Grave’s Disease: Pathophysiology of a Model Autoimmune Disease
Article Information
Marium Nabi1, Rabbiya Noor1,*, Aleena Zahid1, Tuba Zulfiqar1, Ammara Khalid1, Samreen Riaz2
1Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan
2Assistant Professor, Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore-54500, Pakistan
*Corresponding author: Rabbiya Noor, Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore, Pakistan
Received: 21 February 2022; Accepted: 03 March 2022; Published: 05 May 2022
Citation: Marium Nabi, Rabbiya Noor, Aleena Zahid, Tuba Zulfiqar, Ammara Khalid, Samreen Riaz. Grave’s Disease: Pathophysiology of a Model Autoimmune Disease. Archives of Microbiology and Immunology 6 (2022): 149-164
View / Download Pdf Share at FacebookAbstract
Grave's disease is categorized as an autoimmune disease and type V hypersensitivity. It causes the formation of IgG antibodies that stimulates the thyrotropin receptor. It is suggested that in this disease, there is hyper stimulation of the thyroid gland by different clinical researches of this disease that result in thyrocyte hypoplasia (which is also known as goiter) and hyperthyroidism. Vulnerability to factors impacting the defenselessness and triggers for Graves' infection endures, alongside a wide variety in the reaction to hostile thyroid medications, as of now at roughly half of the non-responders. This review article describes the immune genetic, pathophysiological as well as environmental aspects related to this disease. It also emphasizes new research that elucidates the new methods to help resolve this disease-however its various areas of concern still need to be explored more extensively.
Keywords
hyperthyroidism;(TAO); TSHR (TRAb); CTLA-4 haploinsufficiency
Article Details
1. Introduction
The most prevalent reason for hyperthyroidism, with estimated 15-45 persons each year is Grave's disease. It is a complex illness with genetic, environmental, and endogenous variables all playing a role [1]. The condition is most common between the ages of 30 and 50, but it is developed at every age, and women are more frequently affected than males. The reason for hyperthyroidism in Grave's disease is diffused self-antibodies working opposite the thyrotropin receptor (TSHR), which copies the working of TSH and excessive activation of thyroid follicular cells, stimulating thyroid hormone secretion (triiodothyronine and thyroxine), thus making them produce growth of thyroid and vascularization [2, 3]. The mechanisms that cause hyperthyroidism symptoms include anxiety, exhaustion, weakness, palpitations, moist skin, weight loss, and nervousness. Extrathyroidal symptoms include localized dermopathy, ophthalmopathy, edematous- infiltrative changes, acropachy including orbital soft tissues known as thyroid-associated orbitopathy (TAO), an eye disease of the thyroid, also known as Graves ophthalmopathy (GO), of that greater than 90% are caused by Grave's disease. GO, described as a self-immune inflammation condition including the eye orbit, affects roughly 2 people in 10,000 each year and affects 20-45 percent of those with Grave's disease. Though the majority of the individuals are hyperthyroid (90%), people with GO also have euthyroid (5%), or hypothyroid (5%) [4, 5].
The pathogenic autoimmune reaction is shown to be directed towards opposite-reactive self-antigens in the thyroid gland and tissues behind the eyeball. Cytokines and immunological processes are thought to have a role in the pathophysiology of GO. The invasion of tissue by inflammatory cells producing cytokine, along with significant remodeling of the ocular soft tissues, results in a disease phenotype. Conjunctival erythema and orbital tissues, double vision, retracting eyelids, edema and proptosis are all clinical signs and symptoms. GO is classified into three severity levels: low to high, and threatening to the vision. Medication is determined by the harshness of the GO and may involve surgical processes (endoscopic orbital decompression), immunosuppressive, orbital irradiation. Grasping the immune system's function in GO may lead to the development of novel treatment approaches in the future [6, 7].
2. Pathogenesis of Grave's Disease
A self-immune situation of the thyroid gland is Grave's disease. The patients affected by hyperthyroidism are due to the IgG antibodies production against the receptor thyrotropin which copies the results of the thyroid hormone on the thyroid cells, thus stimulating the self-production of thyroxine. Just like GD, at the base of GO is the self-immune response during which the tender T cells, also self-antibodies opposite to a standard self-antigen of the thyroid gland and retro bulbar tissues, play a crucial part [8, 9]. This non-specific antigen could also be the receptor of TSH because it is also expressed on fibroblasts and orbital preadipocytes. Coordination between the ocular changes' degree and therefore the level of stimulating antibodies guiding opposite to TSHR (TRAb) is also reported. Another autoantigen that has been proposed is the growth factor-1 receptor (IGF-1R) which is insulin-like, as GD patient's antibodies may active the IGF-1R. Self-antibodies guided against the receptor take part in the triggering of orbital fibroblasts in GO, and enhanced IGF-1R impregnation is seen in individuals with GD in both thyroid gland and orbiting tissues.
Warwick et al. found that depleting immunoglobulins from GD patients reduced the stimulating action of IGF-1R. Though the antibodies opposite to IGF-1R may be involved in GO formation, there are several problems with the theory. Minich et al. produced a result that does not support the notion which is the presence of circulating antibodies in the blood of a patient worsens GD, nor do they support the utility of a detecting characteristic of the illness. Production of cytokine and its inflammation, synthesis of hyaluronan, myofibrillogenesis, and adipogenesis are the primary mechanisms implicated in the pathophysiology of orbitopathy associated with the thyroid. The circulating adipose tissue and extraocular muscular fiber tissue are the primary sources of continuous inflammation. Active mononuclear cells, like T cells, penetrate the orbital tissues, as do plasmocytes, macrophages, and mast cells to a lesser extent. Cytokines generated by leukocytes, like IFN-, IL-1 (IL-5), and neuregulin (lymphokine produced by active lymphocytes), stimulate glycosaminoglycan production (GAG).
Orbital fibroblasts are triggered by mediators of inflammation (cytokines) or direct cell contact, and they have different morphological and functional characteristics than fibroblasts in other locations. Furthermore, the stimulation of circulatory fibroblasts by TRAb suggests a connection between GD and GO. Activated circulatory fibroblasts proliferate, develop into cells in adipose tissues and myofibroblasts, and perform an important role in outer cellular matrix synthesis. Excess circulatory fibroblast action contributes to orbital tissue growth, remodeling, and fibrosis. The area of tissues around the eyes expands during the dynamic state of orbital alterations as a result of cell infiltration inflammation and edema, leading to a rise in pressure of the intraocular portion. In this consequence, the eyeball extends behind the bony rims of the portion. Furthermore, compression of the optic nerve can develop, leading to neuropathy of the eye, and decreased venous and outflow of lymphatic from the orbit. The last stage (inactive phase) of exophthalmos includes the fibrosis of eye muscle [10, 11].
3. Production of Cytokine and inflammation
The inflammation procedure in orbital tissues guides immune cell transmigration and infiltration, which is similar to the procedure that occurs in the thyroid gland. T cells infiltrate the soft ocular tissue and produce cytokines which aid in tissue responsiveness and remodeling. The early phase of GO is marked by enhanced Th1 lymphocyte activity, which facilitates cell-mediated immunity and produces IL-1, IL-2, TNF-, and IFN [12]. The pro inflammatory cytokines promote the proliferation of fibroblast and the generation of hydrophilic GAG. Moreover, the inflammatory process activates Th2 cells, which produce cytokines such as IL-4, IL-5, IL-10, and IL-13, which activate humoral responses and IgG products. Tissue remodeling and fibrosis define the late phase of GO. Cytokines, chemokines, and growth factors are all produced and have a significant influence on cells in orbital tissues. IFN- stimulates fibroblasts to produce CXCL9, CXCL10, and CXCL11, which promotes lymphocyte migration to orbital tissues.
Furthermore, IFN- increases IL-1 release, and both (synergistically) induce GAG production by orbital fibroblasts. However, unlike IL-1, IFN- suppresses fibroblast adipogenesis. It has been demonstrated that IL-1 stimulates orbital fibroblasts to release CCL5, CCL2, IL-6, IL-8 which are chemical attractants for T and B cells, monocytes, and neutrophils [13]. Fibroblasts of the orbital portion, like thyrocytes, macrophages and lymphocytes represent the costimulatory protein which is CD40. The contact between the ligand (CD154) on T lymphocytes and the molecule of CD40 on the surface of orbital fibroblast increases the synthesis of numerous mediators of inflammation like (IL-8, CCL2, IL-6, IL-1) and the working and spreading of the cells. Prostaglandin E2 (PGE2) promotes the maturation of B lymphocytes, stimulates the fibroblasts of the orbital portion to produce IL-6, and triggers mast cells. The process of recruiting self-reactive T lymphocytes is aided by generally generating or orbiting the adhesive particles whose expressiveness is triggered by cytokines. Interactions between CD49 and CD154, TNF-a, IFN-y, IL-1, and IL-1 increase the expressiveness of adhesive molecules inside the cell (ICAM-1) in fibroblasts of the orbital portion. Adhesive chemicals stimulate and attract T cells, concluding in the responsiveness of cells and the initiation of the active part of the ophthalmopathy. In individuals in the triggering phase of the illness, increased levels of ICAM-1 and L-selectin are being documented.
It is subjected that the reason for the formation of GO is the absence of regulatory T lymphocytes (Tregs) to take control of the inflammation reaction opposite to auto issues (antigens) [40]. Tregs have responsibility for the suppression of the immune response by the release of IL-10 and TGF-β [41]. Under physiological conditions, Tregs destruct self-reactive T lymphocytes, directed against the follicular part of thyroid antigens. Glick et al. discovered that individuals with autoimmune thyroid disease (GD or Hashimoto's disease) who did not receive glucocorticosteroids for at least six months had reduced suppressor activity of Treg cells. According to Klatka et al., patients with GD have a lower number of Tregs and a greater Th17 cell count when compared to healthy participants. Th17 cells are thought to have a substantial role in inflammatory infiltration, as evidenced by their function in autoimmune disorders. The presence of Th17 lymphocytes in the inflammatory infiltration of orbital fat has been documented; however, there is no evidence of the presence of Th17 lymphocytes in the peripheral blood of GO patients [14]
4. Hyaluronan Synthesis
Synthesis of GAG in large concentrations by orbital fibroblasts is the most significant process that takes place in retro-ocular connective tissue. Retrobulbar tissue edema is caused by the accumulation of collagen and hyaluronan acid. In the laboratory, the affected orbital fibroblast producing GAG in higher concentration is treated with IFN- γ 16. Research shows that orbital fibroblast produces hyaluronan acid due to the effect of prostaglandins and some inflammatory response mediators such as TGF-β, and, IGF-1, etc. Regulation of hyaluronan acid is controlled by Hyaluronan synthases, an enzyme produced by IL-1b 17.
5. Adipogenesis and Myofibrillogenesis
Preadipocytes are a part of orbital fibroblasts that is capable of distinguishing between fibroblast and mature adipocytes. Peroxisome proliferator-activated receptors (PPARγ) are nuclear receptors of adipocytes that are thought to be responsible for this expression. (PPARγ) are an important factor of transcription involved in the homeostasis of glucose and lipids. (PPARγ) controls expression of TSHR and also adipogenesis along with rosiglitazone. It also inhibits the secretion of hyaluronan and orbital inflammation. The enzyme cyclooxygenase-2(COX2) is involved in the release of proadipogenic prostaglandin and GO patients, the (COX2) activity is abnormally enhanced that triggers the process of adipogenesis by prostaglandins. The factors Thy1 (CD90) + and Thy1− are distinguished from each other by the action of CD90 glycoprotein. Fibroblasts are accelerated into adipogenesis by some factors such as IL-16, PGD2, and, IL-1β. Research shows that adipogenesis is inhibited by the action of TNF-α and IFNγ 15. The output of these researches concludes that cytokines instead of tissue remodeling and fibrosis are involved in the early phases of ophthalmopathy. The Thy1+ fibroblasts are capable of developing into myofibroblasts. Myofibroblasts are present in muscles and participate in important functions such as collagen accumulation and muscle contractions18. The antiadipogenic factors released by Thy1+ fibroblasts inhibit the differentiation of Thy1− fibroblasts. The introduction of TGF-β and the quantity of Thy1- and Thy1+ causes extraocular muscle and the involvement of adipose tissue in GO patients19.
6. Putative Autoantigens and Potential Treatment
6.1 TSH Receptors
On thyroid epithelium, the antibodies produced affect TSHR and cause hyperthyroidism in patients with the grave disease [24, 31]. Pathogenesis of GO can be demonstrated with the help of a receptor present on the surface of adipose tissue. The enhanced role of TSHR causes orbital adipose tissues in GO patients and also in patients having dermopathy21. The proportion of antibodies secreted against TSHR is directly proportional to the complications in GO patients. TRAb is involved in active GO more than inactive GO. Studies show patients with thyroidal TSHR and extrathyroidal TSHR have some analogous affects20. PDGF-AB and PDGF-BB increase the response produced by orbital fibroblast towards TRAb. The activity of TSHR is lessened by TGF-β. Cytokines and hyaluronan secretion is stimulated by cAMP activated by TSHR and TRAb which also causes orbital fibroblasts [23].
The amplified TSHR response is reported in GO patients preferably in mature fat cells. The contribution of autoantibodies causes adipogenesis not only in the case of hypothyroidism but also in adipose tissues of patients having GO25. Hyaluronan synthesis is caused by TRAb, GD-IgG, and TSH by studying the isolated and purified antibodies of GD-affected persons. The scientist thought that TSHR can help in the treatment of Grave disease as it plays a significant part in GD29.
6.2 Insulin-Like Growth Factor-1 Receptor (IGF-1R)
IGF_1R is an important antigen is expressed in thyrocytes and also in orbital adipose tissues of GD patients. It also participates in immune response, development, and apoptosis. In the immune system, IGF_1R stimulation of B and T lymphocytes [28]. The abnormal concentration of IGF_1R in GO and GD patients. Production of T lymphocytes by the activity of GD-IgG causes orbital fibroblasts in GO patients. The autoimmune response is initiated by a T-cell chemoattractant. Orbital fibroblast is differentiated by IGF-1R into adipocytes.
Thyroid cells undergo metabolism and maturation by the collaboration of TSH and IGF-1 factors. An antibody mostly mentioned as IGF-1R blocking immunogen prevents kinase signaling regulated by the action of TSH 27. The relation between IGF-1R and TSH can be found by this antibody. The function of TSH and IGF-1R in fibrocytes is to prevent other blocking antibodies and is referred to as a breakthrough therapy [30].
7. Role of Natural killer cells in Grave's disease
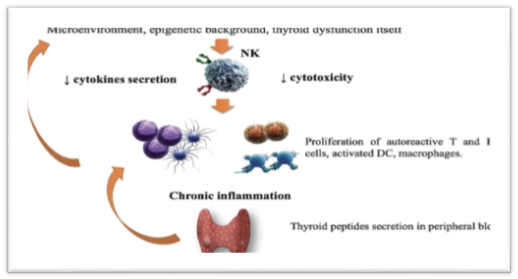
Natural killer cells are the immune cells that are the large granular lymphocytes. They are the subtype of the lymphoid cells that are involved in the defense against viruses and also involved in other cytotoxic activities. About 20% of the lymphoid cells that are circulating in our body are natural killer cells (NK). A decrease in the number of NK shows involvement in many autoimmune disorders.
In different researches, scientists study the role of Natural killer cells in Grave's disease. It is observed that in patients with Grave's disease amount of natural killer cells decreases as compared to the healthy person. According to one hypothesis, it was said that the decrease in the amount of the natural killer cells increases the proliferation of the B cells that cause the production of a large number of autoantibodies that result in the inflammation of the thyroid gland. The Amount of T cells also increases due to it. But still, this hypothesis is contradicted and inconclusive because there are many other immunogenic factors involved in Grave's disease that are still unknown.
According to some scientists, natural killer cells are the secondary factor in Grave's disease, but some said that it is directly related to increasing the chances of having GD. In the following table22, it is clearly shown that the NK and activated NK cells are significantly less than a healthy person.
The blood FT3 and FT4 levels are negatively correlated with the proportion of activated natural killer cells in GD patients. There is a positive relationship between the activated NK cells and serum TSH levels. Abnormalities in NK are found in GD patients but the results are still controversial. Pathogenesis of GD is complicated as it involves many immunogenic factors in which NK cells are also involved in GD.
KIRs (killer Ig-like receptors) by attaching with HLA are involved in the regulation of the function of NK cells by activating or suppressing the function of natural killer cells. Some studies have shown that there is a significant decrease in the amount of KIRs in GD patients as compared to the healthy person which may be the reason for the impaired immune competence of killer cells in Grave's disease patients. But other factors intricate in the guideline of NK cells that maybe result in abnormalities in NK cells and cause GD [26].
The number of natural killer cells that are decreased in Grave's disease patients can be normalized by treating the NK cells with DHEAS (dehydroepiandrosterone sulfate). Anti-thyroid drug treatment can improve the functionality of natural killer cells (impairment of Natural killer cells).
8. Grave's disease and Pregnancy complications
The hormones produced by the thyroid gland are beneficial but an increase or decrease in the amount of these hormones in pregnancy results in many complications. It may result in miscarriage or stillbirth or it may cause the death of the mother if it is not treated timely.
Hyperthyroidism in pregnancy is caused by Grave's disease that can cause many complications like morning sickness which results in vomiting during the pregnancy23. Complications that cause by the mother are:
- Anemia
- Preeclampsia (it happens after the 20th week of pregnancy or after delivery, it is caused by high BP).
- Pulmonary hypertension
- Placental abruption (separation of the uterus from the placenta before birth)
- Heart failure
- Thyroid storm (rare & life-threatening condition)
- Myxedema (result in a coma & even death but is a rare condition)
- Postpartum hemorrhage (PPH) (heavy bleeding after happens within 1 day up to 12 weeks after giving birth).
- Complications that cause in babies are:
- Miscarriage or stillbirth
- Premature birth
- Goiter
- Low birth weight
- Thyroid problems
- Abnormalities in the growth and development of the brain/ nervous system (can result in low IQ).
Infantile myxedema (can cause dwarfism, intellectual disability, and less development of the skills that are needed for daily life).
9. Environmental & dietary factors
Environmental and dietary factors that influence thyroid function and can cause the autoimmune condition like Grave's disease [24] are:
- Iodine consumption
- Radiation
- Poly halogenated biphenyls (PCB's & PBB's)
- Virus infection
- Interferon and iodine-containing drugs
- Smoking
- Pesticides
- Selenium
- Vitamin D deficiency
A person who smokes has 2 fold more chances to develop GD than non-smoker ones. At the same time, the consumption of excess iodine can also result in Grave's disease. In a few years, it will become a major problem as daily consumption of iodine reached 1 mg in a large population in the US. Environmental factors increase the risk of GD up to 20% as they influence the immune response of the person. Radiation also increases the risk of GD, as it also can result in severe thyroiditis and cancer. Selenium is essential for normal body function as it is involved in defense against viruses and also in other functions. The required range of selenium is 60 to 75 micrograms per day if this amount is not taken then a decrease in the number of selenium results in the development of GD [25].
Different viruses are also related to GD, in which retrovirus is mainly included. Virus-like EVB19 is related to GD. But the relation between EVB19 and GD is still unclear and it is under investigation [27].
10. Immunologic perspectives on genetic variables linked to GD
HLAs on chromosome 6 play a critical function in the immune system and are widely variable amongst individuals. HLA-A to -C (MHC class I) particles are found in practically all tissues and present antigens to CD8+ T lymphocytes. HLA-D (MHC class II) particles are typically expressed on antigen presentation cells (APCs), such as dendritic cells or B cells, and contemporaneous antigens to CD4+ T lymphocytes. According to GWAS analysis, they confer the highest vulnerability for GD of all recognized genes. Their sequence variation is mostly due to mutations in the MHC protein's antigen-presenting pocket.
According to one explanation, autoantigens are given to CD4+ T lymphocytes via MHC due to genetic modifications in areas coding for the pocket's structure. In addition, interactions between HLA receptors and autoantigens may open up even additional options. The finding of GD-susceptible variations in the thyroglobulin (TG) gene backs up this theory; just a few TG peptides can tie unequivocally to an infection powerless HLA-DR3 pocket, while others cannot. Additionally, as a result of this, there is less thymic negative selection of autoreactive T cells [28].
10.1 Mutations linked to a reduction in central T cell tolerance
Immune system microorganisms whose TCR distinguishes autoantigens are dispensed with by apoptosis in the thymus, a cycle known as bad determination. TCR articulation just as incessant Lymphocyte flagging is needed for this procedure. TCR flagging, antigen experience responses, and positive and negative choice in the thymus are constrained by CD3, which is situated on all Immune system microorganisms. Transformations in the CD3 quality on fringe Lymphocytes produce low TCR articulation and, thus, broken negative choice of immune system White blood cells in the thymus. There have been nine individuals determined to have familial immune system thyroiditis and CD3 lack up to this point. These patients are more helpless to contaminations because of diminished White blood cell initiation by antigens.
The autoimmune regulator (AIRE) is a transcription factor that guarantees that antigens that are normally found outside the thymus, such as insulin, are expressed there. Organ-specific autoimmunity is caused by human AIRE deficiency, which is assumed to be the outcome of a dysfunctional central immune system. AIRE deficiency is usually related to autoimmune adrenalitis, while AIRE polymorphisms are not [29].
Thymic negative selection occurs when t cells that recognise autoantigens with their tcr perish as a result of activation-induced apoptosis. Fasl does this by attaching to its receptor fas (cd95) and activating caspases eight and three, resulting in t cell death. Connection with fas and fasl transformations brings about immune system lymphoproliferative disorder, an essential immunodeficiency set apart via immune system cytopenia and other immune system signs. Surprisingly, 24 out of 28 GD patients with fas ligation demonstrated impaired in vitro t cell death.
10.2 Mutations linked to a reduction in peripheral T cell tolerance
Treg cells, which stifle autoreactive Lymphocyte enactment in various ways, are found in the fringe blood of solid people and monitored. These Treg cells precise the record factor forkhead box P3 (FOXP3), which is required for Treg cell development and upkeep.
Patients with trisomy 21 had a higher incidence of GD than the general population, as well as an earlier onset of disease and more associated AIDs. Although these patients have a higher number of Treg cells in their peripheral blood with normal proliferation capacity, their cells have a significantly lower inhibitory function, which cannot be attributed solely to the constitutional initiation of the IFN pathway.
Only human Treg cells have a lot of the IL-2 receptor chain (CD25) in the naive (non-activated) state; IL-2 is needed for Treg cells to maintain their normal life cycle. In humans, CD25 loss causes an IPEX-like phenotype. Treg cells have also been discovered in GD patients to be in lesser numbers and to be dysfunctional. A mutation in the CD25 gene was connected to GD among untreated Russians, but these findings could not be replicated in Han Chinese [30].
CTLA-4 acts similarly to programmed cell death protein 1 in that it causes GD when it is blocked. CTLA-4 expression may be reduced by heterozygous germline mutations (CTLA-4 haploinsufficiency). As a result, they have autoimmune and lymphoproliferative illnesses, as well as antibody deficits. Even though the number of peripheral Treg cells in these patients has grown, they are dysfunctional due to reduced CTLA-4 ligand binding and APC CD80 and CD86 trans-endocytosis. Mutations in the machinery that removes cell debris
It is crucial to sufficiently discard junk from apoptosis, rot, and circling safe edifices all through one's life. Mfg-e8 and supplement factor c1q are two proteins that keep up with this framework working [31]. Widespread lupus-like autoimmunity is caused by a malfunction in the apoptotic cell clearance pathway. This has been shown in mice with mfg-e8 deficit and in patients with c1q deficiency who have lupus-like illness. Thyroid function is connected to autoantibodies against C1q in autoimmune thyroid disease.
Multiple cell types release micro vesicles into the bloodstream during apoptosis, which signifies metabolic stress. The figure of micro vesicles (diameter 1 m) in the blood of GD patients was found to be higher in GD patients' blood than in healthy controls' blood and to remain raised following pharmacological therapy, albeit at a reduced level [80]. This idea could lead to the development of a novel biomarker to aid in the treatment of GD patients in the future.
10.3 Risen Interferon (IFN) levels
Although TRAb has been recognized to play a role in hyperthyroidism since 1962, it took nearly another 30 years to completely comprehend the nature of their involvement. Anti-TG and anti-thyroperoxidase antibodies, for example, can present in serum years before autoimmune thyroid disorders manifest, whereas TRAb only appears in serum a few months before a GD diagnosis. This emphasizes how they are only active at the very end of the autoimmune response that leads to the onset of GD. TRAb can stimulate (TSAb), inhibit (TBA), or neutralize (Nab) (TBII). Despite this, approximately 5% of patients (even those with anti-thyroperoxidase antibodies) are antibody-negative.
In light of these discoveries, GD has for quite some time been thought to be a Th2-prompted B cell sickness. The examination depicted underneath, nonetheless, recommends that the beginning stage is constrained by Th1 cells attacking the thyroid. Because IFN-and TNF is delivered by Antigen-presenting cells and B cells in vitro, thyrocytes express C-X-C theme chemokine 10 (CXCL10). By interfacing with its related receptor (CXCR3), CXCL10 is conjectured to draw in the cells, and this impact is more grounded in patients with higher CXCL10 serum levels.
Lymphocytes that cross-respond with B cells to make TSAb have a place with the IgG1 subclass. The way that taking out the qualities for IL-4 or IFN-in paired knockout creatures decreases the advancement of GD by TSH-R-delivering adenoviruses upholds this idea, recommending that both Th1 and Th2 cytokines are fundamental [32].
CXCL10 serum levels are likewise a lot higher in GD patients all through the dynamic period of the sickness, and they decline after ATD therapy in vitro and in vivo, just as with radioactive iodine treatment. Serum CXCL10 levels drop significantly after thyroidectomy, showing that thyrocytes are the chief wellspring of the chemical. This procedure could explain why the thyrocytes of GD patients survive the autoimmune reaction but not those of HT patients. Additionally, an enhanced Th2 response leads to a rise in TBAb production, which includes all IgG subtypes.
One cleverer cytokine, osteopontin (OPN), was found in osteoblasts in 1986, although its job in Help has not been set in stone. OPN is delivered by a scope of cell types, plus chondrocytes, dendritic cells, Lymphocytes, and macrophages, after being set off by supportive of fiery occasions or cytokines (e.g., angiotensin II, TGF-, TNF-, IL-1b, hyperglycemia, nitric oxide, and hypoxia).
11. Immunologic perspectives on epigenetic mechanisms linked to GD
As previously noted, monozygotic twins had a 75% concordance for GD, showing that epigenetic changes play a key role in GD. Epigenetic alterations have been demonstrated to have a major impact on patients with PIDs. Indeed, a recent study of epigenetic alterations in two monozygotic twins who were discordant for antibody deficiency discovered that the twin with antibody deficiency showed an increase in DNA methylation of many genes important for B cell function [33].
Miniature RNAs are additionally engaged with epigenetic control. MicroRNAs are by and large 22 nucleotides in length and hinder quality interpretation by slowing down mRNA, either by severing mRNA when the two arrangements are indistinguishable or by smothering interpretation when the successions are just somewhat indistinguishable. MicroRNA tests were used in one examination to uncover that the amount and capacity of CD4+ CD25+ FOXP3+ Treg cells in the blood of GD patients had altogether diminished. The retinoic corrosive pathway was similarly obstructed in those Treg cells, even though it very well may be reestablished in vitro utilizing the counter-all-trans retinoic corrosive.
Positively charged histones bond tightly to negatively charged DNA and block DNA transcription, according to another suggested epigenetic transformation mechanism. Acetylation of lysine residues at histone ends reduces their positive charge, allowing DNA to move from a packed heterochromatin structure to an unfolded euchromatin one. Acetyl coenzyme-A provides the acetyl groups, and histone acetyltransferase and deacetylase aid in the process. Only one study looked at the degree of histone acetylation in GD, and it found that histone 4 acetylation in PBMCs was reduced. Many other AIDs patients have altered acetylation patterns, thus the clinical significance of this finding is uncertain.
Diverse histone methylation examples may likewise affect fringe resilience. cd247, lck, zap70, cd3d, cd3e, cd3g, ctla4, and cd8a were displayed to have particular examples of hyper-and hypomethylation of histones in cd8+ and cd4+ lymphocytes cleansed from entire blood. Given the different flagging pathways included, this opens up an entirely different universe of immunological guideline prospects. The most powerful kind of epigenetic modification is DNA methylation at the 5' carbon on the pyrimidine ring of cytosine by an assortment of DNA methyltransferases utilizing S-adenosyl-methionine as a co-factor. This methylation is generally irreversible, bringing about chromatin that is more compacted and less quality record. A few quality advertisers are hypomethylated in Helps, which builds records (for instance, PRF1 in SLE). As per a new Chinese case-control exploration of genome-wide methylation, unprocessed female GD patients have essentially lower methylation of the ICAM-1 quality in entire blood leukocytes than typical controls. Therefore, they had more significant levels of ICAM-1 mRNA articulation than solid controls.
The analysts found that expanded ICAM-1 articulation on platelets might play a part in the fiery reaction. In any case, this connection has just been seen in females, and the discoveries should be approved in non-Asian people. Since the majority of the examinations assessed here utilized a case-control configuration, it's difficult to preclude the likelihood that epigenetic changes happened unexpectedly during treatment, were set off by treatment, or those other co-factors, like smoking, and assumed a part. Furthermore, hyperthyroidism can cause epigenetic changes all alone. Yet, we know nothing about any longitudinal exploration that could reveal new insight into this point.
12. Infection-related link
Following a middle of 17 months on the exceptionally dynamic antiretroviral drug, GD has been accounted for to happen all the more generally in HIV patients (HAART). HAART-instigated immunological reconstitution happens because of the huge creation of guileless White blood cells since thymus. IL-7 could be the Immune system microorganism simple of BAFF, as it is significantly expanded in territories of Lymphocyte lymphopenia and is embroiled in the endurance/multiplication of autoreactive White blood cells. In one out of each nine patients, a treatment tries utilizing three dosages of recombinant IL-7 to help fringe Lymphocyte numbers in individuals with White blood cell lymphopenia brought about the enlistment of fundamental lupus erythematosus (SLE)
Yersinia enterocolitica disease was viewed as more normal in GD patients in the last part of the 1970s [42,43,44], albeit cross-responding proteins were not found up to this point [45]. Hargreaves et al [45] displayed in 2013 that TRAb work is gained solely after early forerunner B cells go through physical hyper-change and freak antibodies perceive Y. enterocolitica external film porins A, C, and F. As indicated by the researchers, memory B cells might foster cross-reactivity, permitting them to perceive both the underlying microorganism and the thyroid-animating chemical (TSH) receptor (TSH-R).
Hepatitis C (HepC) infection contamination, just as its connections to cryoglobulinemic vasculitis and Sjögren's illness, inclines toward GD. Shockingly, immune system thyroid illnesses, outstandingly GD, are more normal in HepC patients who have had interferon treatment than in Hepatitis B patients (IFN) One justification for this could be the HepC infection and thyroid proteins show homologies. The connection between type I IFNs and Help is most obviously shown in the interferonopathies, a gathering of monogenic PIDs that part an overexpression of IFN-managed qualities. A significant number of them have chilblain-like lupus-like skin injuries and calcifications in the focal sensory system. In a new investigation of autoimmunity in these patients, an adolescent with a hereditarily demonstrated monogenic interferonopathy was determined to have immune system thyroiditis [34]. It's fascinating to see that patients with trisomy. 21 have a higher pace of GD and give a significant number of indications of interferonopathy in such a manner. It's conceivable that the IFN type I receptor's area on chromosome no. twenty-one has something to do with this. Since IFN-Rs have a bigger quality burden, the IFN pathway might be continually locked in.
13. Mutations affecting B cells in Grave's disease
B-cells are the type of white blood cells that originate from hematopoietic stem cells and mature in the bone marrow. B cells are activated in secondary lymphoid organs -i.e. spleen and lymph nodes. They function in humoral immunity and produce antibodies.
In Grave's disease Thyrotropin Receptor Antibodies (TRAb) become activated against Thyroid Stimulating Hormone Receptors (TSHR) by B- cells that are a characteristic diagnostic feature of this disease [35]. TSHR (Thyroid Stimulating Hormone Receptors) are present on follicular cells and hence when pathognomonic TRAb bind to these receptors, they stimulate it, as a consequence it results in the increased production and secretion of T4 and T3 thyroid hormones. Hence the condition becomes chronic. B-cells also play a role as APC (antigen-presenting cells) as they have BCR receptors also known as transmembrane receptors that help B cells to identify any foreign antigen and initiate an immune response by producing antibodies and thereby these fragments of antigens to CD4+ T cells by MHC Class-II. When an uncommon antigen enters the body B cells act as major APCs as they bear BCR- transmembrane receptors. Here T-cells are involved in the activation of B cells. [36] Hence, sequencing of TAb Thyroid antibodies and B-cell epitopes in TSH thyroid-stimulating hormone enables us to understand the pathophysiology of Grave's Disease. So, B cells produce antibodies in the thyroid gland which can lead to auto reactive B cells infiltration of thyroid tissue. Hence the therapies that reduce B cells in TH are proving to be efficient in their treatment -i.e. anti CD- 20 therapy, which provides strong evidence of B cells involvement in Grave's Disease [37].
14. Role of Regulatory B cells (Breg)
Regulatory B cells participate in the suppression of immune response. They regulate the immune system by different mechanisms -i.e. by producing anti-inflammatory cytokine interleukin-10 (IL-10). B regulatory cells are important in maintaining Treg cell components as they can inhibit Th1 immune response by producing IL-10 during chronic conditions. They also suppress The Th17 cell differentiation indirectly by suppressing the production of inflammatory cytokine by dendritic cells. The evidence is shown by studies that Treg cells get reduced in mice with B cell deficiency. Hence Breg cells and T-cells control the induction of Treg cells. [40] Breg cells also produce Transforming Growth Factor (TGF- beta) that induce CD8+ energy and lead to apoptosis of CD4+ effector T-cells.
14.1 Tolerance in autoimmunity by Breg cells
Studies conducted at a university show that when Breg cells were exposed to an inflammatory cytokine that was at elevated levels, they chronically lead to a reduction of the functional Breg cell populations. Hence they were unable to restore tolerance. Thereby showing Breg cells dysfunction in human diseases [38].
15. Conclusion and future aspects
These studies conducted on Breg cells have helped in the understanding of the future treatment of Grave's disease that will mainly focus on the restoration of immune tolerance. Clarification of Breg cell's involvement in the regulation of immune response can provide information for the development of new B cells mediated therapeutic strategies for AITD (Autoimmune thyroid diseases) -i.e. Grave's disease [39].
16. Bibliography
- Smith TJ and Hegedüs L, "Graves' disease," New England Journal of Medicine 375 (2016): 1552-1565.
- Piantanida E, "Preoperative management in patients with Graves' disease," Gland Surgery 6 (2017): 476-481.
- Lin JD, Yang SF, Wang YH et al., "Associations of melatonin receptor gene polymorphisms with Graves' disease," PLoS One 12 (2017): e0185529.
- Khong JJ, McNab AA, Ebeling PR, Craig JE, and Selva D, "Pathogenesis of thyroid eye disease: review and update on molecular mechanisms," British Journal of Ophthalmology 100 (2016): 142-150, 2016.
- Salvi M, Vannucchi G, Currò N, et al. "Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study," The Journal of Clinical Endocrinology & Metabolism 100 (2015): 422-431, 2015.
- Bahn RS, "Graves' ophthalmopathy," New England Journal of Medicine. 362 (2010): 726-738, 2010.
- Bartley GB, "The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota," Transactions of the American Ophthalmological Society 92 (1994): 477-588.
- Fernando R, Atkins S, Raychaudhuri N, et al., "Human fibrocytes coexpress thyroglobulin and thyrotropin receptor," Proceedings of the National Academy of Sciences 109 (2012): 7427-7432, 2012.
- Bahn R, "The EUGOGO consensus statement on the management of Graves' orbitopathy: equally applicable to North American clinicians and patients," Thyroid 18 (2008): 281-282.
- Sahli E and Gunduz K, "Thyroid-associated ophthalmopathy," Turkish Journal of Ophthalmology 47 (2017): 94-105.
- Wall JR and Lahooti H, "Pathogenesis of thyroid eye disease—does autoimmunity against the TSH receptor explain all cases?" Endokrynologia Polska 62 (2011): 1-7.
- Diana, R. S. Brown, A. Bossowski, et al., "Clinical relevance of thyroid-stimulating autoantibodies in pediatric Graves' disease-a multicenter study," The Journal of Clinical Endocrinology & Metabolism 99 (2014): 1648-1655.
- Place RF, Krieger CC, Neumann S, and Gershengorn MC, "Inhibiting thyrotropin/.
- insulin-like growth factor 1 receptor crosstalk to treat Graves' ophthalmopathy: studies in orbital fibroblasts in vitro," British Journal of Pharmacology, vol. 174, no. 4, pp. 328-340, 2017. .
- Smith J and Hegedüs L, "Graves' disease," New England Journal of Medicine 375 (2016): 1552-1565.
- Piantanida E, "Preoperative management in patients with Graves' disease," Gland Surgery 6 (2017): 476-481.
- Smith TJ, Tsai CC, Shih MJ et al., "Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy," Thyroid 18 (2008): 983-988, 2008.
- Han R and Smith TJ, "T helper type 1 and type 2 cytokines exert divergent influence on the induction of prostaglandin E2 and hyaluronan synthesis by interleukin-1β in orbital fibroblasts: implications for the pathogenesis of thyroid-associated ophthalmopathy," Endocrinology 147 (2006): 13-19.
- Nagayama Y, "Animal models of Graves' hyperthyroidism," Endocrine Journal 52 (2005): 385-394.
- Feldon SE, O'Loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, and Phipps RP, "Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes," The American Journal of Pathology 169 (2006): 1183-1193, 2006.
- Konuk EBY, Konuk O, Misirlioglu M, Menevse A, and Unal M, "Expression of cyclooxygenase-2 in orbital fibro adipose connective tissues of Graves' ophthalmopathy patients," European Journal of Endocrinology 155 (2006): 681-685.
- Iyer S and Bahn R, "Immunopathogenesis of Graves' ophthalmopathy: the role of the TSH receptor," Best Practice & Research Clinical Endocrinology & Metabolism 26 (2012): 281-289.
- Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, et al, "Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: a randomized clinical trial," American Journal of Ophthalmology 195 (2018): 181-190.
- Zhang HQ, Zhao JJ, Zhao YR, et al. Genotype analysis of killer cell immunoglobulin-like receptors in Graves' disease patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chinese Journal of Cellular and Molecular Immunology 25(2009): 699-701.
- Zhang HQ, Zhao JJ, Zhao YR. Association of killer cell immunoglobulin-like receptor gene polymorphisms with Graves 'disease. Chinese Journal of Endocrinology and Metabolism 22 (2006): 130-131.
- https://www.marchofdimes.org/complications/thyroid-conditions-during-pregnancy.aspx#:~:text=If%20you%20have%20Graves'%20disease%20during%20pregnancy%2C%20your%20baby%20is,Miscarriage%20or%20stillbirth
- https://www.jstor.org/stable/3429364
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5357628/
- Weetman AP. Graves' disease. New England Journal of Medicine, 343(2000), 1236-1248.
- Smith, TJ, Hegedüs L. (2016). Graves' disease. New England Journal of Medicine 375 (2016): 1552-1565.
- Volpé R. The pathogenesis of Graves' disease: an overview. Clinics in Endocrinology and Metabolism 7 (1978): 3-29.
- Volpé R. The pathogenesis of Graves' disease: an overview. Clinics in Endocrinology and Metabolism 7 (1978): 3-29.
- Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and the proportion of Th17 cells in intractable Graves' disease. Thyroid 19 (2009): 495-501.
- Gallo D, Piantanida E, Gallazzi M, Bartalena L, Tanda ML, Bruno A, Mortara L. Immunological Drivers in Graves' Disease: NK Cells as a Master Switcher. Frontiers in Endocrinology 11 (2020): 406.
- Kallmann BA, Hüther M, Tubes M, Feldkamp J, Bertrams J, Gries FA, ... & Kolb H. The systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves' disease. Diabetes 46 (1997): 237-243.
- Maravillas-Montero JL, Acevedo-Ochoa E. Human B regulatory cells: the new players in autoimmune disease. Rev Investig Clin 69 (2017): 243-246.
- Miyazaki T, Fujimoto M, Sato S. Regulatory B cells in human inflammatory and autoimmune diseases: from mouse models to clinical research. Int Immunol 27 (2015): 495-504.
- Ray A, Dittel BN. Mechanisms of regulatory B cell function in autoimmune and inflammatory diseases beyond IL-10. J Clin Med 6 (2017): 12.
- ncbi.nlm.nih.gov
- Katz SI, Parker D, Turk JL (October 1974). "B-cell suppression of delayed hypersensitivity reactions". Nature 251 (1974): 550-1.
- m.wikipedia.org