Harms of Momordica charantia L. in Humans; a Systematic Review
Article Information
Armelle Demmers*, 1, Jurriaan J. Mes 2, Roy G. Elbers1, Raymond HH. Pieters3
1Armelle Health Services & Research, Castricum, The Netherlands
2Wageningen Food & Biobased Research, Wageningen University & Research, Wageningen, The Netherlands
3Research group Innovative Testing in Life Sciences & Chemistry, Research Centre for Healthy and Sustainable Living, University of Applied Sciences Utrecht, Utrecht
*Corresponding author: Armelle Demmers. Armelle Health Services & Research, Castricum, Koningsduin 41, 1901 ZV Castricum, The Netherlands,
Received: 18 May 2023; Accepted: 26 May 2023; Published: 12 June 2023
Citation: Armelle Demmers, Jurriaan J. Mes, Roy G. Elbers, Raymond HH. Pieters. Harms of Momordica charantia L. in Humans; a Systematic Review. Fortune Journal of Health Sciences. 6 (2023): 222-236
View / Download Pdf Share at FacebookAbstract
This systematic review aims to evaluate the potential harm of Momordica charantia L. (MC) using data from randomized controlled trials. Databases were searched until December 2020. The PRISMA harms checklist was followed. Data extraction was on aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, adverse effects (AE), reasons for drop out related to the intervention and interaction with other treatment. Two authors independently extracted data and bias was evaluated based on the latest version of the Cochrane risk of Bias Tool (RoB 2). Additional safety data were requested from Health Regulatory Agencies, Herbal Medicine Associations and manufacturers. Seventeen trials met the inclusion criteria. The IRR was calculated for each study ranging from 0.30 (95% CI = 0.12 to 0.75) to 13.00 (95% CI = 0.73 to 230.76) of any adverse events. Under a daily dosage of 6g of MC-derived products no evidence was seen of harms in humans. In case reports that showed serious harm, MC was used in a liquid form. The safety of traditional MC-based supplements appears more guaranteed when produced under strict quality standards.
Keywords
adverse events, diabetes, Momordica charantia, safety, Traditional medicine Asia & Oceania, systematic review
adverse events articles, diabetes articles, Momordica charantia articles, safety articles, Traditional medicine Asia & Oceania articles, systematic review articles
Article Details
List of abbreviations:
AE: adverse events, AF: atrial fibrillation, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, CAERS: centre adverse events reporting system, CI: confidence interval, CFSAN: Center for Food Safety and Applied Nutrition, ECG: electrocardiography, EFSA: European food and safety authority, FDA: food and drug administration, FPG: fast plasma glucose, GABA: gamma-aminobutyric acid, GMP: good manufacturing practice, G6PD: glucose-6-phosphate dehydrogenase, g: gram, Hb: hemaglobin, HbA1c: glycosylated hemoglobin Type A1C, , HP: Hypericum perforatum, I: intervention, I2: chi-square, IRR: incidence rate ratio, MC: Momordica charantia, mcIRBP-19: peptideB19 amino acids mcIRBP-19, mg: milligram, ml: milliliter, Mm: millimoles per litre, mmol/L: millimoles per litre, N= number, OAA: oral diabetic agent, PPG: postprandial glucose, ppm: parts per million, RCT: randomized controlled trial, TCM: traditional Chinese medicine, T2DM: type-2 diabetes mellitus, µg: microgram, u/g: microgram, µmol/L: micromole per litre, WHO: world health organization, wks: weeks, WMD: weighted mean difference, w/v= weight per volume, w/w= weight per weight.
1. Introduction
Traditionally Momordica charantia L. (MC, bitter lemon) is used for eczema, psoriasis, cancer, rheumatism, antiviral activities and to control blood glucose levels [1,2,3]. These indications have been supported with animal and in vitro studies [4,5,6,7,8,9]. Recently, two systematic reviews were conducted to evaluate the effect of MC on control of blood glucose levels in human and these reviews showed conflicting results [10,11]. No statistically significant difference was found in the Cochrane systematic review with regard to the glycaemic control when effects of MC preparations were compared to placebo [10]. On the contrary, in the other systematic review MC formulation was found to significantly reduce fasting plasma glucose (FPG) levels, postprandial glucose (PPG) and haemoglobin A1c (HbA1c) when compared to placebo [11]. The methods of these systematic reviews were different with regard to inclusion of polyherbal supplements, randomization and minimal follow-up time.
Several case reports on safety issues have been published [12,13,14,15] which justifies a closer analysis of safety.
MC seeds contain vicine-like compounds which may induce a hemolytic disorder known as favism [16]. Heart and blood disorders have been observed in animals as well [17,18,19]. Significant increases of liver enzymes have been observed in rats treated with fruit juice and seed extract in different intervention groups [20]. Furthermore, extensive inflammation and cell toxicity occurred in the white adipose tissue of mice subjected to high doses of bitter melon seed oil [21]. Further, reproduction problems have been observed in animal studies [22,23]. Also, in dogs fed with total fruit extract (1.75g/ day oral for 60 days) anti-fertility and anti-spermagenic effects have been found [24]. Various parts of the plant have been shown to cause malformations of embryos in pregnant animals [17,25,26]. It is not yet clear which components may cause these effects, but it seems that specifically two enzymes, β-and α-momorcharins, may be abortifacient [26]. The question arises if these animal findings can be extrapolated to humans and which plant part could cause these effects.
In addition to the possible bioactive substances of the plant, the exact plant material of the product may also play an important role. Most of the adverse events (AE) of herbal products and herbal medicines can be attributed to poor quality, processing and (im)purity of the product [27,28]. This stresses the need for a critical systematic review of the available data on safety of MC use in humans. Here, we evaluate the AE reported after MC use, in relation to the plant material and the daily dosages of the MC-derived products.
2. Methods
The PRISMA harms checklist was followed [29]. When side effects or AE were described as outcome measures in the protocol or the phrase ‘there were no AE’ was reported, the trial was included. When the study report did not mention anything side effects or AE, the study was excluded. Additional safety data were obtained from international drug monitoring agencies, manufacturers and distributors of MC, and herbalist organizations.
2.1 Selection criteria
Randomized controlled trials (RCTs) with any kind of intervention period were included but only those published in English. We included RCTs with healthy participants and with participants displaying any kind of disorder. RCTs mentioning any oral therapy using single MC in any dosage or formulation were included, but studies using a combination with MC and other herbal(s) or food ingredients preparations were excluded. Simultaneously administrated medication was included for analyses as a separate comparison. Interventions in the control group could be: no treatment, placebo, or any other treatment.
2.2 Search methods
We searched the following electronic databases until December 2020: The Cochrane Library, Pubmed and EMBASE. The search plant names were “Momordica charantia” or “bitter melon” or "bitter gourd” or “bittergourd” or "balsam pear" or "bitter squash" or “karela” or “amplaya” or “sopropo” in subject, abstract and keywords. Unpublished or ongoing trials were searched in the electronic databases until December 2020 clinicaltrials.gov and the World Health Organization International Clinical Trials Register Platform. Authors of unpublished trials were asked for data by email. Hand search in Google Scholar, Mendeley, ResearchGate and reference lists of reviews was performed by A.D.
2.3 Data extraction
The results from the searches were independently screened by author A.D. and R.P. Titles and abstracts were scanned on inclusion and exclusion criteria. Full text investigation was performed when titles and abstracts gave insufficient information. In case of disagreement a third author (J.M.) was consulted to reach a final decision.
From the selected studies the following data were extracted:
(a) General study information: authors, publication year, samples sizes, follow- up period, methods of AE assessment; (b) Intervention data: plant material, administered dosages, administration frequency and duration, intervention control group; (c) Results on safety parameters: AST, ALT, creatinine, all reported AE, reasons for drop- out related to the intervention, interaction with other treatment, information about time between intake and adverse-event, number of participants who experienced an AE, follow-up time/time being at risk; (d) Baseline characteristics of subjects: age, sex, body mass index (BMI), diagnose and use of medication.
2.4 Assessment risk of bias
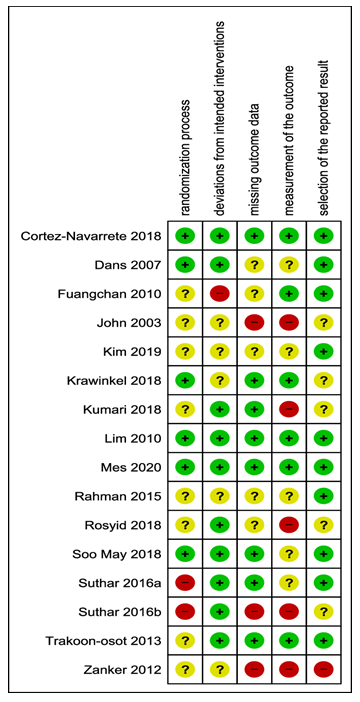
The validity of the study results was assessed by A.D. and J.M. using the latest version of the Cochrane Risk of Bias Tool, a checklist evaluating the validity of studies as to the following five bias parameters; the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result [30]. We assessed bias of starting and adhering to the intervention instead of intention-to-treat because of AE as outcome. For the judgements of the parameter of outcome measurement we rated the methods of AE assessments used in the trials.
2.5 Statistical analyses
We conducted meta-analyses in R-studio with the package Meta [31]. Where statistical pooling was not sensible to clinical heterogeneity, the effect size of each study was separately presented in a forest plot. For continuous data, differences between the control and intervention groups were calculated based on mean difference. AE were analysed as dichotomous data. For comparisons of AE incidence rate ratio (IRR) was calculated with the corresponding 95% confidence intervals (95% CI) using data extracted. The IRR was calculated as follows: dividing the number of AE by the number included participants in the study x time of study period in days.
All reported AE were analysed as total numbers of occurrence because multiple episodes of a side effect could have occurred in one participant. For each reported AE a separate analysis was performed. Similar and most common AE were clustered. Drop-outs were analysed separately and also counted as an AE if the report was clear or if the assumption that the drop-out was related to the intervention was plausible. When a study reported no events, 0.5 was added to all four cells of the 2×2 table. When multiple intervention groups from one study were in a meta-analysis, we combined the groups to create a single pair-wise comparison. We established a cut-off value of 3g per day of MC to analyse as a separate group because most AE were seen in trials with a daily dose of > 3g MC per day [32,33,34].
3 Additional safety data
Additional safety data were obtained from Healthcare Regulatory Agencies, manufacturers and distributors of MC, and herbalist organizations worldwide. Also, we searched for case reports on safety in the databases Pubmed and EMBASE for available literature and to traditional knowledge from ancient use about safety of MC.
4. Results
4.1 Results of the search
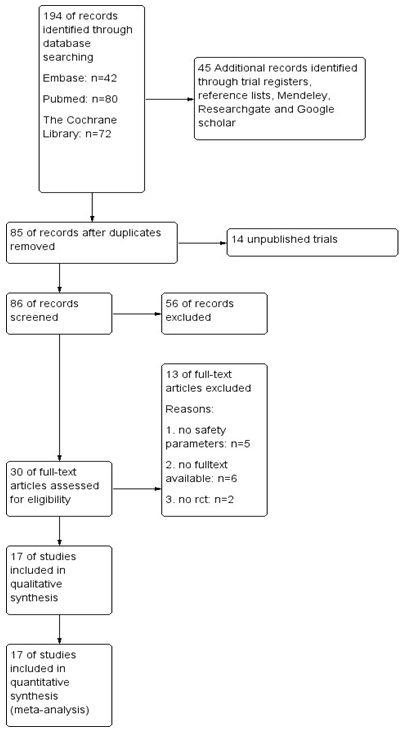
The database searches and additional records gave a total number of 239 studies. After removing duplicates, 85 studies remained. We also included an unpublished trial conducted by author J.M. of this review as it met the inclusion criteria [35]. The flow diagram (Fig. 1) shows that 56 studies did not met the inclusion criteria by reading the title or the abstract and that we retrieved the full-text papers of 30 studies for further assessment. In five of these 30 remaining studies, no safety issues or AE were reported [36,37,38,39,40]. The full-text of six of the 30 studies could not be obtained [41, 42,43,44,45,46]. And were unsuccessful in our efforts to obtain these articles directly from the authors. We were also unsuccessful in getting information from the authors on reports that did not report any safety parameters or AE.
Finally, seventeen RCTs were included for analysis (Table 1). In our analyses of one trial [47] we excluded the intervention group with 100µg chromium and 10mg zinc as intervention next to MC because of the inclusion criteria.
Table 1: Baseline characteristics
|
References |
Sex (male:female) |
Age in years (mean) |
Body Mass Index |
Used medication next to MC |
Diagnose of the participants |
|
Cortez- |
33:67% |
48.5 |
28.9 |
none |
Type-2 diabetes |
|
Navarrete 2018 |
|||||
|
Dans 2007 |
unclear |
59.2 |
26.2 |
100% anti |
Type- 2 diabetes |
|
diabetics |
|||||
|
Fuangchan |
26:74% |
51.8 |
25.1 |
none |
Type- 2 diabetes |
|
2010 |
|||||
|
Hsu 2020 |
unclear |
62.9 |
unclear |
100% anti |
Type- 2 diabetes |
|
diabetics |
|||||
|
John 2003 |
32:68% |
53.2 |
unclear |
100% anti |
Type- 2 diabetes |
|
diabetics |
|||||
|
Kim 2020 |
38:24% |
58.1 |
25.3 |
100% anti |
Type- 2 diabetes |
|
diabetics |
|||||
|
Krawinkel |
46:54% |
47.8 |
29.6 |
none |
prediabetes |
|
2018 |
|||||
|
Kumari 2018 |
unclear |
unclear |
27.7 |
100% anti |
Type-2 diabetes |
|
diabetics |
|||||
|
Lim 2010 |
45:55% |
56.6 |
25.7 |
none |
Type- 2 diabetes |
|
Mes 2020 |
70:30% |
67.8 |
28.7 |
none |
prediabetes |
|
Rahman 2015 |
66:34% |
52 |
25.45 |
none |
Type- 2 diabetes |
|
Rosyid 2018 |
33:67% |
53.7 |
21.6 |
100% anti |
Type-2 diabetes |
|
diabetics, |
with diabetic foot |
||||
|
100% anti |
ulcer |
||||
|
biotics |
|||||
|
Soo May 2018 |
30:70% |
59.9 |
26.9 |
analgesia allowed |
primary knee |
|
osteoarthritis |
|||||
|
Suthar 2016a |
62:38% |
41.3 |
unclear |
89% anti diabetics |
Type- 2 diabetes |
|
Suthar 2016b |
56:44% |
48.55 |
27.29 |
none |
Type- 2 diabetes |
|
Trakoon-osot |
29:71% |
57.9 |
25.7 |
76% anti diabetics |
Type- 2 diabetes |
|
2013 |
|||||
|
Zanker 2012 |
72:28% |
62.6 |
29.3 |
77% anti diabetics |
Type- 2 diabetes |
4.2 Characteristics of included studies
The 17 studies included a total of 1.320 participants, varying from 24 to 142 in number per study and with a total 785 participants in the MC intervention group and 535 participants in the control group. The participants had a mean age between 41.3 and 67.8 years. Baseline characteristics concerning age, sex and BMI of subjects are reported in Table 1. The mean intervention period was 67 days, ranging from a single day (1 dose) to an 112 days intervention period. Four studies did not report details about the production process of the MC derived product [32,48,49,50]. The plant material used in the various studies was very different. Some studies investigated the supposed active constituent charantin which is associated with blood sugar lowering properties [47,51,52,53]. In Rahman et al. the amount of charantin per daily dosage was not clear. For two studies the extraction of MC juice was dried and powdered [54,55]. No information has been reported about the used plant material in four studies [35,51,56,57]. Other details on the included studies can be found in Table 2.
Table 2: Description of included studies
OAA= Oral Anti diabetic agent, I= intervention group MC, N= number of participants, NR= Not reported, wks=weeks, GABA = Y-aminobutyric acid, mcIRBP-19 = specific sequence of 19 amino acids, ppm= parts per million, w/v= weight per volume, w/w= weight per weigh
4.3 Risk of bias
Risk of bias was assessed for the outcome AE (Fig 2). In one study [56] AE were measured by liver and kidney enzymes. We analysed these indicators as separate outcomes, not as AE in general. Therefore, we excluded this trial in the risk of bias assessment. For the domain “outcome measurement”, nine studies were unclear with regard to AE. These were consistently tracked or only reported occasionally [33,42,47,48,49,50,52,55,57]. In one trial, the reporting of AE for the domain “selection of the reported result” was no reporting at all if there were any AE reported or not [47]. We judged this trial as highly biased.
4.4 Analysis of the comparisons
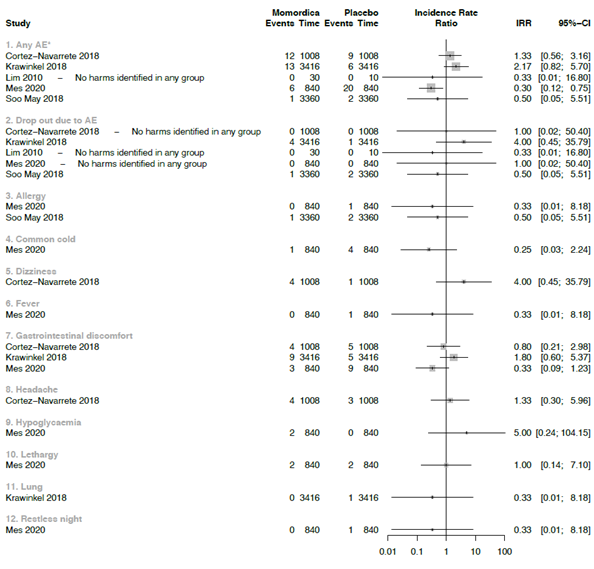
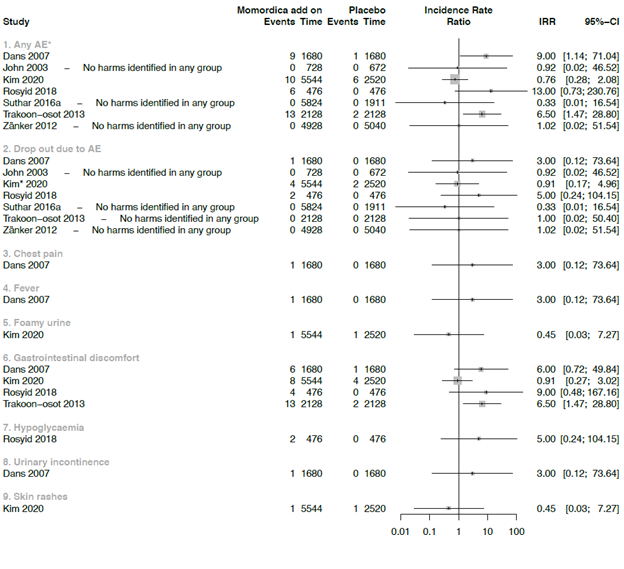
Due the strong clinical heterogeneity between the studies with regard to characteristics of participant, plant material, and dosage, it was not possible to conduct meta-analyses. We decided to graphically present the results in forest plots, but we refrained from estimating pooled summary statistics. Only five RCTs reported a comparison with placebo and these are used in the forest plot of figure 3.
*In Krawinkel et al. the symptoms: loose stools, diarrhea, flatulence, stomach rumbling, nausea or vomiting were reported as mean numbers. For headaches no number was reported and therefore not included in further analysis
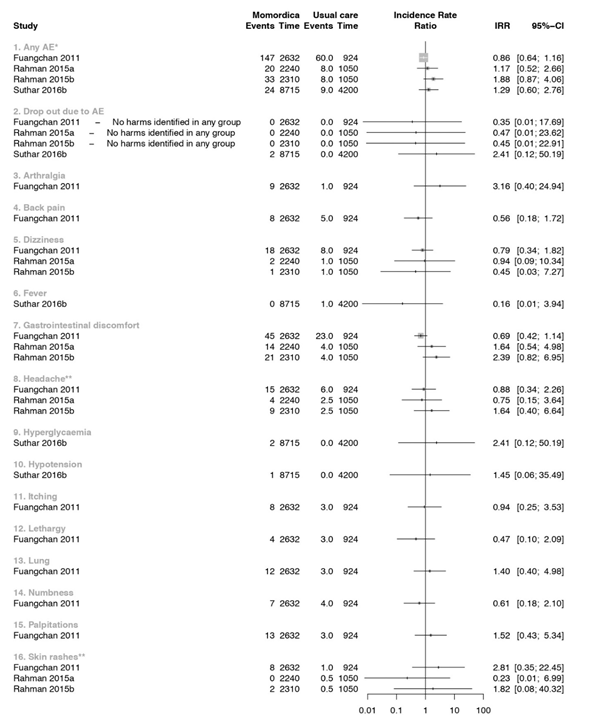
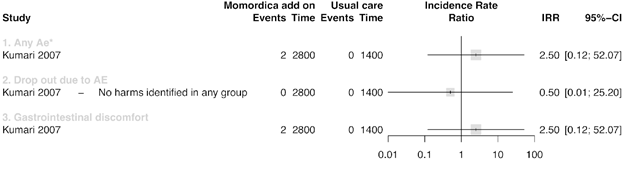
Figure 4 summarizes the results of three studies that compared MC with usual treatment. One study [52] had two intervention arms, one with 2g MC daily (a) and one of 4g MC daily for ten weeks (b). We analysed these two arms separately. In a study that included T2DM patients [51], increased appetite as AE was found after intake of 2g/day MC more frequently than in the groups with 500mg/day and 1g/day compared to the Metformin treatment groups. Other AE were 16 episodes of palpitation. Few of these episodes were associated with hypoglycaemia. These symptoms resolved with rest and did not require any treatment or discontinuation of Metformin or MC. In another trial T2DM participants of 4g/ day MC group had more appetite and headaches compared with 2g/day or Glibenclamide 5mg/day [55].
* In Suthar_b et al. only the total count of the AE was reported and further no specification was given
** The control group of this study was split so the AE were in a decimal (Rahman et al., 2015)
Seven studies were included in the comparison between MC as co-intervention compared with placebo (Fig 5). All seven studies administered anti-diabetic medication as co-intervention. In one of these studies participants used also antibiotics [33]. The IRR was calculated for each study, ranging from 0.33 (95% CI = 0.01 to 16.54) to 13 (95% CI = 0.73 to 230.76) for AE. Two studies showed a large effect on the overall AE outcome. The wide CI’s showed the uncertainty of these IRR’s values. The used plant material in one of the study was prepared from unripe dried fruit without seeds, and administered in a high daily dosage of 6g. At this dosage, more gastrointestinal discomfort with symptoms of diarrhoea and flatulence with an IRR of 6.50 (95% CI = 1.47 to 28.80) was found [53]. In the other study, a daily dosage of 3g fruit with seeds was used [48]. In a study that included people with T2DM and diabetic foot ulcer, two participants dropped -out because of nausea, vomiting and hypoglycaemia. In this study a dose of 6g leaves extract of the MC was used in combination with standard medication [33].
* In this study it is unclear any many participants dropped out due AE (Kim et al., 2020)
In one RCT MC was studied as additional co-intervention next to Metformin and Glibenclamide and compared with standard oral anti-diabetic agents and placebo [57] (Fig 6). Two notifications of gastrointestinal discomfort were the only AE [57].
* The authors of this study reported AE but did not mention in which intervention arm the AE occurred. We assumed that these happened in the MC arm and included them in the data analysed as such
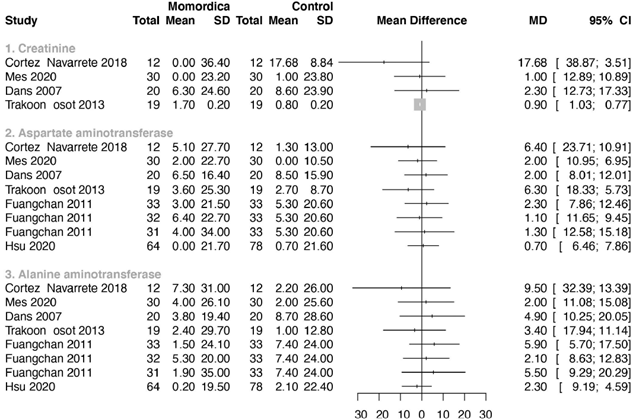
3.5 Safety parameters
Only one study reported the standard deviations of the means [48], for the other studies we imputed these following the methods advised by Cochrane [58]. Figure 7 summarizes the results for the safety parameters. Two studies reported that there was no significant influence of MC on renal and liver function, but did not present detailed numbers [42,59]. In one study creatinine was reported in mmol/L instead of µmol/L [32]. The author confirmed this. In another study the converted creatinine mean value of the control group (247.60µmol/L) seems much too high [56]. We assumed that this reporting was not correct and notified the author. We excluded this study for analysis after a time period with no reaction of this author. ALT and AST were used as outcome parameter in seven studies (Fig 7). In one study no values were reported [47]. No statistical significant differences were found in levels of ALT and AST between groups.
3.5 Results of additional safety data search
In the FDA center for food safety and applied nutrition (CFSAN) AE reporting system (CAERS) we found three reports of AE after using MC. There was a case of a patient in a life-threatening situation, visiting a healthcare provider with symptoms of vomiting, nausea, malaise, hypersensitivity, haemorrhage, haemoptysis, diarrhoea, asthenia and abdominal upper pain. The suspected cause was a dietary supplement of Nature’s Herbs with bitter melon containing 525mg fruit. The second case report is of a 60-year-old patient that visited a healthcare provider with hypertension. The suspected cause was a rapid release supplement of Puritan’s Pride with bitter melon fruit 900mg daily. During the same period, this subject consumed many other products that could be the cause of the symptoms. The last case was a patient with AF who visited a healthcare provider after the use of Puritan’s Pride bitter melon, 450mg rapid release capsules, twice daily. Detailed information about Puritan’s Pride bitter melon could not be found on the manufacturer's website.
VigiBase reports refer to a suspected but not confirmed causal relationship between a drug and an event. In the Vigibase of the WHO, eight cases of adverse reactions to MC were found. In the Pubmed and EMBASE databases we found four case reports. There is a report of a potentially fatal reaction in two young children. They fell into hypoglycaemic coma after drinking tea of MC leaves [12]. Another case report is of a 22-year-old man of AF due to drinking MC juice. He consumed crushed MC [13]. A 42-year-old man admitted to the Emergency Department had complaints of fatigue, intermittent dark urine for 1 week, fever, chills, vomiting and loose black stools for 1 day. He mentioned increased consumption of Chinese MC tea for his hyperlipidemia for 11 days prior to hospitalization. This man was G6PD-deficient and therefore some drugs, foods and chemicals can trigger haemolysis [15]. Another case report involved a 40-year-old man with severe epigastric pain and hematemesis (around 200-300 mL) within half an hour following drinking half a litre of liquid extract of MC [14]. It is unknown how many supplements of MC are used worldwide a year.
4. Discussion
A MC-derived product should be safe for humans and not induce any risk. We performed a systematic review to identify possible harms of MC as supplement based on previously conducted RCTs. Our analysis indicated a strong heterogeneity between the included studies on used plant material, daily dosages, intervention period and participants. Therefore, we created an overview and calculated the IRR separately for each included study in a systematic way but did not combine these results in meta-analyses. Two studies with 6g daily dosage potentially indicate that a too high amount of dried fruit, seeds and leaves might cause a health risk [33,53]. Some of the AE reported by the subjects, like dizziness, headaches, increased appetite, lethargy, lung problems, palpitations, nausea, constipation and vomiting, could be related to the high and fluctuating blood sugar levels as in many studies people with T2DM were included [33,51,55].
A systematic review of RCTs is a good basis for toxicological risk evaluation when enough data can be included and combined in a meta-analysis. The strength of this systematic review is that it was performed with seventeen included RCT’s with the effect size IRR on AE which calculated the harmful effect over time. However, RCTs will not identify any harm on fertility or reproduction problems, which may be a concern since MC has been found to cause reproduction problems and teratogenic effects in animals [17,22,23,24,26]. Also, traditional healers in India and Africa have used the seeds of MC to induce abortions [60]. Bitter melon seeds contain vicine-like compound which may induce G6PD-deficiency resulting in breakdown of red blood cells [16], which is indicative of harmful effects of seeds.
We found four case reports of harm in the databases Pubmed and EMBASE. These case reports of AE were not after intake of MC as dietary supplement but after drinking large amounts of juice or tea. This may suggest that liquid use of MC has a different absorption than when taken as a dietary supplement. The European Food and Safety Authority (EFSA) has concluded: “Safety of an herbal preparation can be presumed when available data would allow concluding that exposure to known levels of the botanical ingredient has occurred in large population groups for many years without reported adverse effects” [61]. The fruits of MC are consumed over decades at different regions in the world without any reported safety concern. So, it can be assumed that when intake does not exceed an equivalent of what can be consumed as vegetable in a meal it would be fair to conclude safety of MC.
Based on our findings concerning the high risk of bias due to missing outcome data and outcome measurement, we would like to stress the importance of systematic collection and detailed reporting of AE during intervention trials. We encountered many incomplete reporting on compliance and reasons for lost to follow-up which can have affected our results. Time to event information could confirm that an reported harm is associated with the intervention. Information about time-to-discontinuation or time-to-withdrawal for each study group was only reported in one trial [48]. Also, information about severity of the complaints were not reported. If numbers of AE were provided, details about on how many days and in how many people events occurred were missing. A consequence of this limited information was that a causal relationship, in line with the Bradford Hill criteria [62], between the AE and the use of MC could not be established
In five studies selected for this systematic review, the researchers did not provide any information on quality assessment of the product and therefore AE due to contaminants could have influenced our results. Good Manufacturing Practice (GMP) is one of the most important tools to ensure quality of pharmaceuticals and herbal medicines [63]. For research but also for safety reasons, GMP should become an important standard for medicinal plants derived products. Plant material can be contaminated with toxic plants containing specific alkaloids [64]. Especially microbial safety is of high importance to include in all these studies as food pathogens like E.coli, Salmonella and Listeria can cause effects within hours after intake [65,66]. A microbiological and pharmaceutical quality and safety assessment on over-the-counter herbal weight loss supplements in Egypt showed that, based on microbial count, 100% of the unapproved weight loss products had poor bacteriological quality [27]. To date, the European Pharmacopoeia TCM working group has elaborated about 80 TCM herbal monographs with ISO standards for over 14 MPs published or under development. These standards provide important references to the quality consistency and safety of MPs [67]. So, with the limitations of this systematic review, no clear evidence was found that MC preparation and available supplements show more harms than placebo or commonly prescribed OAA under a daily dosage of 6g of dried fruit or leaves. Next to that avoid using high amounts of concentrated MC in juice or tea.
5. Conclusion
Comparing collected results and ancient use, MC-based supplements can be assumed to be safe under a daily dosage of 6g. Due to incomplete reporting on compliance and reasons of lost to follow-ups of the participants necessary information is missing to draw a complete conclusion of harms. Causal relationship cannot be confirmed between the AE of the found cases and the intake of MC because of lack of required information. MC is not recommended during birth wish, pregnancy and when breastfeeding based on animal studies. MC is also not recommended for humans with G6PD-deficiency.
Conflict of interest
The authors declare not to have conflicts of interest.
Acknowledgments
The authors have made substantial contributions to the design of the study (AD, JM, RE) acquisition of data (AD, RP), analysis of data (AD, RE), assessment and design of the searches (AD, JM) and drafting the article (AD, JM, RE, RP). The authors have given approval for the final version of this paper to be submitted for publication (AD, JM, RE, RP).
Funding
The project was financially supported by the EFRO project Kansen voor West “Green Health Solutions” (KVW00117) and the Dutch Ministry of Agriculture, Nature and Food Quality project code EU-TU-18007
References
- Grover JK, & Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. Journal of Ethnopharmacology 93 (2004): 123-132.
- Mahomoodally MF & Ramalingum N. An investigation into the consumption patterns, attitude, and perception of Mauritians towards common medicinal food plants. Journal of Herbal Medicine 5 (2015): 99-112.
- Panigrahi HK, Rana R & Padhi MM. Comparative clinical evaluation of Karela (whole fruit) along with Jamun (seeds) Ghana satwa vis-à-vis Ghana satwa of the combination of leaves Bilwa, Neem, Tulsi along with Kalimirch in the management of Madhumeha (Diabetes mellitus). Journal of Research in Ayurveda and Siddha 31 (2010): 41-52
- Bai J, Zhu Y & Dong Y. Response of gut microbiota and inflammatory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats. Journal of Ethnopharmacology 194 (2016): 717-726.
- Bai J, Zhu Y & Dong Y. Obese rats supplemented with bitter melon display marked shifts in the expression of genes controlling inflammatory response and lipid metabolism by RNA-Seq analysis of colonic mucosa. Genes and Genomics 40 (2018): 561-567.
- Bhat GA, Khan HA, Alhomida AS, Sharma P, Singh R, & Paray BA. Correction to: GLP-I Secretion in Healthy and Diabetic Wistar Rats in Response to Aqueous Extract of Momordica charantia. BMC complementary and alternative medicine 18 (2018): 175
- Huang T, Lu K-N, Pai Y-P, Chin Hsu & Huang C. Role of GLP-1 in the Hypoglycemic Effects of Wild Bitter Gourd. Evidence-Based Complementary and Alternative Medicine (2013): 1-13.
- Joseph B & Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pacific Journal of Tropical Disease 3 (2013): 93-102.
- Kim K & Kim HY. Bitter melon (Momordica charantia) extract suppresses cytokineinduced activation of MAPK and NF-κB in pancreatic β-cells. Food Science and Biotechnology 20 (2011): 531-535.
- Ooi CP, Yassin, Z, & Hamid TA. Momordica charantia for type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews (2012): CD007845.
- Peter EL, Kasali FM, Deyno S, Mtewa A, Nagendrappa PB, Tolo CU, et al. Momordica charantia L. lowers elevated glycaemia in type 2 diabetes mellitus patients: Systematic review and meta-analysis. Journal of Ethnopharmacology 231 (2019): 311-324.
- Hulin A, Wavelet M, & Desbordes JM. Intoxication aigue par Momordica charantia (sorrossi). A propos de deux cas. Semaine des bopitaux 64 (1988): 2847-2848.
- Erden I, Ordu S, Erden EC & Caglar SO. A case of atrial fibrillation due to Momordica charantia (bitter melon). Annals of Saudi medicine 30 (2010): 86-87.
- Nadkarni N, D’Cruz S, & Sachdev A. Hematemesis due to bitter melon (Momordica charantia) extract-induced gastric ulcerations. Indian Journal of Gastroenterology: Official Journal of the Indian Society of Gastroenterology 29 (2010): 37-38.
- Mohanty E, Tachamo N, Mir I, Basnet S, Swierczynski S & Foreman D. Bitter Melon Tea Causing Cola Colored Pee! Hospital Medicine (2018): 8-11
- Raman A & Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 2 (2011): 349-362.
- Khan MF, Abutaha N, Nasr FA, Alqahtani AS, Noman OM & Wadaan MAM. Bitter gourd (Momordica charantia) possess developmental toxicity as revealed by screening the seeds and fruit extracts in zebrafish embryos. BMC Complementary and Alternative Medicine 19 (2019): 184.
- Abdillah S, Farida Y, Kartiningsih, Sandhiutami NMD & Mohamad K. Antimalarial activity and toxicity evaluation of the alkaloid-rich fraction of Momordica charantia fruits. International Journal of Pharmaceutical Sciences and Research 10 (2019): 2516-2522.
- Temitope A. Effect of Momordica charantia (Bitter Melon) Leaves on Haemoglobin Concentration in Male Albino Rats. International Blood Research & Reviews 2 (2014): 82-86.
- Tennekoon KH, Jeevathayaparan S, Angunawala P, Karunanayake EH & Jayasinghe KSA. Effect of Momordica charantia on key hepatic enzymes. Journal of Ethnopharmacology 44 (1994): 93-97.
- Chen P-H, Chen G-C, Yang M-F, Hsieh C-H, Chuang S-H, Yang H-L, Chao P-M. Bitter Melon Seed Oil-Attenuated Body Fat Accumulation in Diet-Induced Obese Mice Is Associated with cAMP-Dependent Protein Kinase Activation and Cell Death in White Adipose Tissue. The Journal of Nutrition 142 (2012): 1197-1204.
- Adewale OO, Oduyemi OI, & Ayokunle O. Oral administration oleaf extracts of Momordica charantia affect reproductive hormones of adult female Wistar rats. Asian Pacific Journal of Tropical Biomedicine 4 (2014): S521-S524.
- Odewusi AF, Oyeyemi MO, Olayemi FO, Emikpe B, Ehigie LO, Adisa RA & Olorunsogo OO. Effects of the leaf decoction of Momordica charantia (bitter melon) on Mitochondrial Membrane Permeability Transition Pore (MMPTP) and fertility in normal male albino rats. African Journal of Medicine and Medical Sciences 39 (2010): 47-59.
- Dixit VP, Khanna P & Bhargava SK. Effects of Momordica charantia L. fruit extract on the testicular function of dog. Planta Med 34 (1978): 280-286.
- Uche-Nwachi EO & McEwen C. Teratogenic effect of the water extract of bitter gourd (Momordica Charantia) on the sprague dawley rats. African Journal of Traditional, Complementary and Alternative Medicines 7 (2010): 24-33.
- Yeung HW, LI WW, Chan WY, Law LK & Ng TB. Alpha and beta momorcharins. International Journal of Peptide and Protein Research 28 (2009): 518-524.
- Ahmed N, Nounou MI, Abouelfetouh A & El-Kamel A. Over-the-Counter Herbal Weight Loss Supplements in Egypt: Label Claim, Microbiological (2019).
- Chan K. Some aspects of toxic contaminants in herbal medicines. Chemosphere 52 (2003): 1361-1371.
- Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG,Mulrow C. (2016). PRISMA harms checklist: Improving harms reporting in systematic reviews. The BMJ (352).
- Sterne J, Savovi? J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) 366 (2019): l4898.
- Balduzzi S, Rücker G & Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evidence-Based Mental Health 22 (2019): 153-160.
- Cortez-Navarrete M, Martínez-Abundis E, Pérez-Rubio KG, González-Ortiz M, & Méndez-Del Villar M. Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. Journal of Medicinal Food 21 (2018): 672-677.
- Rosyid FN, Dharmana E, Suwondo A, Heri Nugroho KHS, & Sugiarto. The effect of bitter melon (Momordica charantia L.) leaves extract on TNF-α serum levels and diabetic foot ulcers improvement: Randomized controlled trial. Biomedical and Pharmacology Journal 11 (2018): 1413-1421.
- Soo May L, Sanip Z, Ahmed Shokri A, Abdul Kadir A, & Md Lazin MR. The effects of Momordica charantia (bitter melon) supplementation in patients with primary knee osteoarthritis: A single-blinded, randomized controlled trial. Complementary Therapies in Clinical Practice 32 (2018): 181-186.
- Mes JJ. The Effect of Momordica Charantia Supplementation on Blood Glucose Levels. NCT04090788 (2019).
- Boone CH, Stout JR, Gordon JA, Redd MJ, Church DD, Oliveira LP, et al. Acute effects of a beverage containing bitter melon extract (CARELA) on postprandial glycaemia among prediabetic adults. Nutrition and Diabetes 7 (2017).
- Kasbia GS, Arnason JT, & Imbeault P. No effect of acute, single dose oral administration of Momordica charantia Linn, on glycemia, energy expenditure and appetite: a pilot study in non-diabetic overweight men. J Ethnopharmacol 126 (2009): 127-133.
- Kinoshita H, & Ogata Y. Effect of Bitter Melon Extracts on Lipid Levels in Japanese Subjects: A Randomized Controlled Study. Evidence-based complementary and alternative medicine: eCAM (2018): 4915784.
- Inayat-ur-Rahman, Malik SA, Bashir M, Khan R, & Iqbal M. Serum sialic acid changes in non-insulin-dependent diabetes mellitus (NIDDM) patients following bitter melon (Momordica charantia) and rosiglitazone (Avandia) treatment. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 16 (2009): 401-405.
- Selvakumar G, Shathirapathiy G, Jainraj R, & Yuvaraj Paul P. Immediate effect of bitter gourd, ash gourd, Knol-khol juices on blood sugar levels of patients with type 2 diabetes mellitus: A pilot study. Journal of Traditional and Complementary Medicine 7 (2017): 526-531.
- Amirthaveni M, Premakumari S, Gomathi K, & Ray-yu-Yang. Hypoglycemic Effect of Bitter Gourd (Momordica charantia L) Among Pre Diabetics in India: A Randomized Placebo Controlled Cross Over Study. The Indian Journal of Nutrition and Dietetics 55 (2018): 44-63.
- Kim SK, Jung J, Jung JH, Yoon NA, Kang SS, Roh GS & Hahm JR. Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complementary Therapies in Medicine 52 (2020): 102524.
- Muthumani V, & Ahmed John S. Role of exercise and herbal therapy for HIV infection. Biomedical and Pharmacology Journal 2 (2009): 43-48
- Pongnikorn S, Fongmoon D, Kasinrerk W, Limtrakul PN. Effect of bitter melon (Momordica charantia Linn) on level and function of natural killer cells in cervical cancer patients with radiotherapy. J Med Assoc Thai 86 (2003): 61-8.
- Shi Y-X, Yang Y-L, Huang X-Q, Cao W-W, & Wu L. Effects of kugua jiangtang capsule on blood sugar and lipid in patients with type 2 diabetes. Chinese Journal of Clinical Rehabilitation 8 (2004): 5994-5995.
- Tayyab F, & Lal SS. Comparative study on supplementation effect of Momordica charantia Linn. and Emblica officinalis Gaertn. on lipid profile of type II diabetic patients in Allahabad, Uttar Pradesh, India. Annals of Phytomedicine 5 (2016): 40-42.
- Zänker KS, Mang B, Wolters M & Hahn AK. Personalized Diabetes and Cancer Medicine: A Rationale for Anti-Diabetic Nutrition (Bitter Melon) in a Supportive Setting. Current Cancer Therapy Reviews 8 (2012): 66-77.
- Dans AML, Villarruz MVC, Jimeno CA, Javelosa MAU, Chua J, Bautista R & et al. The effect of Momordica charantia capsule preparation on glycemic control in Type 2 Diabetes Mellitus needs further studies. Journal of Clinical Epidemiology 60 (2007): 554-559.
- John AJ, Cherian R, Subhash HS & Cherian AM. Evaluation of the efficacy of bitter gourd (Momordica charantia) as an oral hypoglycemic agent-a randomized controlled clinical trial. Indian Journal of Physiology and Pharmacology 47 (2003): 363-365.
- Lim ST, Jimeno CA, Razon-Gonzales EB & Velasquez MEN. The MOCHA DM study: The effect of Momordica charantia tablets on glucose and insulin levels during the postprandial state among patients with type 2 diabetes mellitus. Phillippine Journal of Internal Medicine 48 (2010): 19-25.
- Fuangchan A, Sonthisombat P, Seubnukarn T, Chanouan R, Chotchaisuwat P, Sirigulsatien V & et al. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. Journal of Ethnopharmacology 134 (2011): 422-428.
- Rahman IU, Khan RU, Rahman KU & Bashir M. Lower hypoglycemic but higher antiatherogenic effects of bitter melon than glibenclamide in type 2 diabetic patients. Nutrition Journal 14 (2015).
- Trakoon-osot W, Sotanaphun U, Phanachet P, Porasuphatana S, Udomsubpayakul U & Komindr S. Pilot study: Hypoglycemic and antiglycation activities of bitter melon (Momordica charantia L.) in type 2 diabetic patients. Journal of Pharmacy Research 6 (2013): 859-864.
- Suthar AC, Deshmukh A, Babu V, Mohan VS, Chavan MV, Kumar D & et al. Efficacy and safety of Glycebal (PDM011011) capsules as adjuvant therapy in subjects with type 2 diabetes mellitus: An open label, randomized, active controlled, phase II trial. Clinical Diabetology 5 (2016): 88-94.
- Suthar AC, Pai VG, Kadam Y, Tongaonkar A, Kale S, Deshpande AB & et al. Efficacy and Safety of PDM011011 Capsules as Compared to Metformin in Subjects with Type-2 Diabetes Mellitus: An Open-Label, Randomized, Active-Controlled, Multicentric, Phase III Study. Journal of Diabetes Mellitus 06 (2016): 38-48.
- Hsu PK, Pan FFC & Hsieh C Sen. McIRBP-19 of bitter melon peptide effectively regulates diabetes mellitus (DM) patients’ blood sugar levels. Nutrients 12 (2020).
- Kumari S, Dash I, & Behera KK. Therapeutic effect of Momordica charantia on blood glucose, lipid profile and oxidative stress in type 2 diabetes mellitus patients: A randomised controlled trial. Journal of Clinical and Diagnostic Research 12 (2018): BC21-BC25.
- Follmann D, Elliott P, Suh I & Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of clinical epidemiology 45 (1992): 769-773.
- Krawinkel MB, Ludwig C, Swai ME, Yang R-Y, Chun KP & Habicht SD. Bitter gourd reduces elevated fasting plasma glucose levels in an intervention study among prediabetics in Tanzania. Journal of Ethnopharmacology 216 (2018): 1-7.
- Beloin N, Gbeassor M, Akpagana K, Hudson J, de Soussa K, Koumaglo K & et al. Ethnomedicinal uses of Momordicacharantia (Cucurbitaceae) in Togo and relation to its phytochemistry and biological activity. Journal of Ethnopharmacology 96 (2005): 49-55.
- Barlow S, Chesson A, Collins JD, Flynn A, Galli CL, Hardy A, Vannier P. Guidance on safety assessment of botanicals* and botanical preparations** intended for use as ingredients in food supplements. EFSA Journal 7 (2009).
- Hill AB. The environment and disease: association or causation? 1965. Journal of the Royal Society of Medicine 108 (2015): 32-37.
- WHO guidelines on good manufacturing practices (GMP) for herbal medicines (2007).
- De Wit L, Jeurissen S, & Chen W. Risk assessment of herbal preparations containing St John’s wort (2021).
- Alwakeel SS. Microbial and heavy metals contamination of herbal medicines. Research Journal of Microbiology 3 (2008): 683-691.
- Alwakeel SS. The Effect of Mycotoxins found in some Herbal Plant on Biochemical Parameters in Blood of Female Albino Mice. Pakistan Journal of Biological Sciences 12 (2009): 637-642.
- Xiong Y, Gao M, van Duijn B, Choi H, van Horssen F, & Wang M. International policies and challenges on the legalization of traditional medicine/herbal medicines in the fight against COVID-19. Pharmacological Research 166 (2021):