Graphene oxide; Adsorption; Organic, Inorganic; Biological; Radiological contaminants
Article Information
Pascoal Almiro Mazive†, Bo Hu1,2, Haochen Zhu1,2,*, Wenzhi He1,2, Almiro Menino Mazive
†Institute of Environmental and Sustainable Development-Tongji University, 1239 Siping road, Shanghai 200092, China, (UNEP-IESD, Tongji University-Shanghai)
1State Key Laboratory of Pollution Control and Resources Reuse, Key Laboratory of Yangtze River Water Environment, Ministry of Education, College of Environmental Science and Engineering, Tongji University, 1239 Siping Rd., Shanghai 200092, China
2Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, P. R. China
*Corresponding Author: Dr. Haochen Zhu, Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, P. R. China
Received: 09 November 2020; Accepted: 17 November 2020; Published: 29 December 2020
Citation:
Pascoal Almiro Mazive, Bo Hu, Haochen Zhu, Wenzhi He, Almiro Menino Mazive. Graphene Oxide-Based Nanomaterials: The Preparation, Application, and Factors that Affect the Adsorption Capacity on Drinking Water Treatment - Review. Journal of Nanotechnology Research 2 (2020): 060-091.
View / Download Pdf Share at FacebookAbstract
The presence of contaminants in the water is a problem that worries humanity since the consumption of water with inadequate concentrations of contaminants can cause serious problems to human health, and different technologies have been used to reduce or eliminate those contaminants in drinking water. Adsorption has been highlighted because of its low cost and simplicity removal process and, graphene oxide has been reported as excellent adsorbent because it has functional groups that offer stability, antifouling, and hydrophilicity, important to contaminants removal from water. The review of literature related to graphene oxide, water contaminants, and their impact on human health was the basis for the execution of the present work. A thorough analysis of each selected article and critical analysis were made to report the ability to remove contaminants and factors that can contribute positively or negatively to the contaminants removal process. A total of 204 articles were selected, mostly published in the last 5 years, because advances in the use of graphene oxide as adsorbent are reported annually. This article will help to understand better that graphene oxide alone or combined can treat different contaminants from drinking water.
Keywords
Graphene oxide; Adsorption; Organic, Inorganic; Biological; Radiological contaminants
Graphene oxide articles Graphene oxide Research articles Graphene oxide review articles Graphene oxide PubMed articles Graphene oxide PubMed Central articles Graphene oxide 2023 articles Graphene oxide 2024 articles Graphene oxide Scopus articles Graphene oxide impact factor journals Graphene oxide Scopus journals Graphene oxide PubMed journals Graphene oxide medical journals Graphene oxide free journals Graphene oxide best journals Graphene oxide top journals Graphene oxide free medical journals Graphene oxide famous journals Graphene oxide Google Scholar indexed journals Adsorption articles Adsorption Research articles Adsorption review articles Adsorption PubMed articles Adsorption PubMed Central articles Adsorption 2023 articles Adsorption 2024 articles Adsorption Scopus articles Adsorption impact factor journals Adsorption Scopus journals Adsorption PubMed journals Adsorption medical journals Adsorption free journals Adsorption best journals Adsorption top journals Adsorption free medical journals Adsorption famous journals Adsorption Google Scholar indexed journals Organic articles Organic Research articles Organic review articles Organic PubMed articles Organic PubMed Central articles Organic 2023 articles Organic 2024 articles Organic Scopus articles Organic impact factor journals Organic Scopus journals Organic PubMed journals Organic medical journals Organic free journals Organic best journals Organic top journals Organic free medical journals Organic famous journals Organic Google Scholar indexed journals Inorganic articles Inorganic Research articles Inorganic review articles Inorganic PubMed articles Inorganic PubMed Central articles Inorganic 2023 articles Inorganic 2024 articles Inorganic Scopus articles Inorganic impact factor journals Inorganic Scopus journals Inorganic PubMed journals Inorganic medical journals Inorganic free journals Inorganic best journals Inorganic top journals Inorganic free medical journals Inorganic famous journals Inorganic Google Scholar indexed journals Biological articles Biological Research articles Biological review articles Biological PubMed articles Biological PubMed Central articles Biological 2023 articles Biological 2024 articles Biological Scopus articles Biological impact factor journals Biological Scopus journals Biological PubMed journals Biological medical journals Biological free journals Biological best journals Biological top journals Biological free medical journals Biological famous journals Biological Google Scholar indexed journals Radiological contaminants articles Radiological contaminants Research articles Radiological contaminants review articles Radiological contaminants PubMed articles Radiological contaminants PubMed Central articles Radiological contaminants 2023 articles Radiological contaminants 2024 articles Radiological contaminants Scopus articles Radiological contaminants impact factor journals Radiological contaminants Scopus journals Radiological contaminants PubMed journals Radiological contaminants medical journals Radiological contaminants free journals Radiological contaminants best journals Radiological contaminants top journals Radiological contaminants free medical journals Radiological contaminants famous journals Radiological contaminants Google Scholar indexed journals nanomaterials articles nanomaterials Research articles nanomaterials review articles nanomaterials PubMed articles nanomaterials PubMed Central articles nanomaterials 2023 articles nanomaterials 2024 articles nanomaterials Scopus articles nanomaterials impact factor journals nanomaterials Scopus journals nanomaterials PubMed journals nanomaterials medical journals nanomaterials free journals nanomaterials best journals nanomaterials top journals nanomaterials free medical journals nanomaterials famous journals nanomaterials Google Scholar indexed journals photocatalytic articles photocatalytic Research articles photocatalytic review articles photocatalytic PubMed articles photocatalytic PubMed Central articles photocatalytic 2023 articles photocatalytic 2024 articles photocatalytic Scopus articles photocatalytic impact factor journals photocatalytic Scopus journals photocatalytic PubMed journals photocatalytic medical journals photocatalytic free journals photocatalytic best journals photocatalytic top journals photocatalytic free medical journals photocatalytic famous journals photocatalytic Google Scholar indexed journals hygienic articles hygienic Research articles hygienic review articles hygienic PubMed articles hygienic PubMed Central articles hygienic 2023 articles hygienic 2024 articles hygienic Scopus articles hygienic impact factor journals hygienic Scopus journals hygienic PubMed journals hygienic medical journals hygienic free journals hygienic best journals hygienic top journals hygienic free medical journals hygienic famous journals hygienic Google Scholar indexed journals dysentery articles dysentery Research articles dysentery review articles dysentery PubMed articles dysentery PubMed Central articles dysentery 2023 articles dysentery 2024 articles dysentery Scopus articles dysentery impact factor journals dysentery Scopus journals dysentery PubMed journals dysentery medical journals dysentery free journals dysentery best journals dysentery top journals dysentery free medical journals dysentery famous journals dysentery Google Scholar indexed journals
Article Details
1. Introduction
Water is a crucial element of human survival [1, 2] and development [3]. Clean water is a ?nite natural resource, and water consumption is soaring due to the rapid growth of the population [4, 5], industrialization [6-14] and critical disposition of water pollution [15]. Because of the problems illustrated in the current paragraph, the majority of issues that humankind is facing in this area are interlinked to the water quality or quantity concerns and, owing to these water problems, the severe impact on the human health includes lack of better sanitation, exposure to the pathogens via the recreation or food chain, etc. [16].
In recent years, innovative solutions have been devised in attempts to solve these issues for water environmental remediation [17]. For example, graphene-based nanomaterials [18] have exhibited many exciting properties. These include adsorption of metal and organic dyes, antimicrobial capability, and photocatalytic degradation of organic molecules [19]. Because of its unique structure (two-dimensional material) [20-30], graphene oxide (GO) have been intensively investigated [31], for drinking water treatment process.
GO can be obtained from the oxidation of graphite [32-34] and its exfoliation [35]. GO is conventionally prepared by chemical oxidation of graphite using Brodie, Staudenmaier, Hummers methods [2,36-41], or some variations of these methods and subsequent exfoliation of graphite oxide using various reduction techniques like thermal, microwave, laser, etc. [42]. Graphene oxide can be combined with other nanomaterials such as CuO [43], Fe−Mg (hydr) Oxide [44], FeOx [45], MoS2 [46], Ag [47], TiO2 [48], Fe3O4 [49], ZnO [50], to form nanohybrids material, to remove contaminants from water efficiently. The present work will focus on collecting relevant information from studies already done related to the most used graphene oxide preparation methods, its application, and factors that affect the adsorption capacity on drinking water treatment. The work will be useful to the researchers associated with water treatment using graphene oxide because they may have relevant information in this review article.
1.1. General understanding of contaminants in drinking water
Several are the causes (intrinsic and extrinsic) that contribute to the presence of contaminants in water. Ingesting unrecommended contaminant concentrations such as biological, organic, inorganic, and radiological can create collateral damage to living organisms such as plants, humans, animals, etc. Inorganic contaminants such as Fluoride, arsenic [51], lead, copper, mercury, chromium, cadmium [52, 53] may have geological or anthropogenic, natural deposits and industrial practices [54] as sources and, can cause health consequences such as cancer, human health, aesthetic [16] and skeletal deformity [51]. Organic contaminants such as pharmaceuticals [55], pesticides, synthetic and natural hormones, industrial compounds, and personal care products, among others [56], usually the sources are agricultural, public hygienic, industrial and hospital, can cause cancer, hormonal disruptions and nervous system disorder [54] to the human body. Biological contaminants such as pathogenic organisms (bacteria, protozoan, and viruses) [53] are often caused by human and animal fecal waste [57], wastewater from sewage [54]. The contaminants' presence in drinking water can cause dysentery, cholera, and gastroenteritis can damage the nervous system (neurotoxins) and skin [54]. Radiological contaminants such as Uranium [58], alpha particles, beta particles, and photon emitters, radium 226 and radium 228 (combined) [54] can come from natural sources [58], some industrial waste [54] and can cause cancer [58], skin and nervous system toxicity [53]. In particular, graphene oxide (GO) nanosheet (oxygenated graphene sheets bearing carboxyl, hydroxyl, and epoxide functional groups) o?er an extraordinary potential for making functional nanocomposite materials with high chemical stability, strong hydrophilicity, and excellent antifouling properties, all of which are promisingly exploited in water treatment processes. Most water-related applications of Graphene oxide have focused on its adsorptive [19] mechanism to remove different contaminants.
2. Methodology
The objetive of the review is to understand better the synthesis of graphene oxide, its evolution, and the different possible applications in removing biological, organic, inorganic, and radiological contaminants in the water. A total of 204 articles were mostly obtained through the Web of Science and analyzed, discussed, and compared according to the type of contaminants to be removed from the water and consider different factors that may improve water pollutants removal. A critical analysis of some literature was also made based on the experience and detailed explanation reported by other authors in different literature works. To better report up-to-date information, of the 204 articles selected, the overwhelming majority were published between 2015-2020.
3. Graphene Oxide Evolution and Preparation
Several methods are used to produce graphene oxide, which provides distinctly different materials with a significant difference in the relative abundance of various functional oxygen-containing groups. The most common of them are Hummers and Brodie's methods [59-61], Staudenmaier method [62-64], Hofmann method [65, 66], and other modified methods such as modified Staudenmaier's method [67], modified Hofmann method [68], modified Hummer [69-74] and improved Hummer [75-77]. These standard methods have evolved since their discovery to the current date (see Figure. 1); they differ from each other because of the involved chemicals, oxidation time, and environmental safety; for example, C. Schafhaeutl, Brodie, Staudenmaier, and Hofmann's methods use strong chemicals, produce toxic gases and long oxidation time, compared to Hummers and Improved hummers methods. The choice of the procedure to be used depends on each methods advantages and disadvantages (see Table 1) and the purpose of the produced GO.
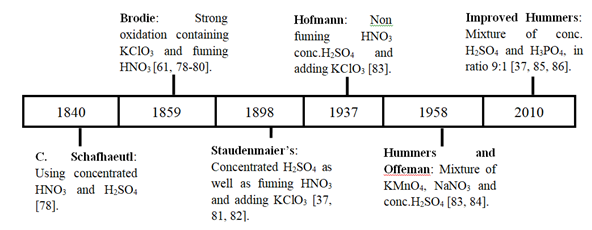
Figure 1: Evolution and difference between methods used to prepare GO using chemical oxidation.
Since the works of literature, [87-91] and [92] shows the synthesis procedure of Graphene oxide using Brodie method, Staudenmaier method, Hofmann method, Hummers method, Marcano Improved Hummers method and modified Hummers method respectively. They provide distinctly different materials with a significant difference in the relative abundance of various functional oxygen-containing groups, signi?cantly different properties [59], and different structures obtained. The distribution of the types of oxygen-containing groups available could affect the performance of the GO (or its exfoliated form, graphene oxide) materials in specific applications [83].
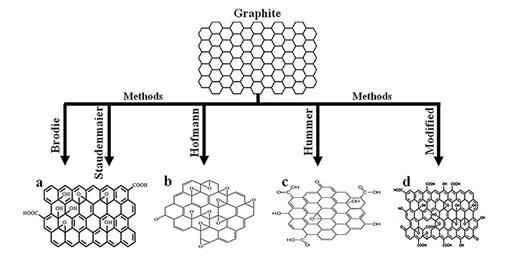
Figure 2: Structure of graphene oxide produced using(a)Brodie and Staudenmaier methods, reprinted with the permission of [93]; (b)Hofmann method, reprinted from the literature [94] with the permission of Spring Nature and Copyrights Clearance Center – license number 4917670969786;(c)Hummer method, reprinted from the literature [95] according to MDPI policy and;(d)Modified method, reprinted from the literature [96] with permission of Elsevier and Copyrights Clearance Center – license number 4917460128617.
Different structures of Graphene oxide (see Figure 2) produced using different methods is shown a few defects [59] and less oxygen [82] (see Figure 2a), more oxygen compared to Figure 2a (see Figure. 2b) and a higher proportion of C=O bond type (see Figures 2c and 2d). The O−C=O bond type occurrence indicates a significant presence of the carboxyl group [83].
|
Methods |
Advantages |
Disadvantages |
|
C. Schafhaeutl |
The earliest method [78]. |
Not clear about the type of graphitic oxidation [78]. |
|
Brodie |
Less aggressive [97]. |
Very long reaction time-up to several days; Have certain risk of self-ignition or explosion [98]; Lower sorption capacity and lower activity [59]. |
|
Staudenmaier |
Highly oxidized Graphene oxide in a single reaction vessel [99], compared to Brodie method. |
Large particles and lower interlayer spacing [100]; Hazard and time consumer [101]. |
|
Hofmann |
Avoid the usage of corrosive fuming HNO3 [102]; High degree of oxidation, small particles, compared to Staudenmaier method [100]. |
Risk of explosion [65, 103, 104]. |
|
Hummer |
Few hours to complete the reaction; Avoid the evolution of explosive ClO2 [105]; Eliminate the formation of acid fog [106]; Non-toxicity [107, 108], compared to above methods. |
Toxic gases are produced [105, 109, 110]; The residual Na+ and NO3- ions are difficult to be removed from wastewater formed [106]; Allows the diffusion of oxidizing agents into the interlayer spaces of graphite [111]. |
|
Improved Hummer [37]. |
Eliminate the generation of toxic gases, avoiding the NaNO3 [106, 112-114]; Large amount of oxidized graphite is provided [115-117]. |
Separation and purification processes are tedious processes [115]. |
|
Modified Hummer |
Improved level of oxidation and, therefore, product performance [115]. |
Production of NOx gases [102, 115]. |
|
Improved method [118]. |
Less acid used; Reduce the cost of waste-acid treatment as well as lowering the impact on the environment. |
The temperature must be well controlled to reduce the safety risk. |
|
Simplified Hummer [119]. |
Oxidized graphene without the use of a strong oxidation agent. |
- |
Table 1: Advantages and disadvantages of the Graphene oxide preparation methods, including some hummer’s modified methods.
4. Strategies for Graphene Oxide Application
Many techniques have been utilized for water puri?cations, in which the adsorption is known as a simple, effective, and economical method to achieve high-performance water puri?cation due to its high ability to remove various soluble and insoluble organic and inorganic contaminants [120]. Graphene-based nanocomposites and its derivatives [52] are developed as the impressive adsorbent materials owing to its outstanding features [121]. Graphene oxide (GO) as the most studied graphene-derivatives, is a precursor material in graphene preparation and has a large number of carboxyl, hydroxyl [122-124], carbonyl, and epoxy carboxyl groups on its surface [125-127], which are responsible for binding both organic and inorganic species [128]. Graphene oxide is hugely hydrophilic and has oxygen functional groups on the structure [8, 129-137], making it ideal to be used in aqueous solution [125]. Water ?ow is afforded due to the spacing between nanosheets, which is typical of the order of 0.3-0.7 nm [138]. It is ideal for water permeation while blocking the transport of larger molecules [139]. Figure 3a shows the adsorption mechanism that graphene oxide has. It is possible to see that only water can pass from the graphene oxide layer; the large and small particles are blocked and not allowed to pass the GO layer.
Many studies are made to prove the significant advantages that graphene oxide has in removing the contaminants from water. Graphene oxide, by itself, is an attractive adsorbent and becomes increasingly interesting in the removal of contaminants when combined with other nanomaterials, forming nanohybrids. To better understand the importance of graphene oxide in the contaminants removal process, table 2 shows different technologies and performance of graphene oxide to remove contaminants from the water.
4.1 Strategies for using graphene oxide to remove bacterias from water
Pathogens are microscopic, single-celled organisms that thrive in diverse environments, including water. To remove the harmful ones, LIU et al [140] studied the antibacterial activity of different graphene-based materials. They found that the produced graphene oxide had higher antibacterial activities than other studied materials (graphite oxide, graphite, and reduced graphene oxide), under similar concentration and incubation conditions. According to them, the reason for GO's excellent bacteria adsorption is related to the size [141] of diameter (small diameter has higher antibacterial activity than larger diameter). Another reason is related to stable dispersions with small nanosheets (because of carboxyl, hydroxyl, and epoxy groups introduced on graphene oxide sheets), thus o?ers more opportunities to interact with cells for cell deposition. They showed that the incubation time and nanomaterials concentration to remove E. coli from water have a relationship. The loss viability increases from 49.1 ± 6.0% after 1h incubation to 69.3 ± 6.1% after 2h, and further increases to 81.5 ± 3.9% after 3h and 89.7 ± 3.1% after 4h. The loss of E.coli viability jumps from 10.5 ± 6.6% at the graphene oxide concentration of 5 μg/mL to 91.6 ± 3.2% at 80 μg/mL. The majority of E. coli was killed after incubation with graphene oxide at 80 μg/mL concentration.
Ming et al [142] studied the adhesion of bacteria to a graphene oxide film. They tested the interaction between graphene oxide film with several bacteria, such as Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Shewanella-MR 1 (Shewanella), and Bacillus. They studied the ability to graphene oxide to get electrons to adhere to bacteria (see Figure 3b). As an electron acceptor, graphene oxide was easier to get electrons from Shewanella than that of the other bacteria studied; it was why Shewanella's adherence to the GO ?lm was low (below 3%). Moreover, the strategy of impressed current provided new insight into the increase in the adherence of bacteria. In this study, they also found that exerting a positive current would draw the electrons from graphene oxide and enhance graphene oxide ability to obtain electrons from bacteria, resulting in effective adsorption of bacteria on graphene oxide ?lms. The ease that graphene oxide has to adhere to bacteria comes from the nature of graphene oxide to get electrons from bacteria. They also reported the dependency between the mass of graphene oxide and adherence effects toward di?erent bacterias. Was possible to understand that when the graphene oxide ?lm mass was in the range of 0.398mg to 3.184mg, the adherence percentages for E.coli changed from 20% to more than 100%, while they changed only from 18.4% to 31.4% for S.aureus, from 12.0% to 52.3% for Bacillus, and from 0.8% to 3.1% for ShewanellaMR-1.
Akhavan and Ghaderi, [143] studied the toxicity against bacteria of graphene oxide nanowalls achieved by electrophoretic deposition of Mg2+ graphene oxide nanosheets. They reported that the bacterias were effectively damaged by direct contact with the nanowalls, very sharp edges of the, resulting in inactivation of the bacteria by the nanowalls; The cell membrane of S. aureus bacteria was more damaged, compared to the cell membrane of E. coli. Assigned the more resistance of the E. coli bacteria against the direct contact interaction with the nanowalls as compared to the S. aureus bacteria to the existence of an outer membrane in the structure of Gram-negative E.coli bacteria and the lack of such an outer membrane in the form of Gram-positive S. aureus bacteria.
Rajapaksha et al [43] studied a hybrid GO-CuO to remove waterborne pathogenic bacteria (E. coli and S. Typhimurium bacteria). The resulting GO-CuO nanocomposite was found to be an extremely effective antibacterial nanomaterial, signi?cantly inhibiting the growth of Escherichia coli and S. Typhimurium bacteria. The GO-CuONP nanocomposite revealed a moderate concentration-dependent antibacterial activity, with the inhibition increasing as a function of substrate concentration. The observed GO-CuONP could dramatically inhibit the growth of E. coli and S. Typhimurium. The study shows that the ideal concentration of GO-CuONP for S. Typhimurium removal was 2mg/ml and 3mg/ml to remove E.coli; the removal capacity was ~98% and ~88%, respectively. The antibacterial activity of the GO−CuONP nanocomposite could arise from the combined effect of both the native graphene oxide and the surface deposited CuONP. However, it appears that the derived activity is mostly affected by the inherent antibacterial of the CuONPs; this means that the GO primarily acts as a sca?old structure. Were widely demonstrated that the results obtained are unsurprising because of the antibacterial activities of both CuO [144-147] and GO [148], are known.
Song et al [149] studied the antibacterial properties of GO-Ag nanocomposites. Even requiring more investment, the combination exhibited great antibacterial activity, and the bactericidal process was controlled within 25 minutes. They found that the antibacterial behavior of GO-Ag against both bacterial strains (E. coli and S. aureus) was not only a dose-dependent process but also a time-dependent contact one. At 25 minutes, the antibacterial rates for E. coli reached 89.72%, 97.83%, 99.99%, and 99.99%, while the antibacterial rates for S. aureus were 70.32%, 95.53%, 95.70%, and 97.65%, when the concentrations of GO-Ag were 40, 120, 200 and 280 mg/L, respectively. GO-Ag was much more destructive to the cell membrane of E. coli than that of S. aureus, which could also provide evidence for the result mentioned above that antibacterial activity of GO-Ag against E. coli was more effective. Cell membranes were then ?rst to be affected when the cells were exposed to harmful material. Negatively charged lipids quickly took up positively charged silver ions on cell membranes because of electrostatic attraction.
Li et al [150] studied the multifunctional combination between Lysozyme (Lys), Tannic acid (TA) and GO, to remove bacterias from water. The results displayed high effciency on the killing bacteria, both Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. aureus), and enhanced osteogenic di?erentiation. The results obtained can be justified by the natural characteristics from used materials; for example, graphene oxide kills bacteria through multiple ways: cutting the bacteria membrane by its sharp edge, inducing oxidative stress and, TA kills the bacteria through several mechanisms too, such as interacting with biomolecules and metal ions within bacteria, increasing the cell membrane permeability, destabilizing the cytoplasmic membrane, and changing protein to lipid ratios in the membrane. The incorporated GO, Lys, and TA play multimode in killing bacteria (see Figure 3c) because of their respective and di?erent antibacterial mechanism. Under such multiple and di?erent antibacterial mechanisms, if even one component was out of action, the antibacterial effect could be o?set by another element, leading to high effciency in killing bacteria.
4.1.1 effect of pH to remove bacterias from water: In addition to the contact time, size, and the concentration of the material in use, it was reported by Song et al [149] that the GO-Ag showed better antibacterial activity against both E.coli and S.aureus bacteria under the acidic condition in comparison to the disinfection of the same water at higher pH value. For E.coli, the antibacterial rates were 81.68%, 75.5% and 70.5%, and for S.aureus, the antibacterial rates were 58.23%, 45.96% and 40.6%, when the pH values were 5.5, 7 and 8.5, respectively. Different concentrations of silver ions released from AgNPs at varying pH values can explain the obtained results.
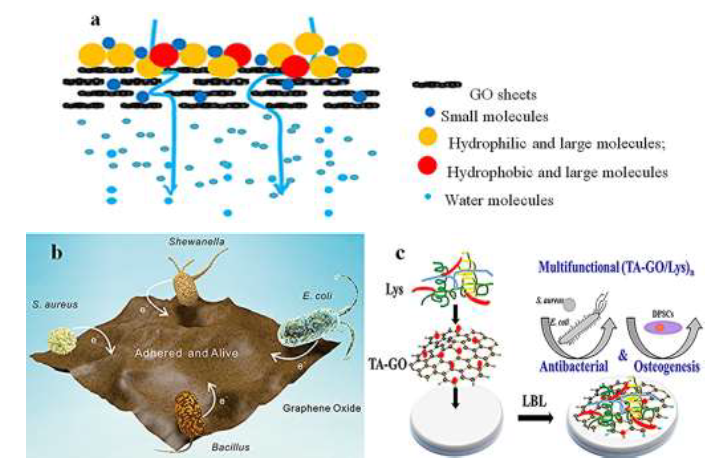
Figure 3:(a)Mechanisms of GO adsorption, reprinted and modified from the literature [31] with permission of Elsevier and Copyrights Clearance Center-license number 4917471105182;(b)Schematic diagram of bacteria adhered on GO, reprinted with permission from literature [142], Copyright 2020, American Chemical Society, and;(c)schematic diagram of multifunctional (TA-GO/ Lys)n to remove bacterias, reprinted with permission from literature [150] Copyright 2020, American Chemical Society.
4.2 Strategies for using GO to remove different inorganic contaminants from water
4.2.1 Heavy metals removal from water: Among the inorganic contaminants, heavy metals [151] and metalloids are the most harmful because they have high toxicity at low concentrations [152, 153]. Metal ions can easily interact with graphene derivatives because of the π-systems and carboxyl and hydroxyl groups on the edges and surfaces [154-156]. Graphene oxide has been studied to remove different types of inorganic contaminants from the water. Ain et al [74] have reported that magnetic graphene oxide can remove heavy metals (Pb+2, Cr+3, Cu+2, Zn+2, and Ni+2 ions) from water. The carbon material ability to adsorb metal cations from the solution depends on a range of parameters, including the mixture's pH and ionic strength [157]. The adsorption capacity of magnetic graphene oxide (MGO) was found to be 200 mg/g for Pb+2, 24.330 mg/g for Cr+3, 62.893 mg/g for Cu+2, 63.694 mg/g for Zn+2 and 51.020 mg/g for Ni+2. The MGO increases by increased pH. High pH, the functional groups are deprotonated, and the surface of MGO becomes more negatively charged. More metal ions are attracted and attached to the surface of MGO due to the strong electrostatic [158] attraction, and the removal of effciency is increased.
4.2.2 Arsenic removal from water: Arsenic is one of the highly toxic and carcinogenic chemical elements, and its contamination in natural waters has become a global problem [159]. Among the four different oxidation states of arsenic, As3+ is ten times more toxic than As5+ and seventy times more toxic than methylated arsenic compounds [160]. To remove those toxins from water, Wu et al [161] synthesized a magnetic Fe@Cu&GO composite by successive growth of Fe3O4 and CuO on the surface of GO sheets to remove arsenic (III/ V) from water and, they saw that graphene oxide increased the adsorption rate of As(V) and As(III) compared with Fe@Cu adsorption rate. They also compared the adsorbents results obtained from other research and concluded that the combination they made (Fe@Cu&GO) presents better As(III/ V) removal from water. The maximum amounts of adsorbed As(III) and As(V) were 70.36 and 62.60mg/g, respectively. Pourbeyram et al [162] found high amounts of adsorbed As3+ and As5+ in the combination of graphene oxide and zirconium (GO-Zr) nano-composite. The maximum adsorption capacity found in the study was 212.33mg/g and 232.52mg/g for As3+ and As5+, respectively.
β-FeOOH incorporated carboxylic graphene oxide nano-composite β-FeOOH@GO-COOH nano-composite has removed 100% arsenic ions from water. The composite has shown tremendous adsorption effciency even after 20 successive adsorption-desorption cycles and adsorbed >80% of both As(III) and As(V). The maximum adsorption capacity for β-FeOOH@GO-COOH was found to be 77.5 and 45.7 mg/g for As(III) and As(V) ions, respectively. Figure 4a shows the bonding between β-FeOOH@GO-COOH, As(III), As(V), and the stretching vibrations of As–O group in the As–O–Fe linkage at the surface of the β-FeOOH@GO-COOH composite. The adsorption behavior depends not only on the charge properties of the adsorbent surface but also on the specific interactions between functional groups on the adsorbent surface and the adsorbed species [163].
4.2.3 Mercury removal from water: Due to its volatility, persistence, and bioaccumulation, mercury (Hg) has been considered as one of the most toxic metals [164], which can affect the health of human beings [165]. To remove mercury from polluted water, Bao et al [166] reported in their research the higher Hg2+ adsorption capacity of Thiol-functionalized magnetite/ graphene oxide (MGO). Its ability reached 289.9 mg/g in the solution with an initial Hg2+ concentration of 100mg/l. The adsorption capacity could be attributed to the affinity of Hg2+ by magnetite nanocrystals and thiol groups. Henriques et al [167] observed that GO macrostructures are a beneficial material on the sorption of Hg(II) from water solution. The different chemical surface modi?cations performed showed that the combination of oxygen and nitrogen functional groups (3DGON) increases by 96% the removal effciency of Hg (II).
Kabiri et al [168] studied a functionalized graphene-based composite with a unique 3D architecture composed of graphene nanosheets decorated with αFeOOH nanoparticles and porous diatom silica microparticles and, the results showed high-effciency adsorption of Hg ions in water. The adsorption performance for Hg removal was achieved with an adsorption capacity of ?800 mg/g (at 400 mg/l Hg2+). Rathore and Biswas [169] showed that GO@SnS2 exhibits highly selective and e?cient removal of Hg(II) from the water with a capacity of 342.02 ± 8.02mg/g. The highly accessible Hg(II) binding sites, S2- from SnS2 and COO– on the GO surface, play a synergistic role in the effective Hg(II) capture. It can sequester 99% Hg(II) selectively even in the presence of Na(I), K(I), Cs(I), Rb(I), Mg(II), Co(II), Cu(II), Ni(II), Zn(II), Pb(II), Cd(II), Mn(II), Fe(III) and As(III) with an extremely high separation factor.
4.2.4 Fluoride removal from water: Fluorine, one of the 14 necessary elements to the human body and has a positive effect on human in a specific concentration (0.7mg/l), but excess fluoride intake (1.5mg/l) [170] is harmful to human [171] and aquatic organisms [172]. To Remove fluoride ion from polluted water using GO, Chakra et al [173] reported in their study the combination between GO and zinc oxide nanocomposite. The adsorption capacity of GO-ZnO nanocomposites for fluoride was 16.608 mg/g. The increase in the removal of fluoride point outs that the fluoride adsorption perhaps occurs due to the diffusion of intriguing position inside the pores on the adsorbent surface. The fluoride ions removal from the water rises with an increase in agitation time to a small degree. Prabhu et al [174] reported an alternative adsorbent for ?uoride removal, assembling nano-sized hydroxyapatite onto graphene oxide (GO-nHAp). 44.068mg/g was said to be the highest adsorption. Liu et al [175] reported the maximum adsorption capacity of 17.67mg/g in the study about de?uoridation by rice spike-like akaganeite anchored graphene oxide. Shang et al [176] enhanced ?uoride uptake by bimetallic hydroxides (Zr-La) anchored in cotton cellulose (CC)/ graphene oxide composites (Zr-La-CC/GO). They reported that ?uoride uptake was signi?cantly increased from 45.1mg/g to 81.3mg/g with an increased pH from 2.5 to 3.0. Zr-La-CC/GO nanocomposites exhibited higher ?uoride adsorption at lower pH conditions. It was reasoned that it could selectively adsorb the ?uoride ions onto the positively charged Zr and La hydroxides and formed Zr-F and La-F complexes in the inner-sphere composites(see Figure. 4b). They protonated the Zr/La species in an acidic environment, which favors F- or HF adsorption based on electrostatic attraction. At the equilibrium stage, the ?uoride uptake capacity onto Zr-La-CC/ GO nanocomposites was about 38.8mg/g. Wang et al [177] studied the synthesis of (ZrO2-Al2O3)/GO nanocomposite for high defluoridation. The results show a maximum fluoride adsorption capacity of 62.2mg/g and adsorption ability of 13.80mg/g when the F- equilibrium concentration is 1mg/l. Figure 4c indicates that all three components (ZrO2, Al2O3, and GO), prepared by the sonochemical method and incorporated together, can significantly improve adsorption capacity.
4.2.5 Copper removal from water: Cu2+ ion is an essential ion in biological systems, such as various redox processes and enzyme functions. De?cient or excessive Cu2+ ion levels are usually associated with some severe disorders [178]. Copper excess may severely affect the ecological cycle and, subsequently, human health [179], and the recommended concentration in drinking water has to be below 2mg/l [180]. To maintain potable water with recommended copper concentrations, Huang et al [181] studied a molecular beacon and GO-based fluorescent biosensor for Cu2+ detection; Zhang et al [182] studied the synthesis of nitrogen-functionalized graphene oxide for copper adsorbent; Wang et al [183], learned a graphene quantum dots as a fluorescent sensing platform for highly Cu2+ detection and, Shao et al [184], studied a nanoscale zero-valent iron decorated on bentonite/ graphene oxide for removal of copper ions. They found ~50nM as detection limit, 26.7451mg/g as adsorption capacity, 0.226μΜ as detection limit, and 184.5mg/g as maximum adsorption limit, respectively.
4.2.6 Other important factors to consider to remove inorganic contaminants from water
4.2.6.1 effect of contact time: The contact time required to achieve more excellent performance to remove heavy metals from water depends on the combination of materials or the type of material used. For example, Ain et al [74] reported that the best results of removing heavy metals (Pb+2, Cr+3, Cu+2, Zn+2, and Ni+2 ions) from water, was verified from 25 to 35 minutes. The study reported by Pourbeyram et al [162] shows that the adsorption of As(V) on the nanocomposite increased rapidly during the ?rst 5 minutes and gradually increased and ?nally reached equilibrium after 10 min. In the same condition, the removal of As(III) took some more time than As(V). The study made by Rathore and Biswas [169] reported results close to 100% of Hg(III) removal, although the long contact time required (close to 1500 minutes) to achieve the maximum reduction of Hg(III). The reference [173] illustrates fluoride removal in the range of 0-240 minutes. The fluoride percentage values were escalating linearly up to 40 minutes; afterward, it remains nearly invariable, signifying the accomplishment of adsorption equilibrium; suddenly, at 200minutes, it showed an increase in value up to 240 minutes. Therefore 240 minutes showed the highest defluoridation (83%). The study made by Prabhu et al [174] reports an increased defluoridation capacity in the contact time from 5 to 25 minutes, resulted in an increase in the adsorption capacity from 78% to 96%, which indicates that the number of active sites gradually occupied. The sorbent's defluoridation capacity was almost saturated at 25 minutes and reached a maximum of 96%.
4.2.6.2 effect of pH: pH is the most crucial factor in the liquid-solid adsorption procedure [162]. To get satisfactory results in removing pollutants, it is essential to know the ideal pH to be used. It's important to realize that the same or different materials may require different pH to remove various water contaminants. The study from reference [161] demonstrates the adsorption capacity of As(III) and As(V) over a pH range of 3 to 10 and reveals that the use of different pH can efficiently remove As(III) and As(V). For example, to remove the maximum of As(V) was necessarily verified an acid pH (pH=3), and the maximal removal of As(III) was with pH in the range of 8 to 10, which was explained by the possibility that more OH− competed with arsenate anions for active sites at higher pH conditions. The effect of ?uoride adsorption shown by Prabhu et al [174] indicates that the maximum ?uoride uptake between the pH ranges 3–7 reached saturation. The pillar-like support of graphene oxide materials would make the bene?t of the growth of hydroxyapatite materials using a large number of functional groups like -OH, -COOH through hydrogen bonding and van der Waals forces of attraction which would attract more ?uoride ions in acidic condition by electrostatic attraction (96%). The adsorption capacity reported by Liu et al [175] towards fluoride showed a similar uptake rate when pH values ranged from 2.1 to 10.4, with the maximum adsorption rate around 80%. The adsorption of fluoride results in an apparent drop from pH 11.1 to 12.7, even dropping from 45.8% to17.6%. In the removal of Cu(II) and Cr(VI), Kumar et al [179] reported that the best results of adsorption capacity were ~200mg/g and ~340mg/g, with pH=5 and pH=3, respectively. According to reference [184], the pH of the solution was among the most critical factors that affected the removal effciency of Cu(II) for the surface speciation of the adsorbent in contact with the solution and speciation of Cu(II) in solution. The removal effciency of Cu(II) increased with the change in pH from 2 to 6. They also found that Cu(II) absorption depends mostly on pH, with higher sorption of cations at high pH and higher sorption of anions at low pH. The removal effciency of Cu(II) was very low; only 3% to 10% of Cu(II) had been removed when the pH was 2. One of the main factors was that fewer surface active sites of adsorbents could be accessible to Cu(II) because at low pH was possible to see the competition between Cu(II) and (H+). In acidic environments, iron particles can be corroded by (H+), resulting in fewer iron particles reacting with Cu(II). When the pH value of the solution was 5, the removal effciency of Cu(II) reached a relatively high result (86%), and the maximum adsorption was 93% at pH=6.
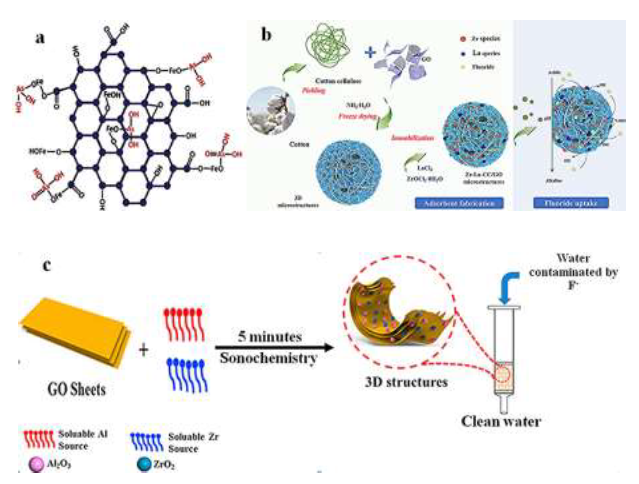
Figure 4:(a)bonding between β-FeOOH@GO-COOH, As(III) and As(V), reprinted from reference [163] with the permission of Elsevier and Copyrights Clearance Center – license number 4918011449709;(b)Zr-La-CC/GO nanocomposites fabrication and adsorption schemes, reprinted from reference [176] with permission of Elsevier and Copyrights Clearance Center – license number 4917470394440; (c)(ZrO2-Al2O3)/GO fabrication and its performance, reprinted and modified from reference [177] with permission of Elsevier and Copyrights Clearance Center – license number 4917461236318.
4.2.6.3 effect of concentration: Henriques et al [167] showed a direct proportionality between increasing the sorbent concentration and the efficient removal of Hg(II). The results show that higher percentages of Hg(II) removal are obtained with increased sorbent concentration, from 33% to 96% for doses 1 to 20 mg/l, respectively. It was evident that the increase of sorbent mass leads to a larger number of available coordination sites improving the removal effciency of the sorbent. Whereas, the study reported by Shao et al [184] presents an indirect proportionality with the sorbent and removal effciency concentration. The removal effciency of Cu(II) shows that the initial concentration of Cu(II) greatly influenced the removal effciency. When the initial Cu (II) concentration was 75 mg/l, the removal effciency was the best, which reached 93%, and the adsorbent decreased with the increase of the initial concentration; this was due to the limited adsorption sites of the iron particles, and before the high concentrations of Cu(II) was adsorbed completely, the iron particles had reached a state of adsorption saturation.
4.3 Strategies for using graphene oxide to remove different organic contaminants from water
Organic water contaminants (e.g., pharmaceuticals and personal care products) are constantly emerging with the rapid advances in various industry sectors. Many of these emerging contaminants are commonly considered micropollutants and have potential adverse health effects [185], thereby raising severe public concerns about water safety [186]. Geng et al [187] studied humic acid removal using Fe3O4/TiO2-N-GO sonocatalyst with ultrasound assistance. The removal effciency was 80% for sono-adsorption, and 93% for sono-photocatalysis. The increasing removal effciency was contributed by cavitation effect caused by ultrasonic waves, and resulted in the formation of more OH and other radical species, allowing more humic acid to be removed. Tan et al [188] demonstrated a highly sensitive and selective ?uorescent biosensor based on GO-hydrogel for antibiotic detection. GO hydrogels were prepared by physically mixing GO solution with adenosine. The fast gelation of the graphene oxide dispersion in the presence of adenosine may contribute to the electrostatic interactions and strong hydrogen bonding between the adenosine and the GO nanosheets. They related that adenosine contains more than one nitrogen (N)-containing functionality and can accept protons from the carboxylic acid groups (– COOH) of the GO sheets to participate in acid-base-type electrostatic attraction. Adenosine and aptamer were served together as cross-linkers between the GO sheets, as illustrated in Figure 5a. The aptamer can recognize and bind to their cognate targets with high speci?city and af?nity; these functional hydrogels were prepared primarily to target detection and adsorbed the aptamer probe on the GO sheets via the strong π–π stacking interactions. Thoroughly immersed in the GO-based functional hydrogel in antibiotic solutions, the aptamer would prefer to capture the antibiotic molecule to form a target-aptamer complex (see Figure. 5b). The recoveries were acceptable and calculated to be in the range of 96–117%.
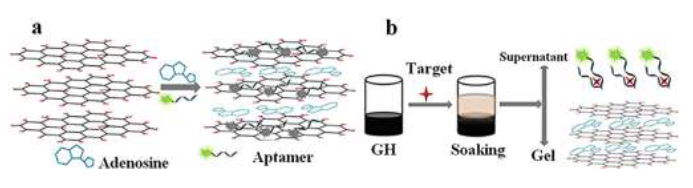
Figure 5:Schematic of the fabrication of(a)GO hydrogel; (b)the mechanism of selective detection of antibiotics, reprinted and modified from reference [188] with the permission of Elsevier and Copyrights Clearance Center – license number 4917621275241.
4.4 effect of support membrane to enhance graphene oxide properties in pollutants removal from water
Support membranes always have a place in the field of adsorption [165]; polymer macromolecules, such as polyvinylidene ?uoride (PVDF), polyacrylonitrile (PAN), polyethersulfone (PES), polysulfone (PSF) and other materials have been used, owing to their respective advantages [8] such as flux, fouling resistance and life span [35]. The existence of graphene oxide in the membranes induce remarkable properties due to its intrinsic hydrophilicity, compatibility with the polymeric matrices, considerable negative zeta potential, and mechanical and thermal stabilities [10]. Zhao et al [31] reported that the PVDF support membrane by itself could remove dissolved organic carbon (DOC); at 120 minutes, the maximum removal was ~30%. When modified, the PVDF with GO's presence, the DOC maximum removal, was ~70%. They also reported the removal improvement to UV254, and the results were ~58% and ~70% for PVDF and PVDF-GO, respectively. The adsorption amount of membranes increased with the increasing number of GO layers, which might indicate the deposition of GO layers adsorption in the ?ltration process, which further improved the DOC rejection. It implied that the fouling layers on the modi?ed membranes could improve the DOC rejection through size exclusion and also with increasing ?ltration time.
Hu and Mi [21] reported a study of graphene oxide membranes' assembly via electrostatic interaction. The hydrolyzed polyacrylonitrile (hPAN) support was ?rst immersed in the poly(allylamine hydrochloride) (PAH) solution to attach positively charged PAH and then in GO solution to deposit negatively charged GO on top of PAH (see Figure 6a), thus completing the assembly of the ?rst GO–PAH bilayer on each side of the hPAN support (see Figure 6b). They used the Characterization techniques to con?rm the successful assembly of multiple GO-PAH bilayers, understanding the structure of the GO membrane and quantify their composition and thickness. In low ionic strength solutions, the graphene oxide membrane retained a tight structure and exhibited a high rejection of sucrose (~99%). Wu et al [25] developed a SiO2–GO nanohybrid/ polysulfone membrane for egg albumin rejections and pure water ?uxes. They compared the performance of different inorganic nanoparticles (Psf, SiO2/Psf, GO/Psf, SiO2-GO/Psf). They reported that all of the hybrid membranes have a higher water ?ux than pure PSF membrane, and SiO2–GO/Psf hybrid membrane exhibited the excellent enhancement. The SiO2–GO nanohybrid possesses extremely high hydrophilicity due to the synergistic effect of nano-sized SiO2 and GO. SiO2–GO nanohybrid demonstrated good dispersion and improved compatibility with the PSF matrix, contributing to the highly maintained egg albumin rejection. They also found that the egg albumin rejection rates of membranes decreased slightly with increasing amounts of SiO2–GO particles, staying at a relatively high level ( ?98%).
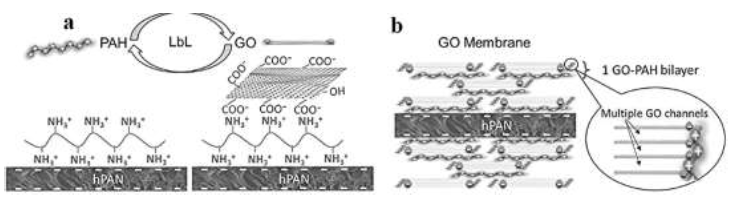
Figure 6:Schematic diagram of(a)LbL assembly of a GO membrane by alternately soaking an hPAN support substrate;(b)deposit of a prescribed number of graphene oxide–PAH bilayers on both sides of hPAN, reprinted from reference [21] with permission of Elsevier and Copyrights Clearance Center – license number 4917481264067.
|
Material/ Technology |
Contaminants to be removed |
Removal capacity: (mg/g) or (%) |
Ref. |
|
EDTA-magnetic GO |
Pb2+ Hg2+ Cu2+ |
96.6% 96.1% 94.3% |
[189] |
|
PVK-GO |
Pb2+ |
888mg/g |
[190] |
|
GO-Magnetic Chitosan |
Zn (II) |
71.4 mg/g |
[191] |
|
GO-AgNPs-MS |
E. Coli S. Aureus |
99.8% 99.3% |
[192] |
|
SMGO |
Uranium - U(VI) |
95.2% |
[193] |
|
Cu-GOS |
E. Coli |
?99.9% |
[194] |
|
CuO-GO |
Nitroaromatics |
85% |
[195] |
|
GO-Ag |
E. Coli |
85.6% |
[196] |
|
GO-γ-Fe2O3
|
1-naphthol Bisphenol A Atrazine Dibutylphthalate |
680mg/g 360mg/g 200mg/g 200mg/g |
[197] |
|
TiO2-GOFe3O4 |
Microcystin-LR |
65-100% |
[198] |
|
MGO-Sr |
Phosphate ions Nitrate ions |
238.09mg/g 357.14mg/g |
[199] |
|
Ox-GO-Zr |
Fluoride |
9.70mg/g |
[200] |
|
PPY-GONC |
Cr (VI) |
625mg/g |
[201] |
|
GO |
TC |
323mg/g |
[202] |
|
Sp-GO/PLL |
E. Coli B. Subtilis |
61% 59% |
[203] |
|
Fe3O4@DTSA-GO |
Hg (II) |
283.5mg/g |
[204] |
|
GO-TiO2 |
Carbamazepine Caffeine |
99% |
[29] |
Table 2:Technologies and performance in the removal of pollutants using graphene oxide.
5. Critical Observation
Water is an essential and essential good for living beings. To obtain the potable water (safe for human consumption) still a real challenge for the whole world, especially for the less developed countries. With the discovery of new water treatment technologies using nanomaterials such as graphene oxide, are given different solutions to safely remove contaminants from the water with minimal possible water recontamination. The underdeveloped countries less use advanced technologies to remove pollutants from the water because the solutions proposed by many researchers to purify water are not economically viable. For example, the study showed by Song et al [149] even with fantastic results in removing bacteria, the nanomaterials used require more investment (are expensive); it shows that their study is useful only for developed countries.
Using the same concentration and incubation conditions, LIU et al [140] reported that the graphene oxide showed to be the best nanomaterial to remove bacterias from water; but, the study reveals only the time and concentration-dependent and does there mention about the possible pH dependence, as reported by Ming et al [142]. Akhavan and Ghaderi [143] reported that higher bacterial toxicity shown was related more to sharpening the nanowalls edges. But, Rajapaksha et al [43] contradicting, said that the higher bacterial toxicity demonstrated in a similar study was not related to their sharp edges but related to the mechanism relied upon the contact between the graphene oxide and the bacterial cells. Another reason which eliminates the number of sharp edges present on the exposed GO surface was that the bacterial toxicity could be achieved using a Langmuir-Blodgett-deposited GO ?lm. According to the justifications presented by the two references, we agree with the connection shown by Rajapaksha et al [43] because many reasons could be related to the process to adsorb bacterias from water and, we should never limit ourselves to a single hypothesis, as happened with the literature Akhavan and Ghaderi [143].
The reference Shao et al [184] reports an indirect proportionality between copper ions removal and the concentration of the used material, which contrasts with what many kinds of literature such as Henriques et al [167] and Zeng et al [172] reported in their studies on mercury and fluoride removal, respectively. The indirect proportionality showed by Shao et al [184] can be related to the type of material and contaminants to be removed from the water. The same study did not report copper removal behavior in concentrations below 75mg/l, which could better understand the relationship between the material concentration and copper removal from water.
6. Conclusion
Using graphene oxide as adsorbent, biological, organic, inorganic, and radiological contaminants can be removed from water until safe for human consumption concentrations. Different methods used to prepare graphene oxide may have a positive or negative impact on the environment and adsorption capacity. Graphene oxide itself absorbs pollutants from water, but the adsorption capacity is improved when the GO is combined with one or more nanomaterials, forming nanohybrids. It was noted that the support membrane's presence also increases the adsorption capacity of contaminants that GO has. Factors such as pH, adsorption time, and concentration of adsorbent material play a significant role in removing different pollutants. Different combinations of GO with other nanomaterials showed a higher capacity of adsorption of contaminants at different pH, time, and concentrations. In implementing water treatment projects using GO, it is essential to understand all factors that can allow us to achieve maximum adsorption of pollutants.
Conflict of Interest
The authors declared that they have no conflicts of interest to this work.
Aknowledgments
This work was supported by Natural Science Foundation of Shanghai (20ZR1462900), National Natural Science Foundation of China (21603164), Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07207004) and Shanghai Science and Technology Innovation Action Plan (19DZ1204304).
References
- Ji Yanli, Weijie Qian, Yawei Yu, et al. Recent developments in nanofiltration membranes based on nanomaterials. Chinese Journal of Chemical Engineering 25 (2017): 1639-1652.
- Anand Anisha, Binesh Unnikrishnan, Ju-Yi Mao, et al. Graphene-based nanofiltration membranes for improving salt rejection, water flux and antifouling-A review. Desalination 429 (2018): 119-133.
- Liu Ji, Min Gao, Dewu Jin, et al. Assessment of Groundwater Quality and Human Health Risk in the Aeolian-Sand Area of Yulin City, Northwest China. Exposure and Health (2019).
- Han Yi, Zhen Xu, Chao Gao. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Advanced Functional Materials 23 (2013): 3693-3700.
- Vatankhah Hooman, Conner C Murray, Jacob W Brannum, et al. effect of pre-ozonation on nanofiltration membrane fouling during water reuse applications. Separation and Purification Technology 205 (2018): 203-211.
- Karami Pooria, Behnam Khorshidi, Mick McGregor, et al. Thermally stable thin film composite polymeric membranes for water treatment: A review. Journal of Cleaner Production 250 (2020): 119447.
- Zhang Peng, Ji-Lai Gong, Guang-Ming Zeng, et al. Enhanced permeability of rGO/S-GO layered membranes with tunable inter-structure for effective rejection of salts and dyes. Separation and Purification Technology 220 (2019): 309-319.
- Yang Shujuan, Qinfeng Zou, Tianhao Wang, et al. effects of GO and MOF@GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane. Journal of Membrane Science 569 (2019): 48-59.
- Koulivand Habib, Afsaneh Shahbazi, Vahid Vatanpour, et al. Development of carbon dot-modified polyethersulfone membranes for enhancement of nanofiltration, permeation and antifouling performance. Separation and Purification Technology 230 (2020): 115895.
- Halakoo Elnaz, Feng X. Layer-by-layer assembly of polyethyleneimine/graphene oxide membranes for desalination of high-salinity water via pervaporation. Separation and Purification Technology 234 (2020): 116077.
- Zhang Hai-Zhen, Zhen-Liang Xu, Hao Ding, et al. Positively charged capillary nanofiltration membrane with high rejection for Mg2+ and Ca2+ and good separation for Mg2+ and Li+. Desalination 420 (2017): 158-166.
- Wu Chunrui, Sihua Liu, Zhongyang Wang, et al. Nanofiltration membranes with dually charged composite layer exhibiting super-high multivalent-salt rejection. Journal of Membrane Science 517 (2016): 64-72.
- Xing L, Kong M, Xie X, et al. Feasibility and safety of papermaking wastewater in using as ecological water supplement after advanced treatment by fluidized-bed Fenton coupled with large-scale constructed wetland. Sci Total Environ 699 (2020): 134369.
- Khorshidi B, Thundat T, Fleck BA, et al. A Novel Approach Toward Fabrication of High Performance Thin Film Composite Polyamide Membranes. Sci Rep 6 (2016): 22069.
- Nasrollahi Nazanin, Vahid Vatanpour, Soheil Aber, et al. Preparation and characterization of a novel polyethersulfone (PES) ultrafiltration membrane modified with a CuO/ZnO nanocomposite to improve permeability and antifouling properties. Separation and Purification Technology 192 (2018): 369-382.
- Raghav Sapna, Ritu Painuli, Dinesh Kumar. Threats to Water: Issues and Challenges Related to Ground Water and Drinking Water. In Mu Naushad (ed.), A New Generation Material Graphene: Applications in Water Technology. Springer International Publishing: Cham (2019): 1-19.
- Cheng Xi Quan, Lu Shao, Cher Hon Lau. High flux polyethylene glycol based nanofiltration membranes for water environmental remediation. Journal of Membrane Science 476 (2015): 95-104.
- Ahmad SZNWN Wan Salleh, Ismail AF, Yusof N, et al. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 248 (2020): 126008.
- Hu M, Mi B. Enabling graphene oxide nanosheets as water separation membranes. Environ Sci Technol 47 (2013): 3715-3723.
- Xing Jian, Bingyao Deng, Qingsheng Liu. effect of graphene nanoplatelets on the performance of polyphenylene sulfide composites produced by melt intercalation. High Performance Polymers 30 (2017): 519-526.
- Hu Meng, Baoxia Mi. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. Journal of Membrane Science 469 (2014): 80-87.
- Li D, Muller MB, Gilje S, et al. Processable aqueous dispersions of graphene nanosheets. Nature Nanotechnology 3 (2008): 101-105.
- Pan Xiaoru, Jiahui Ji, Nana Zhang, et al. Research progress of graphene-based nanomaterials for the environmental remediation. Chinese Chemical Letters 31 (2020): 1462-1473.
- Awad Abdelrahman M, Rem Jalab, Abdelbaki Benamor, et al. Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview. Journal of Molecular Liquids 301 (2020): 112335.
- Wu Huiqing, Beibei Tang, Peiyi Wu. Development of novel SiO2-GO nanohybrid/ polysulfone membrane with enhanced performance. Journal of Membrane Science 451 (2014): 94-102.
- Fathizadeh Mahdi, Weiwei L Xu, Margaret Shen, et al. Antifouling UV-treated GO/PES hollow fiber membranes in a membrane bioreactor (MBR). Environmental Science: Water Research and Technology 5 (2019): 1244-1252.
- Vatanpour Vahid, Abbas Shockravi, Hamed Zarrabi, et al. Fabrication and characterization of anti-fouling and anti-bacterial Ag-loaded graphene oxide/polyethersulfone mixed matrix membrane. Journal of Industrial and Engineering Chemistry 30 (2015): 342-352.
- Chen P, Yan LT. Physical principles of graphene cellular interactions: computational and theoretical accounts. J Mater Chem B 5 (2017): 4290-306.
- Awfa Dion, Mohamed Ateia, Manabu Fujii, et al. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Research 142 (2018): 26-45.
- Wu Jiang-Bin, Miao-Ling Lin, Xin Cong, et al. Raman spectroscopy of graphene-based materials and its applications in related devices. Chemical Society Reviews 47 (2018): 1822-1873.
- Zhao Jingjing, Yu Yang, Chen Li, et al. Fabrication of GO modified PVDF membrane for dissolved organic matter removal: Removal mechanism and antifouling property. Separation and Purification Technology 209 (2019): 482-490.
- You Y, Jin XH, Wen XY, et al. Application of graphene oxide membranes for removal of natural organic matter from water. Carbon 129 (2018): 415-419.
- Yin Jun, Guocheng Zhu, Baolin Deng. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 379 (2016): 93-101.
- Soroush Adel, Wen Ma, Yule Silvino, et al. Surface modification of thin film composite forward osmosis membrane by silver-decorated graphene-oxide nanosheets. Environmental Science: Nano 2 (2015): 395-405.
- Ambre Jyoti P, Kiran B Dhopte, Parag R Nemade, et al. High flux hyperbranched starch-graphene oxide piperazinamide composite nanofiltration membrane. Journal of Environmental Chemical Engineering 7 (2019): 103300.
- Backes Claudia, Amr M Abdelkader, Concepción Alonso, et al. Production and processing of graphene and related materials. 2D Materials 7 (2020): 022001.
- Marcano DC, Kosynkin DV, Berlin JM, et al. Improved Synthesis of Graphene Oxide. ACS Nano 4 (2010): 4806-4814.
- Cao Ning, Zhang Y. Study of Reduced Graphene Oxide Preparation by Hummers Method and Related Characterization. Journal of Nanomaterials 2015 (2015): 1-5.
- Petit Camille, Mykola Seredych, Teresa J. Bandosz. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. Journal of Materials Chemistry 19 (2009): 9176.
- Wang Shaobin, Hongqi Sun, Ang HM, et al. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chemical Engineering Journal 226 (2013): 336-347.
- Alam Syed Nasimul, Nidhi Sharma, Lailesh Kumar. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO). Graphene 06 (2017): 1-18.
- Kumar Rajesh, Rajesh K Singh, Vinod Kumar, et al. Functionalized Nanosize Graphene and Its Derivatives for Removal of Contaminations and Water Treatment - A New Generation Material Graphene: Applications in Water Technology. Springer International Publishing (2019): 133-185.
- Rajapaksha Piumie, Samuel Cheeseman, Stuart Hombsch, et al. Antibacterial Properties of Graphene Oxide–Copper Oxide Nanoparticle Nanocomposites. ACS Applied Bio Materials 2 (2019): 5687-5696.
- Huang Dayong, Boxuan Li, Min Wu, et al. Graphene Oxide-Based Fe–Mg (Hydr)oxide Nanocomposite as Heavy Metals Adsorbent. Journal of Chemical and Engineering Data 63 (2018): 2097-2105.
- Su Hui, Zhibin Ye, Nuri Hmidi. High-performance iron oxide-graphene oxide nanocomposite adsorbents for arsenic removal. Colloids and Surfaces A: Physicochemical and Engineering Aspects 522 (2017): 161-172.
- Zhang Peng, Ji-Lai Gong, Guang-Ming Zeng, et al. Novel loose GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure. Journal of Membrane Science 574 (2019): 112-123.
- Akther Nawshad, Sherub Phuntsho, Yuan Chen, et al. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. Journal of Membrane Science 584 (2019): 20-45.
- Wang Zhuqing, Aiguo Wu, Lucio Colombi Ciacchi, et al. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 8 (2018): 65.
- Ren Suyu, Jing Tao, Ying Cui, et al. Preparation and characterization of hydrophilic polydopamine-coated Fe3O4/oxide graphene imprinted nanocomposites for removal of bisphenol A in waters. Korean Journal of Chemical Engineering 35 (2018): 1836-1843.
- Singh Pardeep, Pooja Shandilya, Pankaj Raizada, et al. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arabian Journal of Chemistry 13 (2020): 3498-3520.
- Fawell J, Nieuwenhuijsen MJ. Contaminants in drinking water. Br Med Bull 68 (2003): 199-208.
- Tatarchuk Tetiana, Mohamed Bououdina, Basma Al-Najar, et al. Green and Ecofriendly Materials for the Remediation of Inorganic and Organic Pollutants in Water-A New Generation Material Graphene: Applications in Water Technology. M. Naushad. Cham, Springer International Publishing (2019): 69-110.
- Edzwald JK. Water quality and treatment-chapter 2 (health and aesthetic aspects of drinking water). American water works association 6 (2010):1-2.100.
- Sharma S, Bhattacharya A. Drinking water contamination and treatment techniques. Applied Water Science 7 (2016): 1043-1067.
- Bilal M, Rasheed T, Mehmood S, et al. Mitigation of environmentally-related hazardous pollutants from water matrices using nanostructured materials-A review. Chemosphere 253 (2020): 126770.
- Vieira Octávia, Rui S Ribeiro, Marta Pedrosa, et al. Nitrogen-doped reduced graphene oxide-PVDF nanocomposite membrane for persulfate activation and degradation of water organic micropollutants. Chemical Engineering Journal 402 (2020): 126117.
- Abu Hasan, Hassimi, Mohd Hafizuddin Muhammad, et al. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. Journal of Water Process Engineering 33 (2020): 101035.
- Guidelines for drinking-water quality: incorporating 1st and 2nd addenda 1 (2008).
- Kuzenkova Anastasiia S, Anna Yu Romanchuk, Alexander L Trigub, et al. New insights into the mechanism of graphene oxide and radionuclide interaction. Carbon 158 (2020): 291-302.
- Seredych Mykola, Albert V Tamashausky, Teresa J. Bandosz. Graphite Oxides Obtained from Porous Graphite: The Role of Surface Chemistry and Texture in Ammonia Retention at Ambient Conditions. Advanced Functional Materials 20 (2010): 1670-1679.
- Talyzin Alexandr V, Guillaume Mercier, Alexey Klechikov, et al. Brodie vs Hummers graphite oxides for preparation of multi-layered materials. Carbon 115 (2017): 430-440.
- Zygouri Panagiota, Theodoros Tsoufis, Antonios Kouloumpis, et al. Synthesis, characterization and assessment of hydrophilic oxidized carbon nanodiscs in bio-related applications. RSC Advances 8 (2018): 122-131.
- Pavoski Giovani, Thuany Maraschin, Fabiana de Carvalho Fim, et al. Few Layer Reduced Graphene Oxide: Evaluation of the Best Experimental Conditions for Easy Production. Materials Research 20 (2016): 53-61.
- Hontoria-lucas C, Lopez-peinado AJ, Lopez-gonzalez J de D, et al. Study of oxygen-containing groups in a series of graphite oxides: physical and chemical characterization. Carbon 33 (1995): 1585-1592.
- Poh HL, Sanek F, Ambrosi A, et al. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 4 (2012): 3515-3522.
- Hofmann U, König E. Untersuchungen über Graphitoxyd. Z. Anorg. Allg. Chem 234 (1937): 311-336.
- Hussain Intesar R, Dayang Radiah AB, Faten H Kamil, et al. Physical Properties of Reduced Graphite Oxide Prepared via Chemical Reduction by Using Ammonia solution as a Reducing Agent. IOP Conference Series: Materials Science and Engineering 454 (2018): 012136.
- Khan Qaiser Ali, Ahmed Shaur, Tayyab Ali Khan, et al. Characterization of reduced graphene oxide produced through a modified Hoffman method. Cogent Chemistry 3 (2017): 80.
- Soares Yago, Elyff Cargnin, Mônica Naccache, et al. Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions. Fluids 5 (2020): 41.
- Velasco-Hernández A, Esparza-Muñoz RA, Moure-Flores FJ de, et al. Synthesis and characterization of graphene oxide - TiO2 thin films by sol-gel for photocatalytic applications. Materials Science in Semiconductor Processing 114 (2020): 105082.
- Zaman A, Orasugh JT, Banerjee P, et al. Facile one-pot in-situ synthesis of novel graphene oxide-cellulose nanocomposite for enhanced azo dye adsorption at optimized conditions. Carbohydr Polym 246 (2020): 116661.
- Abbasi Reza, Jafar Mostafavi Amjad, Hamed Nosrati, et al. Synthesis and characterization of PEGylated iron and graphene oxide magnetic composite for curcumin delivery. Applied Organometallic Chemistry 34 (2020).
- Thakur Suman, Niranjan Karak. Green reduction of graphene oxide by aqueous phytoextracts. Carbon 50 (2012): 5331-5339.
- Ain Qurat-Ul, Muhammad Umar Farooq, Muhammad Irfan Jalees. Application of Magnetic Graphene Oxide for Water Purification: Heavy Metals Removal and Disinfection. Journal of Water Process Engineering 33 (2020): 101044.
- Tene T, Tubon Usca G, Guevara M, et al. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials (Basel) 10 (2020).
- Ma J, Ping D, Dong X. Recent Developments of Graphene Oxide-Based Membranes: A Review. Membranes (Basel) 7 (2017).
- Yuan Rui, Jing Yuan, Yanping Wu, et al. Efficient synthesis of graphene oxide and the mechanisms of oxidation and exfoliation. Applied Surface Science 416 (2017): 868-877.
- Tiwari Santosh, Mishra Raghvendra, Ha Sung, et al. Evolution of Graphene Oxide and Graphene: From Imagination to Industrialization. ChemNanoMat 4 (2018): 598-620.
- Jeong HK, Lee YP, Lahaye RJWE, et al. Evidence of Graphitic AB Stacking Order of Graphite Oxides. Journal of the American Chemical Society 130 (2008): 1362-1366.
- Aguilar-Bolados H, Contreras-Cid A, Yazdani-Pedram M, et al. Synthesis of fluorinated graphene oxide by using an easy one-pot deoxyfluorination reaction. J Colloid Interface Sci 524 (2018): 219-226.
- Vrettos Katerina, Niki Karouta, Panagiotis Loginos, et al. The Role of Diamines in the Formation of Graphene Aerogels. Frontiers in Materials 5 (2018).
- Wong Colin Hong An, Ond?ej Jankovský, Zden?k Sofer, et al. Vacuum-assisted microwave reduction/exfoliation of graphite oxide and the influence of precursor graphite oxide. Carbon 77 (2014): 508-517.
- Chua CK, Sofer Z, Pumera M. Graphite oxides: effects of permanganate and chlorate oxidants on the oxygen composition. Chemistry 18 (2012): 13453-13459.
- Chen Ji, Yingru Li, Liang Huang, et al. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 81 (2015): 826-834.
- Lojka M, Lochman B, Jankovsky O, et al. Synthesis, Composition, and Properties of Partially Oxidized Graphite Oxides. Materials (Basel) 12 (2019).
- Gandhi Muniyappan Rajiv, Subramanyan Vasudevan, Atsushi Shibayama, et al. Graphene and Graphene-Based Composites: A Rising Star in Water Purification - A Comprehensive Overview. ChemistrySelect 1 (2016): 4358-4385.
- Jankovský Ond?ej, Petr Marvan, Michal Nová?ek, et al. Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Applied Materials Today 4 (2016): 45-53.
- Eng AYS, Poh HL, Šan?k F, et al. Searching for Magnetism in Hydrogenated Graphene: Using Highly Hydrogenated Graphene Prepared via Birch Reduction of Graphite Oxides. ACS Nano 7 (2013): 5930-5939.
- Nová?ek Michal, Ond?ej Jankovský, Jan Luxa, et al. Tuning of graphene oxide composition by multiple oxidations for carbon dioxide storage and capture of toxic metals. Journal of Materials Chemistry A 5 (2017): 2739-2748.
- Paulchamy B, Arthi G, Lignesh Bd. A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial. Journal of Nanomedicine & Nanotechnology 06 (2015):
- Deemer EM, Paul PK, Manciu FS, et al. Consequence of oxidation method on graphene oxide produced with different size graphite precursors. Materials Science and Engineering B 224 (2017): 150-157.
- Eng Alex Yong Sheng, Adriano Ambrosi, Chun Kiang Chua, et al. Unusual Inherent Electrochemistry of Graphene Oxides Prepared Using Permanganate Oxidants. Chemistry - A European Journal 19 (2013): 12673-12683.
- Adetayo Adeniji, Damilola Runsewe. Synthesis and Fabrication of Graphene and Graphene Oxide: A Review. Open Journal of Composite Materials 09 (2019): 207-229.
- Anku William W, Ephraim M Kiarii, Rama Sharma, et al. Photocatalytic Degradation of Pharmaceuticals Using Graphene Based Materials - A New Generation Material Graphene: Applications in Water Technology (2019): 187-208.
- Su M, Chen X, Zhang L, et al. Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties. Nanomaterials (Basel) 10 (2020).
- Gupta V, Sharma N, Singh U, et al. Higher oxidation level in graphene oxide. Optik 143 (2017): 115-124.
- Botas Cristina, Patricia Álvarez, Patricia Blanco, et al. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 65 (2013): 156-164.
- Muzyka Roksana, Monika Kwoka, ?ukasz Sm?dowski, et al. Oxidation of graphite by different modified Hummers methods. New Carbon Materials 32 (2017): 15-20.
- Yin Fei, Jianchen Hu, Zhenglin Hong, et al. A review on strategies for the fabrication of graphene fibres with graphene oxide. RSC Advances 10 (2020): 5722-5733.
- Jankovsky O, Novacek M, Luxa J, et al. Concentration of Nitric Acid Strongly Influences Chemical Composition of Graphite Oxide. Chemistry 23 (2017): 6432-6440.
- Orsu Prabhakar, Arun Koyyada. Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: a comprehensive review. Materials Today Communications 22 (2020): 100823.
- Padmajan Sasikala S, Lim J, Kim IH, et al. Graphene oxide liquid crystals: a frontier 2D soft material for graphene-based functional materials. Chem Soc Rev 47 (2018): 6013-6045.
- Jankovsky O, Simek P, Klimova K, et al. Towards graphene bromide: bromination of graphite oxide. Nanoscale 6 (2014): 6065-6074.
- Wang Lu, Zden?k Sofer, Jan Luxa, et al. Nitrogen doped graphene: influence of precursors and conditions of the synthesis. J. Mater. Chem. C 2 (2014): 2887-2893.
- Alkhouzaam Abedalkader, Hazim Qiblawey, Majeda Khraisheh, et al. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceramics International 46 (2020): 23997-24007.
- Chen Ji, Bowen Yao, Chun Li, et al. An improved Hummers method for eco-friendly synthesis of graphene oxide.Carbon 64 (2013): 225-229.
- Abd-Elhamid AI, Aly HF, Hesham AM Soliman, et al. Graphene oxide: Follow the oxidation mechanism and its application in water treatment. Journal of Molecular Liquids 265 (2018): 226-237.
- Shao Guilin, Yonggen Lu, Fangfang Wu, et al. Graphene oxide: the mechanisms of oxidation and exfoliation. Journal of Materials Science 47 (2012): 4400-4409.
- Nanda S, Atirek Gaur, Rajendra Kumar Duchaniya. Synthesis, properties and applications of graphene oxide: an overview. World Scientific News 143 (2020): 17-27.
- Trikkaliotis Dimitrios G, Athanasios C Mitropoulos, George Z Kyzas. Low-cost route for top-down synthesis of over- and low-oxidized graphene oxide. Colloids and Surfaces A: Physicochemical and Engineering Aspects 600 (2020): 124928.
- Hou Yonggang, Shenghua Lv, Leipeng Liu, et al. High-quality preparation of graphene oxide via the Hummers method: Understanding the roles of the intercalator, oxidant, and graphite particle size. Ceramics International 46 (2020): 2392-2402.
- Turkaslan EB, Aydin FM. Optimizing parameters of graphene derivatives synthesis by modified improved Hummers. Math Meth Appl Sci (2020): 1-8.
- Chang Wei-Ting, Yu-Hao Chao, Chen-Wei Li, et al. Graphene oxide synthesis using microwave-assisted vs modified Hummer's methods: Efficient fillers for improved ionic conductivity and suppressed methanol permeability in alkaline methanol fuel cell electrolytes. Journal of Power Sources 414 (2019): 86-95.
- Khan M Bilal, Mohd Parvaz, Zishan Husain Khan. Graphene Oxide: Synthesis and Characterization. Advanced Structured Materials 83 (2017): 1-28.
- Lavin-Lopez Maria del Prado, Amaya Romero, Jesus Garrido, et al. Influence of Different Improved Hummers Method Modifications on the Characteristics of Graphite Oxide in Order to Make a More Easily Scalable Method. Industrial and Engineering Chemistry Research 55 (2016): 12836-12847.
- Lojka Michal, Adéla Ji?í?ková, David Sedmidubský, et al. Fast synthesis of graphite oxide via modified chlorate route 1988 (2018): 020025.
- Shuai Shirong, Yu Liu, Cong Zhao, et al. Improved synthesis of graphene oxide with controlled oxidation degree by using different dihydrogen phosphate as intercalators. Chemical Physics 539 (2020): 110938.
- Luo D, Zhang F, Ren Z, et al. An improved method to synthesize nanoscale graphene oxide using much less acid. Materials Today Physics 9 (2019): 100097.
- Kudus Muhammad Helmi Abdul, Muhammad Razlan Zakaria, Hazizan Md Akil, et al. Oxidation of graphene via a simplified Hummers method for graphene-diamine colloid production. Journal of King Saud University - Science 32 (2020): 910-913.
- Wang Y, Guo L, Qi P, et al. Synthesis of Three-Dimensional Graphene-Based Hybrid Materials for Water Purification: A Review. Nanomaterials (Basel) 9 (2019).
- Carmalin Sophia A, Tanvir Arfin, Eder C Lima. Recent Developments in Adsorption of Dyes Using Graphene Based Nanomaterials-A New Generation Material Graphene: Applications in Water Technology. Springer International Publishing: Cham (2019): 439-471.
- Liu L, Bai H, Liu J, et al. Multifunctional graphene oxide-TiO(2)-Ag nanocomposites for high performance water disinfection and decontamination under solar irradiation. J Hazard Mater 261 (2013): 214-223.
- Toh Shaw Yong, Kee Shyuan Loh, Siti Kartom Kamarudin, et al. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chemical Engineering Journal 251 (2014): 422-434.
- Zhou Lin, Huijun Jiang, Shaohua Wei, et al. High-effciency loading of hypocrellin B on graphene oxide for photodynamic therapy. Carbon 50 (2012): 5594-5604.
- Zhao Y, Liu Y, Zhang X, et al. Environmental transformation of graphene oxide in the aquatic environment. Chemosphere 262 (2020): 127885.
- Nuji? Marija, Habuda-Stani? M. Toxic Metal Ions in Drinking Water and effective Removal Using Graphene Oxide Nanocomposite - A New Generation Material Graphene: Applications in Water Technology. Springer International Publishing: Cham (2019): 373-395.
- Pytlakowska Katarzyna, Micha? Pilch, Barbara Hachu?a, et al. Pisarski. Energy dispersive X-ray fluorescence spectrometric determination of copper, zinc, lead and chromium species after preconcentration on graphene oxide chemically modified with mercapto-groups. Journal of Analytical Atomic Spectrometry 34 (2019): 1416-1425.
- Gurz?da Bartosz, Ahmed Subrati, Patryk Florczak, et al. Two-step synthesis of well-ordered layered graphite oxide with high oxidation degree. Applied Surface Science 507 (2020): 145049.
- Dikin DA, Stankovich S, Zimney EJ, et al. Preparation and characterization of graphene oxide paper. Nature 448 (2007): 457-460.
- Chen J, Lv L, Li Y, et al. Preparation and evaluation of Bletilla striata polysaccharide/graphene oxide composite hemostatic sponge. Int J Biol Macromol 130 (2019): 827-835.
- Wang Y, Li Z, Wang J, et al. Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29 (2011): 205-212.
- Tang Duosi, Jingjing Liu, Xiaomei Yan, et al. Graphene oxide derived graphene quantum dots with different photoluminescence properties and peroxidase-like catalytic activity. RSC Advances 6 (2016): 50609-50617.
- Peng Fumin, Tao Luo, Lingguang Qiu, et al. An easy method to synthesize graphene oxide–FeOOH composites and their potential application in water purification. Materials Research Bulletin 48 (2013): 2180-2185.
- Zhao J, Yang Y, Li C, et al. Impacts of mono/divalent cations on the lamellar structure of cross-linked GO layers and membrane filtration performance for different DOM fractions. Chemosphere 237 (2019): 124544.
- Singh Virendra, Daeha Joung, Lei Zhai, et al. Graphene based materials: Past, present and future. Progress in Materials Science 56 (2011): 1178-1271.
- Bala Saranya, Nithya D, Mohan Doraisamy. Exploring the effects of graphene oxide concentration on properties and antifouling performance of PEES/GO ultrafiltration membranes. High Performance Polymers 30 (2017): 375-383.
- Sajjad S, Khan SA Leghari, et al. Study of Graphene Oxide Structural Features for Catalytic, Antibacterial, Gas Sensing, and Metals Decontamination Environmental Applications. ACS Appl Mater Interfaces 9 (2017): 43393-43414.
- Buelke Chris, Ali Alshami, James Casler, et al. Evaluating graphene oxide and holey graphene oxide membrane performance for water purification. Journal of Membrane Science 588 (2019): 117195.
- Lawler J. Incorporation of Graphene-Related Carbon Nanosheets in Membrane Fabrication for Water Treatment: A Review. Membranes (Basel) 6 (2016).
- Liu S, Zeng TH, Hofmann M, et al. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 5 (2011): 6971-6980.
- Barrios Ana C, Wang Y, Gilbertson LM, et al. Structure-Property-Toxicity Relationships of Graphene Oxide: Role of Surface Chemistry on the Mechanisms of Interaction with Bacteria. Environ Sci Technol 53 (2019): 14679-14687.
- Ming Jiang, Duo Sun, Jingping Wei, et al. Adhesion of Bacteria to a Graphene Oxide Film. ACS Applied Bio Materials 3 (2019): 704-712.
- Akhavan O, Ghaderi E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS NANO 4 (2010): 5731-5736.
- Allaker Robert Patrick. Nanoparticles and the Control of Oral Biofilms. (2013): 203-27.
- Alfredo Nelson V, Rodríguez-Hernández Juan. Antimicrobial Polymeric Nanostructures (2017): 85-115.
- Luna Ismat Zerin, Lutfun Naher Hilary, Sarwaruddin Chowdhury AM, et al. Preparation and Characterization of Copper Oxide Nanoparticles Synthesized via Chemical Precipitation Method. OALib 2 (2015): 1-8.
- Hans M, Erbe A, Mathews S, et al. Role of copper oxides in contact killing of bacteria. Langmuir 29 (2013): 16160-16166.
- Xue Jinkai, Sara BinAhmed, Zhaoxing Wang, et al. Bacterial Adhesion to Graphene Oxide (GO)-Functionalized Interfaces Is Determined by Hydrophobicity and GO Sheet Spatial Orientation. Environmental Science & Technology Letters 5 (2017): 14-19.
- Song B, Zhang C, Zeng G, et al. Antibacterial properties and mechanism of graphene oxide-silver nanocomposites as bactericidal agents for water disinfection. Arch Biochem Biophys 604 (2016): 167-176.
- Li Huaqiong, Chenyuan Gao, Lin Tang, et al. Lysozyme (Lys), Tannic Acid (TA), and Graphene Oxide (GO) Thin Coating for Antibacterial and Enhanced Osteogenesis. ACS Applied Bio Materials 3 (2020): 673-684.
- Peng Cheng, Yihao Shen, Xuefei Wu, et al. Heavy Metals, Nitrogen, and Phosphorus in Sediments from the First Drinking Water Reservoir Supplied by Yangtze River in Shanghai, China: Spatial Distribution Characteristics and Pollution Risk Assessment. Water, Air, and Soil Pollution 231 (2020).
- Kumar A, Schreiter IJ, Wefer-Roehl A, et al. Production and Utilization of Biochar From Organic Wastes for Pollutant Control on Contaminated Sites (2016): 91-116.
- Kumar Rudra, Thiruvelu Bhuvana, Ashutosh Sharma. Nickel tungstate–graphene nanocomposite for simultaneous electrochemical detection of heavy metal ions with application to complex aqueous media. RSC Advances 7 (2017): 42146-42158.
- Huang H, Liao L, Xu X, et al. The electron-transfer based interaction between transition metal ions and photoluminescent graphene quantum dots (GQDs): a platform for metal ion sensing. Talanta 117 (2013): 152-157.
- Liu Wei, Danjun Wang, Razium Ali Soomro, et al. Ceramic supported attapulgite-graphene oxide composite membrane for efficient removal of heavy metal contamination. Journal of Membrane Science 591 (2019): 117323.
- Saeedi-Jurkuyeh Alireza, Ahmad Jonidi Jafari, Roshanak Rezaei Kalantary, et al. A novel synthetic thin-film nanocomposite forward osmosis membrane modified by graphene oxide and polyethylene glycol for heavy metals removal from aqueous solutions. Reactive and Functional Polymers 146 (2020): 104397.
- Rosillo-Lopez Martin, Christoph G. Salzmann. Highly efficient heavy-metal extraction from water with carboxylated graphene nanoflakes. RSC Advances 8 (2018): 11043-11050.
- Xu Lili, Hongbo Suo, Jianling Wang, et al. Magnetic graphene oxide decorated with chitosan and Au nanoparticles: synthesis, characterization and application for detection of trace rhodamine B. Analytical Methods 11 (2019): 3837-3843.
- Zhu Jin, Zimo Lou, Yu Liu, et al. Adsorption behavior and removal mechanism of arsenic on graphene modified by iron-manganese binary oxide (FeMnOx/RGO) from aqueous solutions. RSC Advances 5 (2015): 67951-67961.
- Ramesha GK, SampathS. In-situ formation of graphene–lead oxide composite and its use in trace arsenic detection. Sensors and Actuators B: Chemical 160 (2011): 306-311.
- Wu Kun, Chunyang Jing, Jin Zhang, et al. Magnetic Fe3O4@CuO nanocomposite assembled on graphene oxide sheets for the enhanced removal of arsenic(III/V) from water. Applied Surface Science 466 (2019): 746-756.
- Pourbeyram Sima, Sara Alizadeh, Somayeh Gholizadeh. Simultaneous removal of arsenate and arsenite from aqueous solutions by graphene oxide-zirconium (GO-Zr) nanocomposite. Journal of Environmental Chemical Engineering 4 (2016): 4366-4373.
- Chen ML, Sun Y, Huo CB, et al. Akaganeite decorated graphene oxide composite for arsenic adsorption/removal and its proconcentration at ultra-trace level. Chemosphere 130 (2015): 52-58.
- Zhang Xingang, Zhigao Dai, Shuyao Si, et al. Ultrasensitive SERS Substrate Integrated with Uniform Subnanometer Scale Hot Spots Created by a Graphene Spacer for the Detection of Mercury Ions. Small 13 (2017): 1603347.
- Yu Jin-Gang, Bao-Yu Yue, Xiong-Wei Wu, et al. Removal of mercury by adsorption: a review. Environmental Science and Pollution Research 23 (2015): 5056-5076.
- Bao Jian, Fu You, Bao Zhihao. Thiol-functionalized magnetite/graphene oxide hybrid as a reusable adsorbent for Hg2+ Nanoscale Research Letters 8 (2013): 486.
- Henriques B, Goncalves G, Emami N, et al. Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. J Hazard Mater 301 (2016): 453-461.
- Kabiri Shervin, Diana NH Tran, Martin A Cole, et al. Functionalized three-dimensional (3D) graphene composite for high effciency removal of mercury. Environmental Science: Water Research and Technology 2 (2016): 390-402.
- Rathore Ekashmi, Kanishka Biswas. Selective andppb level removal of Hg(ii) from water: synergistic role of graphene oxide and SnS2. Journal of Materials Chemistry A 6 (2018): 13142-13152.
- Mohapatra M, Anand S, Mishra BK, et al. Review of fluoride removal from drinking water. J Environ Manage 91 (2009): 67-77.
- M Anju, Renuka NK. Graphene-dye supramolecular assembly for parts per trillion level F− monitoring. Materials Research Bulletin 110 (2019): 50-56.
- Zeng Yifan, Yingwen Xue, Shuhao Liang, et al. Removal of fluoride from aqueous solution by TiO2 and TiO2–SiO2 Chemical Speciation and Bioavailability 29 (2016): 25-32.
- Chakra CS, sai kumar VS, Madhuri S, et al. Adsorption studies and fluoride removal from aqueous solutions by graphene oxide - zinc oxide nanocomposite. Digest journal of nanomaterials and biostructures 14 (2019): 183-192.
- Prabhu SM, Elanchezhiyan SS, Lee G, et al. Assembly of nano-sized hydroxyapatite onto graphene oxide sheets via in-situ fabrication method and its prospective application for defluoridation studies. Chemical Engineering Journal 300 (2016): 334-342.
- Liu Yuyang, Jiaxin Lv, Wei Jin, et al. Defluoridation by rice spike-like akaganeite anchored graphene oxide. RSC Advances 6 (2016): 11240-11249.
- Shang Y, Wang Z, Xu X, et al. Enhanced fluoride uptake by bimetallic hydroxides anchored in cotton cellulose/graphene oxide composites. J Hazard Mater 376 (2019): 91-101.
- Wang Qi, Pinghua Chen, Xiong Zeng, et al. Synthesis of (ZrO2-Al2O3)/GO nanocomposite by sonochemical method and the mechanism analysis of its high defluoridation. Journal of Hazardous Materials 381 (2020): 120954.
- Peng XX, Bao GM, Zhong YF, et al. Highly selective detection of Cu(2+) in aqueous media based on Tb(3+)-functionalized metal-organic framework. Spectrochim Acta A Mol Biomol Spectrosc 240 (2020): 118621.
- Kumar Rajeev, Barakat MA, Md Abu Taleb, et al. A recyclable multifunctional graphene oxide/SiO2@polyaniline microspheres composite for Cu(II) and Cr(VI) decontamination from wastewater. Journal of Cleaner Production 268 (2020): 122290.
- Bandara T, Xu J, Potter ID, et al. Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural wastes. Chemosphere 254 (2020): 126745.
- Huang J, Zheng Q, Kim JK, et al. A molecular beacon and graphene oxide-based fluorescent biosensor for Cu(2+) detection. Biosens Bioelectron 43 (2013): 379-383.
- Zhang Kexin, Haiyan Li, Xingjian Xu, et al. Facile and Efficient Synthesis of Nitrogen-Functionalized Graphene Oxide as a Copper Adsorbent and Its Application. Industrial & Engineering Chemistry Research 55 (2016): 2328-2335.
- Wang Fengxiang, Zhenyan Gu, Wu Lei, et al. Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions. Sensors and Actuators B: Chemical 190 (2014): 516-522.
- Shao J, Yu X, Zhou M, et al. Nanoscale Zero-Valent Iron Decorated on Bentonite/Graphene Oxide for Removal of Copper Ions from Aqueous Solution. Materials (Basel) 11 (2018).
- He J, Li Y, Cai X, et al. Study on the removal of organic micropollutants from aqueous and ethanol solutions by HAP membranes with tunable hydrophilicity and hydrophobicity. Chemosphere 174 (2017): 380-389.
- Oh Yoontaek, Dana L Armstrong, Casey Finnerty, et al. Understanding the pH-responsive behavior of graphene oxide membrane in removing ions and organic micropollulants. Journal of Membrane Science 541 (2017): 235-243.
- Geng Nannan, Wei Chen, Hang Xu, et al. Preparation of Fe3O4/TiO2-N-GO sonocatalyst and using for humic acid removal with the assist of ultrasound. Materials Science in Semiconductor Processing 102 (2019): 104593.
- Tan B, Zhao H, Du L, et al. A versatile fluorescent biosensor based on target-responsive graphene oxide hydrogel for antibiotic detection. Biosens Bioelectron 83 (2016): 267-273.
- Bolisetty S, Peydayesh M, Mezzenga R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev 48 (2019): 463-487.
- Smith Sean C, Debora F Rodrigues. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 91 (2015): 122-143.
- Kazemi E, Dadfarnia S, Haji Shabani AM, et al. Synthesis, characterization, and application of a Zn (II)-imprinted polymer grafted on graphene oxide/magnetic chitosan nanocomposite for selective extraction of zinc ions from different food samples. Food Chem 237 (2017): 921-928.
- Deng CH, Gong JL, Zhang P, et al. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J Colloid Interface Sci 488 (2017): 26-38.
- Choi YL, Choi JS, Lingamdinne LP, et al. Removal of U(VI) by sugar-based magnetic pseudo-graphene oxide and its application to authentic groundwater using electromagnetic system. Environ Sci Pollut Res Int 26 (2019): 22323-22337.
- Deng CH, Gong JL, Zeng GM, et al. Graphene sponge decorated with copper nanoparticles as a novel bactericidal filter for inactivation of Escherichia coli. Chemosphere 184 (2017): 347-357.
- Zhang K, Suh JM, Lee TH, et al. Copper oxide-graphene oxide nanocomposite: efficient catalyst for hydrogenation of nitroaromatics in water. Nano Converg 6 (2019): 6.
- Ahmed Ali, Fekri Abdulraqeb, Javed Alam, et al. Graphene oxide-silver nanosheet-incorporated polyamide thin-film composite membranes for antifouling and antibacterial action against Escherichia coli and bovine serum albumin. Journal of Industrial and Engineering Chemistry 80 (2019): 227-238.
- Sinha A, Jana NR. Graphene-based composite with gamma-Fe2O3 nanoparticle for the high-performance removal of endocrine-disrupting compounds from water. Chem Asian J 8 (2013): 786-791.
- Liang Yulu, Xiwen He, Langxing Chen, et al. Preparation and characterization of TiO2–Graphene@Fe3O4 magnetic composite and its application in the removal of trace amounts of microcystin-LR. RSC Adv 4 (2014): 56883-56891.
- Sereshti H, Zamiri Afsharian E, Esmaeili Bidhendi M, et al. Removal of phosphate and nitrate ions aqueous using strontium magnetic graphene oxide nanocomposite: Isotherms, kinetics, and thermodynamics studies. Environmental Progress and Sustainable Energy 39 (2020): 13332.
- Prabhu Subbaiah Muthu, Sd Elanchezhiyan, Giehyeon Lee S, Sankaran Meenakshi. Defluoridation of Water by Graphene Oxide Supported Needle-Like Complex Adsorbents. Journal of Inorganic and Organometallic Polymers and Materials 26 (2016): 834-844.
- Tolkou Athanasia K, Ioannis A Katsoyiannis, Anastasios I Zouboulis. Removal of Arsenic, Chromium and Uranium from Water Sources by Novel Nanostructured Materials Including Graphene-Based Modified Adsorbents: A Mini Review of Recent Developments. Applied Sciences 10 (2020): 3241.
- Li MF, Liu YG, Zeng GM, et al. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 226 (2019): 360-380.
- Filina Anya, Nariman Yousefi, Mira Okshevsky, et al. Antimicrobial Hierarchically Porous Graphene Oxide Sponges for Water Treatment. ACS Applied Bio Materials 2 (2019): 1578-1590.
- Khazaei Mohammad, Simin Nasseri, Mohammad Reza Ganjali, et al. Selective removal of mercury(II) from water using a 2,2-dithiodisalicylic acid-functionalized graphene oxide nanocomposite: Kinetic, thermodynamic, and reusability studies. Journal of Molecular Liquids 265 (2018): 189-198.
