischemia is fixed. Since HSPB1 has been shown to stop E2F-4 expression in human lung fibroblast cells [23] and AATF has been linked to the transcription of E2F target genes and cell death in anticancer drugs [22], it is tempting to think that both proteins play a role in the DOX-induced pyroptosis of cardiomyocytes. The goal of this study was to see if reducing the amount of HSPB1 expression or increasing the amount of AATF expression in HL-1 cardiomyocytes could stop the pyroptosis caused by DOX. So, the lower expression of HSPB1 or AATF that we saw after DOX exposure could be a new therapeutic target for preventing DOX-caused damage to the heart.
2. Materials and Methods
2.1 Cell culture
HL-1 cardiomyocytes came from the ATCC. They were grown in DMEM medium (Gibco, USA) with 10% fetal bovine serum (FBS) (Gibco, USA). As described in earlier reports [11], it was kept at 37 degrees Celsius in a humid environment with 5% CO2.
2.2 Cell viability assay
Five thousand HL-1 cells were planted in 96-well culture plates. After 9 hours, 5 μM DOX was added to see what effect it would have on the cells' ability to live. The cells' ability to live was then tested with a cell counting kit-8 assay kit (CCK8, Bimake, China), following the instructions from the manufacturer. We used an InfiniteTM M200 Microplate reader (Tecan, Mannedorf, Switzerland) to check the OD at 450 nm [24].
2.3 LDH release assay
We used a commercial test kit (Beyotime, Shanghai, China) that was made for this purpose to find out how much of this enzyme was being released. After the HL-1 cells were treated with 5 μM DOX for 9 hours, the supernatant was taken out and 120 l was added to a 96-well plate, with 60 l going into each well. After 30 minutes of incubation at room temperature and in the dark, we measured the absorbance at 490 nm [25].
2.4 Microscopy imaging
Cells were grown in six-well plates and treated for 9 hours with 5 μM DOX before their shape was looked at. As an added bonus, bright field images were taken with a Nikon TE2000 microscope (Nikon, Japan) [11].
2.5 Cell transfection
SiRNAs that target HSPB1 (si-HSPB1), AATF (si-AATF), and a negative control (si-Ctrl) were put into HL-1 cells that were 30% to 50% full (Wuhan, China). For example, we were able to get AATF plasmids from Youbio (Changsha, China). To make the AATF expression vector, pcDNA3.1-AATF, the full wild-type AATF encoding sequence was cloned into pcDNA3.1. As a negative control, we used empty vectors to put into cells. When the HL-1 cells were between 60% and 70% full, plasmids were added. HL-1 cells were planted in six-well plates the day before transfection [26] to get ready for the process. We put DNA into HL-1 cells by using Lipofectamine 2000 reagent as the manufacturer told us to (Invitrogen, Carlsbad, CA, USA). DOX was added to the cells 48 hours after transfection.
2.6 Co-immunoprecipitation
The ice-cold IP lysis solution from Thermo Fisher Scientific in the United States was used to get lysates out of HL-1 cells. The lysate was put into a microcentrifuge tube and spun for 10 minutes at 2500 rpm. After the protein concentration was found, the supernatant was put into a new microcentrifuge tube. To sum up, protein A/G PLUS-Agarose (Santacruz Biotechnology) was mixed with lysate and target antibody, CA, USA) over a period of 24 hours at a temperature of 4°C [27]. After allowing the combination to settle for a full 24 hours, the following day it was centrifuged at 2500 rpm for ten minutes, and the complex rinsed in phosphate buffer solution. We carried out this procedure a minimum of three times, each time using anti-AATF antibody as a decoy to capture HSPB1 protein and normal IgG (Catalog #5946, Cell Signaling Technology, USA) as a control. In order to provide positive controls, lysates taken from control cells and cells that had been treated with DOX were subjected to the same processing as the Co-IP samples (Input). In order to conduct an investigation of the co-immunoprecipitated protein using Western blotting, the AATF antibody was used.
2.7 Western blot analysis
Before doing Western blotting, RIPA lysates that were treated with phosphatase and protease inhibitors led to the cleavage of HL-1 cardiomyocytes. The Bradford assay was used to tally up the amount of protein present in the sample. After separating thirty grams of protein on SDS-polyacrylamide gels with a concentration of 12%, the protein was electroblotted onto Immobilon® PVDF membranes (Merck KGaA, Darmstadt, Germany). After being blocked for a total of 1.5 hours at room temperature with skim milk containing 5% fat, the membranes were then subjected to an overnight treatment with the antibody at a temperature of 4 degrees Celsius. After that, the membrane was treated with a secondary antibody that had been conjugated with horseradish peroxidase for two hours at room temperature [28]. This secondary antibody was goat anti-rabbit IgG from Proteintech in China. During the development process, chemiluminescence (ECL) signals were continuously monitored and analyzed using Image LabTM (Cwbio, Beijing, China). Tubulin is a protein that has the ability to properly control loads. Antibodies used for Western blotting are detailed in Table 1.
Co-immunoprecipitation and Western blotting may be carried out using any of the antibodies that are shown in Table 1.
Table 1: Antibodies used for Co-IP/Western blot
|
Name |
Description |
Manufacturer |
|
Anti-Tubulin |
Rabbit polyclonal, 55 kDa |
Proteintech (11224-1-AP) |
|
Anti-GSDME |
Rabbit monoclonal, 55; 34 kDa |
Abcam (ab215191) |
|
Anti-Pro-CASP3 |
Rabbit polyclonal, 35 kDa |
CST (9662S) |
|
Anti-Cleaved-CASP3 |
Rabbit monoclonal, 17 kDa |
CST (9664S) |
|
Anti-HSPB1 |
Rabbit monoclonal, 25kDa |
Abcam (ab155987) |
|
Anti-AATF |
Rabbit monoclonal, 70 kDa |
Abcam (ab233546) |
2.8 Statistical analysis
GraphPad Prism 6 was used throughout the process of doing the statistical analysis. The results are shown as the mean as well as the standard deviation of at least three different experiments. When comparing the means of two different groups, we used the Student's t test with two tails, and when comparing the means of multiple different groups, we used the one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. In order for the P-value to have statistical significance, it must be lower than 0.05.
3. Results:
3.1 In the wake of DOX-induced pyroptosis of cardiomyocytes, HSPB1 expression is shown to be increased.
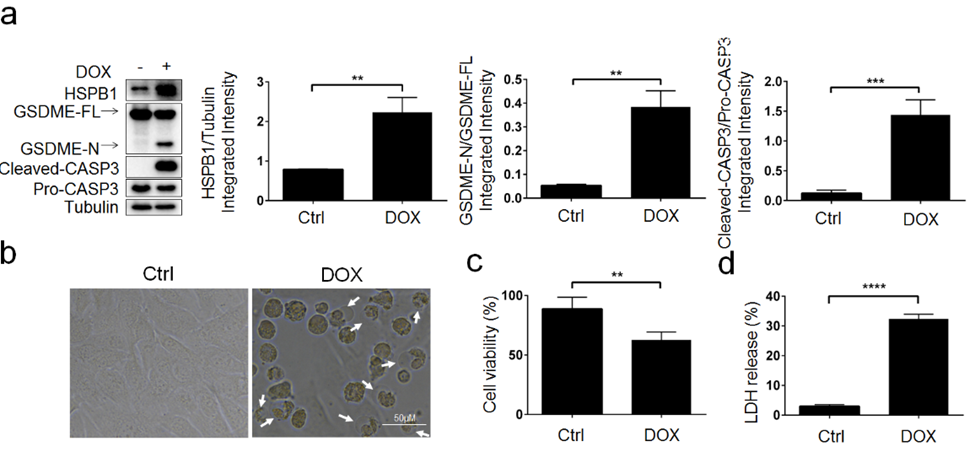
To begin, we investigated the effect that DOX had on the expression of HSPB1 in HL-1 cardiomyocytes so that we could determine whether or not this protein is involved in the DOX-induced pyroptosis. On the other hand, DOX markedly elevated the expression of HSPB1, GSDME-N, and cleaved-CASP3 in HL-1 cardiomyocytes, as shown by Western blotting (P<0.01, P<0.01, and P<0.001, respectively) (Figure 1a). The pyroptotic shape (Figure 1b) of HL-1 cardiomyocytes, lower cell viability (Figure 1c, P<0.01), and greater LDH release (Figure 1d, P<0.0001) are examples of evidence that support the hypothesis that HSPB1 plays a role in the DOX-induced cardiac mortality.
Figure 1: HSPB1 is up-regulated in cardiomyocyte pyroptosis induced by DOX. HL-1 cells were treated with doxorubicin (DOX) at 5 μM for 9 h to assess: a The expression of HSPB1, GSDME-N, and Cleaved-CASP3 was examined by Western blot analysis (N=3). b Representative microscopic images. White arrowheads indicate pyroptotic cells. c Cell viability(N=3). d The LDH content (N=3); **P< 0.01, ***P< 0.001, ****P< 0.0001 compared with indicated groups.
3.2 It is required for doxorubicin to interact with HSPB1 in order to trigger pyroptosis in cardiomyocytes.
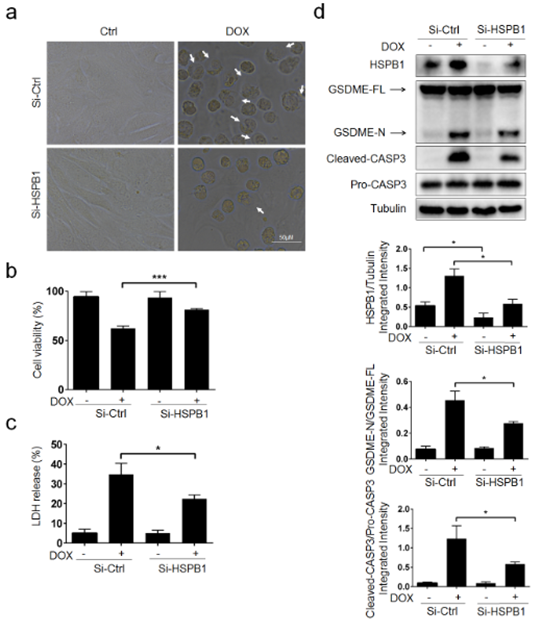
Because of this, we decided to use small interfering RNA targeting HSPB1 (Si-HSPB1) in HL-1 cardiomyocytes to investigate whether or not DOX stimulates pyroptosis through HSPB1. We discovered that DOX-treated cells exhibited reduced pyroptotic cell death (Figure 2a, P<0.001), were more viable (Figure 2b, P<0.001), and emitted less lactate dehydrogenase (LDH) when HSPB1 was inhibited using siRNA. These results were statistically significant (Figure 2c, P<0.05). Additionally, Si-HSPB1 had a strong inhibitory effect on the caspase-3 cleavage produced by GSDME-N and DOX (Figure 2d). In conclusion, we have come to the realization that HSPB1 is essential for the pyroptosis that is caused by DOX in cardiomyocytes.
Figure 2: HSPB1 is required for DOX-induced pyroptosis in HL-1 cardiomyocytes. HL-1 cells were pretreated with Si-HSPB1 (HSPB1 siRNA) or NC (negative control) for 48 h, followed DOX (5 μM) treatment for 9 h to determine: a Representative microscopic images. White arrowheads indicate pyroptotic cells. b Cell viability (N=3). c The LDH content (N=3). d The protein levels of HSPB1, GSDME-N, Cleaved-CASP3 were examined by Western blot analysis (N=3); *P < 0.05, ***P< 0.001 compared with indicated groups.
3.3 After DOX has been delivered, there is a possibility that HSPB1 will interact with AATF inside cardiomyocytes.
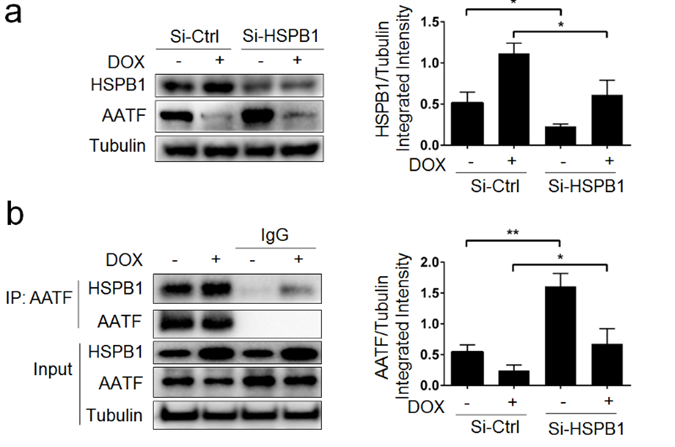
Some people, as a consequence of the DOX therapy, have hypothesized that HSPB1 and AATF are linked to one another by means of a third party [22, 23]. Additional research was done to look at the possibility that DOX-induced HSPB1 may interact with AATF. As can be observed in Figure 3a, treatment with doxorubicin resulted in a significant reduction in AATF expression. Following administration of DOX, suppression of HSPB1 by Si resulted in dramatically increased levels of AATF expression in comparison to Si-Ctrl (Figure 3a, P<0.05). After treatment with DOX, it was shown by a co-immunoprecipitation research that HSPB1 has the potential to bind to AATF (Figure Following treatment with DOX, we reach the conclusion that HSPB1 and AATF may interact in cardiomyocytes.
Figure 3: HSPB1 interacts with AATF in cardiomyocytes after DOX treatment. HL-1 cells were pretreated with Si-HSPB1 or NC for 48 h, followed DOX (5 μM) treatment for 9 h. a The protein levels of HSPB1 and AATF were examined by Western blot analysis (N=3). b The expression of HSPB1 and AATF was examined by Co-immunoprecipitation (N=3); *P < 0.05, **P< 0.01 compared with indicated groups.
3.4 It is possible that AATF promotes cardiomyocyte pyroptosis by acting as a mediator for DOX and HSPB1.
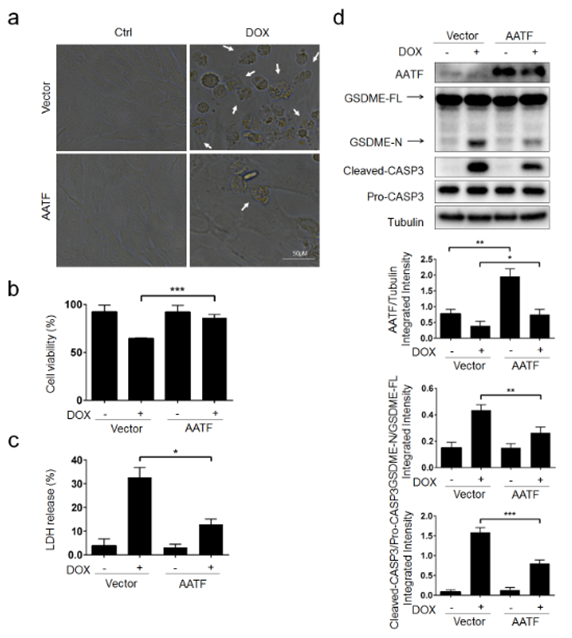
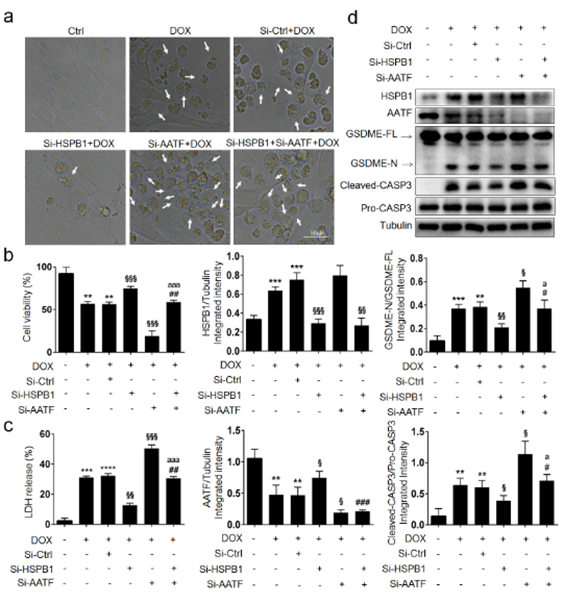
Our findings offer the first evidence that directly demonstrates HSPB1's role in regulating AATF expression (Figure 3a). It is speculated that there is a regulatory relationship between HSPB1 and AATF, however this has not been shown. After a reduction in HSPB1 and AATF expression, we treated HL-1 cardiomyocytes with DOX to study the function of AATF in DOX/HSPB1-induced pyroptosis. This was done in order to determine whether or not pyroptosis is caused by DOX or HSPB1. Cell viability (Figure 5b, P<0.01), pyroptotic cell count (Figure 5a), and LDH release (Figure 5c) were all very comparable between the Si-HSPB1+Si-AATF+ and DOX groups (Figure 5c, P<0.01). In comparison to the other two groups, the Si-Ctrl + DOX group demonstrated these distinct distinctions. Inhibition of AATF had no impact on the expression of HSPB1, while downregulation of HSPB1 increased AATF expression (Figure 5 d, P>0.05). This suggests that HSPB1 may have a negative influence on AATF. However, HSPB1 downregulation did not increase AATF expression. During DOX-induced pyroptosis in cardiomycytes, the activation of AATF is prevented from occurring thanks to HSPB1's actions. Inhibiting AATF, HSPB1 may increase pyroptosis.
Figure 4: DOX-induced cardiomyocyte pyroptosis is through AATF. HL-1 cells were transfected with AATF plasmid (AATF) or empty vector for 48 h, followed DOX (5 μM) treatment for 9 h to assess: a Representative microscopic images. White arrowheads indicate pyroptotic cells. b Cell viability (N=3). c The LDH content (N=3). d The protein levels AATF, GSDME-N, Cleaved-CASP3 were examined by Western blot analysis (N=3); *P < 0.05, **P< 0.01, ***P< 0.001 compared with indicated groups.
3.5 AATF may hold the DOX/HSPB1-induced pyroptosis of cardiomyocytes.
There is now evidence that HSPB1 controls AATF expression, as we have demonstrated (Figure 3a) . There may be a regulatory relationship between HSPB1 and AATF, but we cannot be certain. In order to evaluate the role of AATF in DOX/HSPB1-induced pyroptosis, we first downregulated HSPB1 and AATF expression and then treated HL-1 cardiomyocytes with DOX. Number of pyroptotic cells (Figure 5a), cell viability (Figure 5b, P<0.01), and LDH release (Figure 5c) were all similar between the Si-HSPB1+Si-AATF+ group and the DOX group (Figure 5c, P<0.01). These variances appeared when the Si-Ctrl + DOX group was compared to the other two. Notably, HSPB1 expression was unaffected by AATF inhibition, but HSPB1 inhibition enhanced AATF expression (Figure 5d, P>0.05), indicating that HSPB1 may adversely impact AATF. As a consequence, HSPB1 reduces AATF during DOX-induced pyroptosis in cardiomycytes. Therefore, HSPB1 may increase pyroptosis by blocking AATF.
Figure 5: Inhibition of HSPB1 and AATF restores the DOX-induced cardiomyocyte pyroptosis. HL-1 cells were transfected with Si-HSPB1, Si-AATF (AATF siRNA) and NC for 48 h, followed DOX (5 μM) treatment for 9 h to examine: a Representative microscopic images. White arrowheads indicate pyroptotic cells. b Cell viability (N=3). c The LDH content (N=3). d The expression of HSPB1, AATF, GSDME-N and Cleaved-CASP3 was examined by Western blot analysis (N=3); **P<0.01, ***P<0.001, ****P<0.0001 compared with control; §P< 0.05, §§P< 0.01, §§§P< 0.001 compared with DOX+NC; #P< 0.05, ##P< 0.01,###P< 0.001 compared with DOX+Si-HSPB1; aP < 0.05, aaaP < 0.001 compared with DOX+Si-AATF.
4. Discussion
Evidence from the past shows that enhancing the therapeutic effectiveness of DOX needs removing or lowering its cardiotoxicity [29, 30]. DOX, an anthracycline antibiotic, is commonly employed in frontline anti-tumor therapy owing to its effectiveness against a broad spectrum of human malignancies. This covers not only solid tumors but also Hodgkin's disease, breast cancer, acute lymphoblastic leukemia, juvenile leukemia, and lung cancer. Metastatic cancer [31]. DOX-induced cardiotoxicity, which is linked with poor results, restricts its usage in the treatment of malignant tumors. The anti-cancer medication DOX has widespread use [32]. DOX-induced cardiotoxicity manifests in two distinct time periods: the acute phase and the chronic phase. Chronic cardiac toxicity is the first kind and it often manifests itself a year after the toxicity of chemotherapy has subsided, leading to heart failure. The second kind, chronic delayed cardiotoxicity, often manifests itself long after chemotherapy treatment has ended (sometimes decades later) [33]. Finding a strategy to lessen DOX-induced cardiotoxicity while raising DOX effectiveness remains an unmet need.
Recent investigations have linked DOX-induced pyroptosis in the development of DOX-induced cardiomyopathy [5]. There are six unique forms of cardiomyocyte death (apoptosis, autophagy, necrosis, necroptosis, pyroptosis, and ferroptosis). Over the last several years, researchers have revealed many pathogenic pathways of DOX cardiotoxicity [34, 35]. DNA damage, cytokine release or regulation, mitochondrial malfunction, activation of the apoptotic/necrotic cascade, and pyroptosis are all examples of these. Cells undergo pyroptosis, or programmed death, in response to harmful stimuli. Inflammation is characterized by the proliferation and death of cells as well as the release of inflammatory mediators including interleukin-1 and interleukin-18. Activation of GSDMD is controlled by one of four caspases (CASP1, CASP4, or CASP11), whereas activation of GSDME is controlled by caspase 3 [36]. In this investigation, we discovered that 9 hours of treatment with DOX (5 μM) led to a substantial increase in the expression of cleaved-pyroptoic-associated proteins and a considerable number of cells with altered shape (cell membrane blebbing) compared to the control group (Cleaved-CASP3 and GSDME-N). Our prior research [28] established that DOX promotes cardiomyocyte pyroptosis through activation of the CASP3/GSDME pathway. Even while DOX is known to trigger pyroptosis in cardiomyocytes, the precise chemical mechanism by which this occurs is still unclear. Therefore, we postulate that inhibiting pyroptosis may help reduce DOX-induced cardiac injury.
Based on their molecular weights, humans have discovered 97 different heart shock proteins (HSPs) [37]. By attaching to partly denatured proteins and preventing them from permanently aggregating, small HSPs may function as molecular chaperones to maintain protein homeostasis in healthy cells [38]. Increased levels of heat shock proteins like HSP90 have been linked to cell death processes such apoptosis in hepatoma cells, autophagy in osteosarcoma cells, and pyroptosis in THP-1 cells [39-43]. HSPB1's status as an HSPs has been established [14], and its involvement in apoptosis and autophagy has been shown. There is fresh insight into the effects of HSPB1 through CASP3 activation, as suggested by the idea that HSPB1 assists in CASP3-associated apoptosis and tumor treatment [17, 18]. Even while HSPB1 interacts with a wide variety of proteins involved in pyroptosis, including CASP3, its precise role in this process is still unclear. Due to our interest in the function of HSPB1 in DOX-induced pyroptosis, we downregulated its expression in HL-1 cardiomyocytes. Increased HSPB1 expression in cardiomyocytes may attenuate the pyroptotic cleavage caused by CASP3, GSDME, and DOX. The apoptosis antagonizing transcription factor (AATF) is a transcription factor that stimulates the transcription of many genes involved in controlling gene expression. AATF prevents apoptosis, causes cell cycle arrest, autophagy, and DNA repair, and may be protective because of these effects [19, 20]. According to Welker et al. [44], AATF may prevent apoptosis and enhance cell proliferation, making it a crucial molecule in Kras-induced lung cancer. There may be a connection between HSPB1 and AATF, since both molecules interact with many E2F family members [22, 23]. Even though AATF's role in cell pyrolysis has not been proven, we found evidence suggesting a connection between HSPB1 and this transcription factor. Crucially, the research showed that AATF prevented cardiomyocytes from undergoing pyroptosis in response to DOX. Our Western blot and Co-IP data also hints to a possible connection between HSPB1 and AATF, albeit the nature of this interaction has to be clarified in future research. Our results demonstrate for the first time that HSPB1 may augment DOX-induced cardiomyocyte pyroptosis. Furthermore, we demonstrate that AATF possesses anti-pyroptotic characteristics and that HSPB1 may inhibit AATF after DOX treatment.
Given that HSPB1 has been shown to both inhibit AATF and enhance DOX-induced cardiomyocyte pyroptosis, it is tempting to consider it as a therapeutic target.
Acknowledgements
We would like to thank all the colleagues in our research team for technical support.
Funding
This research was supported by the National Natural Science Foundation of China Grants 82170242 and 81570454 (to D.Y.).
Conflicts of interest
There are no actual or potential conflicts of interest in their respective fields of expertise.
Availability of data and materials
This published research has all of the necessary data.
Authorial assistance
In the manuscript, YT is the one who really performs the experiments that he and DY devised. YT is in charge of all the statistical analysis and graphing. The original draft was written by YT, and DY revised it. The final manuscript has been reviewed and approved by all writers.
Ethical review board clearance and informed consent.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Publishing is not restricted.
Author details
Xiangya School of Pharmacy, Central South University, Changsha 410008, Hunan, China. Department of Cardiology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Cardiovascular Key Laboratory of Zhejiang Province, Hangzhou 310009, China.
Abbreviations:
DOX (doxorubicin), HSPB1 (Heat shock protein beta-1), AATF (apoptosis antagonizing transcription factor), GSDME (gasdermin E), Cleaved-CASP3 (cleaved-caspase 3).
References
- Prathumsap N, Shinlapawittayatorn K, Chattipakorn SC, et al. Effects of doxorubicin on the heart: From molecular mechanisms to intervention strategies. European journal of pharmacology 866 (2020): 172818.
- Longhi A, Ferrari S, Bacci G, et al, S. Long-term follow-up of patients with doxorubicin-induced cardiac toxicity after chemotherapy for osteosarcoma. Anti-cancer drugs 18 (2007): 737-744.
- Fernandez-Chas M, Curtis MJ, Niederer SA. Mechanism of doxorubicin cardiotoxicity evaluated by integrating multiple molecular effects into a biophysical model. British journal of pharmacology 175 (2018): 763-781.
- Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Progress in cardiovascular diseases 49 (2007): 330-352.
- Ma W, Wei S, Zhang B, et al. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Frontiers in cell and developmental biology 8 (2020): 434.
- Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunological reviews 265 (2015): 130-142.
- Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends in biochemical sciences 42 (2017): 245-254.
- Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 18 (2020): 941-955.
- Rogers C, Fernandes-Alnemri T, Mayes L, et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature communications 8 (2017): 14128.
- Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547 (2017): 99-103.
- Zheng X, Zhong T, Ma Y, et al. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life sciences 242 (2020): 117186.
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circulation research 83 (1998): 117-132.
- Wang X, Chen M, Zhou J, et al. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review). International journal of oncology 45 (2014): 18-30.
- Shan R, Liu N, Yan Y, et al. Apoptosis, autophagy and atherosclerosis: Relationships and the role of Hsp27. Pharmacological research 166 (2021): 105169.
- Wang Y, Liu J, Kong Q, et al. Cardiomyocyte-specific deficiency of HSPB1 worsens cardiac dysfunction by activating NFκB-mediated leucocyte recruitment after myocardial infarction. Cardiovascular research 115 (2019): 154-167.
- Liu L, Zhang X, Qian B, et al. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. European journal of heart failure 9 (2007): 762-769.
- Guo Y, Ziesch A, Hocke S, et al. Overexpression of heat shock protein 27 (HSP27) increases gemcitabine sensitivity in pancreatic cancer cells through S-phase arrest and apoptosis. Journal of cellular and molecular medicine 19 (2015): 340-350.
- Choi SK, Kam H, Kim KY, et al. Targeting heat shock protein 27 in cancer: A druggable target for cancer treatment ? Cancers 11 (2019): 1195.
- Srinivas AN, Suresh D, Mirshahi F, et al. Emerging roles of AATF: Checkpoint signaling and beyond. Journal of cellular physiology 236 (2021): 3383-3395.
- Iezzi S & Fanciulli M. Discovering Che-1/AATF: a new attractive target for cancer therapy. Frontiers in genetics 6 (2015): 141.
- Toldo S, Marchetti C, Mauro AG, et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. International journal of cardiology 209 (2016): 215-220.
- Bruno T, De Nicola F, Iezzi S, et al. Che-1 phosphorylation by ATM/ATR and Chk2 kinases activates p53 transcription and the G2/M checkpoint. Cancer cell 10 (2006): 473-486.
- Park AM, Tsunoda I, Yoshie O. Heat shock protein 27 promotes cell cycle progression by down-regulating E2F transcription factor 4 and retinoblastoma family protein p130. The Journal of biological chemistry 293 (2018): 15815-15826.
- Tan G, Huang C, Chen J, et al. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. Journal of hematology & oncology 13 (2020): 149.
- Qiu T, Pei P, Yao X, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell death & disease 9 (2018): 946.
- Hao L, Ren M, Rong B, et al. TWEAK/Fn14 mediates atrial-derived HL-1 myocytes hypertrophy via JAK2/STAT3 signalling pathway. Journal of cellular and molecular medicine 22 (2018): 4344-4353.
- Xu H, Guan N, Ren YL, et al. IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC nephrology 19 (2018): 140.
- Zeng C, Duan F, Hu J, et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox biology 34 (2020): 101523.
- Haupt LP, Rebs S, Maurer W, et al. Doxorubicin induces cardiotoxicity in a pluripotent stem cell model of aggressive B cell lymphoma cancer patients. Basic research in cardiology 117 (2022): 13.
- Wang Y, Yan S, Liu X, et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell death and differentiation 29 (2022): 1982-1995.
- Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440 (2006): 237-241.
- Sager HB, Heidt T, Hulsmans M, et al. Targeting Interleukin-1β Reduces Leukocyte Production After Acute Myocardial Infarction. Circulation 132 (2015): 1880-1890.
- Sandanger Ø, Ranheim T, Vinge LE, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia reperfusion injury. Cardiovascular research 99 (2013): 164-174.
- Zhang L, Fan C, Jiao HC, et al. Calycosin Alleviates Doxorubicin-Induced Cardiotoxicity and Pyroptosis by Inhibiting NLRP3 Inflammasome Activation. Oxidative medicine and cellular longevity (2022): 1733834.
- Li W, Wang X, Liu T, et al. Harpagoside Protects Against Doxorubicin-Induced Cardiotoxicity via P53-Parkin-Mediated Mitophagy. Frontiers in cell and developmental biology 10 (2022): 813370.
- Zeng C, Wang R and Tan H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. International journal of biological sciences 15 (2019): 1345-1357.
- Menu P, Pellegrin M, Aubert JF, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell death & disease 2 (2011): 137.
- Krishnan SM, Dowling JK, Ling YH, et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. British journal of pharmacology 173 (2016): 752-765.
- Tanaka Y, Nagoshi T, Yoshii A, et al. Xanthine oxidase inhibition attenuates doxorubicin-induced cardiotoxicity in mice. Free radical biology & medicine 162 (2021): 298-308.
- Zhou Z, Li X, Qian Y, et al. Heat shock protein 90 inhibitors suppress pyroptosis in THP-1 cells. The Biochemical journal 477 (2020): 3923-3934.
- Xu Q, Tu, J, Dou C, et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Molecular cancer 16 (2017): 178.
- Xiao X, Wang W, Li Y, et al. HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma. Journal of experimental & clinical cancer research : CR 37 (2018): 201.
- Bulek K, Zhao J, Liao Y, et al. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. The Journal of clinical investigation 130 (2020): 4218-4234.
- Qi J, Yu XJ, Shi XL, et al. NF-κB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovascular toxicology 16 (2016): 345-354.