Granuloma in a Baclofen Intrathecal Infusion Catheter in a Patient with Spinal Cord Injury: Review of a Case Report
Article Information
Ángela Palencia-Vidal1*, Sebastián Salvador-De La Barrera1, Aurora De La Iglesia-López2, María E. Ferreiro-Velasco1, Antonio Montoto-Marqués1,3, Ricardo Vázquez-Fernández1, Antonio Rodríguez-Sotillo1,3
1Unidad de Lesionados Medulares, Instituto de investigación Biomédica de A Coruña (INIBIC). Complejo hospitalario Universitario de A Coruña, A Coruña, Spain.
2Coordinadora de la Unidad del Dolor, servicio de Anestesiología y Reanimación. Instituto de investigación Biomédica de A Coruña (INIBIC). Complejo hospitalario Universitario de A Coruña, A Coruña, Spain.
3Departamento de Fisioterapia, Medicina y Ciencias Biomédicas, Universidad de A Coruña, Spain.
*Corresponding Author: Ángela Palencia-Vidal, Unidad de Lesionados Medulares, Instituto de investigación Biomédica de A Coruña (INIBIC). Complejo hospitalario Universitario de A Coruña, A Coruña, Spain
Received: 08 May 2025; Accepted: 15 June 2025; Published: 26 June 2025
Citation: Angela Palencia-Vidal, Sebastian Salvador-De La Barrera, Aurora De La Iglesia- Lopez, Maria E. Ferreiro-Velasco, Antonio Montoto- Marques, Ricardo Vazquez-Fernandez, Antonio Rodriguez-Sotillo. Granuloma in a Baclofen Intrathecal Infusion Catheter in a Patient with Spinal Cord Injury: Review of a Case Report. Archives of Clinical and Medical Case Reports. 9 (2025): 132-136.
View / Download Pdf Share at FacebookAbstract
Spasticity, a common complication in patients with spinal cord injury, can lead to loss of function and reduced quality of life. Although oral baclofen attenuates spasticity, its effects are usually modest, and adverse effects can be significant. Intrathecal baclofen infusion therapy has emerged as an effective and safe option for patients with severe spasticity refractory to conventional oral treatment. This reversible procedure allows for precise dosing and achieves high local concentrations in the spinal cord, minimizing systemic side effects. Although generally safe, intrathecal therapy can be associated with complications, with granuloma formation being one of the least frequent.
We present the case of a woman with post-traumatic paraplegia at the T3 level, classified as AIS A, who developed severe spasticity refractory to conventional oral treatment. Following the implantation of a baclofen intrathecal infusion system, the patient experienced progressive and severe pain attributed to an inflammatory granuloma or mass at the catheter tip. Complete replacement of the system resulted in complete resolution of the pain, demonstrating the effectiveness of this intervention. Granuloma formation at the catheter tip associated with intrathecal baclofen infusion is a rare complication with a non-specific clinical presentation and a pathogenesis that remains unclear. Magnetic resonance imaging (MRI) is the diagnostic tool of choice.
Keywords
Intrathecal pump therapy; Spasticity; Inflammatory mass; Baclofen; Spinal cord injury
Intrathecal pump therapy articles; Spasticity articles; Inflammatory mass articles; Baclofen articles; Spinal cord injury articles
Article Details
1. Introduction
Spasticity is a common consequence in patients with spinal cord injury (SCI), sometimes leading to loss of function, pain and impaired quality of life. Its incidence is estimated to be around 65-78% one year after injury. In SCI, spasticity typically presents with a global distribution, making regional or systemic treatment preferable [1]. Although oral baclofen reduces spasticity of both spinal and cerebral origin, its effects are usually moderate, and the adverse effects (sedation, drowsiness, cardiorespiratory depression) can be significant [2]. Intrathecal baclofen infusion therapy is a safe and effective alternative for patients with severe spasticity refractory to conventional oral treatment.
It is a reversible and adjustable procedure that allows for precise drug dosing. Direct administration of baclofen into the subarachnoid space provides a higher concentration at the site of action (GABAB receptors in the spinal cord). Effective baclofen levels are achieved in the cerebrospinal fluid (CSF), while plasma concentrations remain up to 100 times lower than those produced by oral administration, thereby reducing potential systemic side effects [3]. With intrathecal administration, the doses required to manage spasticity vary widely between patients. The baclofen dose ranges from 50 to 900 micrograms (µg) per day, with gradual titration over the first year of treatment [2].
It is a generally safe and well-tolerated therapy, although catheter-related complications have been reported, including rare cases of granuloma formation. These represent an inflammatory response of the tissue surrounding the catheter, and can cause pain, loss of therapeutic effect and, in severe cases, even neurological damage [4-6]. We present a case of a patient who, after implantation of a baclofen intrathecal infusion system, developed progressively worsening chronic pain, which became intense and disabling and resolved following complete system replacement. MRI revealed an image consistent with granuloma/inflammatory mass at the catheter tip.
2. Case Report
We present the case of a 51-year-old woman with post-traumatic paraplegia secondary to a spinal cord injury at the T3 level, classified as AIS A following a traffic accident. As part of the polytrauma, she sustained a burst fracture of the vertebral body and posterior elements of T5, for which vertebral arthrodesis with T3-T7 osteosynthesis was performed. She was diagnosed with a T3 AIS A spinal cord injury according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [7].
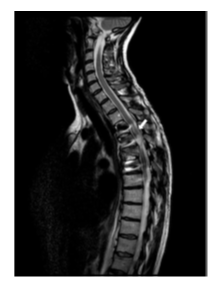
She was admitted to the Spinal Cord Injury Unit for six months. During her stay, she presented with severe lower limb spasticity refractory to systemic treatment (baclofen and tizanidine), as well as intermittent dorsal pain. MRI revealed signal alterations in the spinal cord compatible with dorsal myelomalacia from T5 to T3 (Figure A).
After hospital discharge, progressive worsening of lower limb spasticity and recurrent urinary tract infections (UTIs) were observed. In addition, the patient reported neuropathic pain in the abdominal region radiating to the lower back. The oral dose of baclofen increased to 35 mg every 8 hours (105 mg/day) and tizanidine 2 mg every 8 hours, but after five months, treatment proved to be largely ineffective. Therefore, an intrathecal baclofen test dose of 100 µg was administered, yielding a positive response, and implantation of an intrathecal infusion system was indicated.
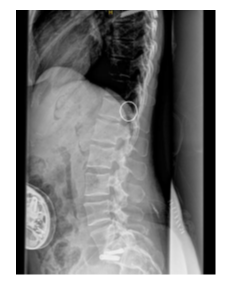
A Synchromed™ II 20mL pump was implanted for intrathecal baclofen infusion at a concentration of 2000 µg/ml. An Ascenda™ intrathecal catheter was placed at the upper border of the T12 vertebra (Figure B).
Intrathecal baclofen infusion dose of 140 µg/day in a single continuous flow and gradually increased until optimal spasticity control was achieved with a daily dose of 195 µg/day, with Ashworth scale 0 and Penn scale 0.
Over the period of four years and five months, the patient maintained good spasticity control without requiring any increase in baclofen dosage. However, she developed neuropathic pain in the abdominal region radiating to the lower back, which was refractory to treatment and subjectively exacerbated during episodes of UTIs.
Pain initially improved with pregabalin 150mg every 12 hours. An attempt was made to reduce the dose to 75mg every 12 hours, but after a period of stabilisation, the pain intensity worsened. Analgesic treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and a trial of gabapentin were attempted, but moderate-to-severe pain persisted.
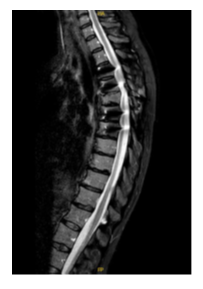
Several months later the patient returned with worsening pain and increased abdominal spasms. The dose of intrathecal baclofen was increased to 225 µg/day. Among the tests requested, a plain spinal X-ray was performed, which showed sclerosis of the vertebral endplate immediately below the dorsal arthrodesis (T7–T8) and the intrathecal catheter positioned within the vertebral canal at T12. Subsequently, a thoracolumbar MRI was performed (Figure C, D) revealing a millimetric (8mm) arachnoid collection/loculation at the level of T12, coinciding with the distal tip of the catheter.
Figure C: STIR MRI sequence. Within the malacic and atrophic spinal cord at the T4 level, a syringohydromyelic cyst measuring 13 x 10 x 6 mm is identified. Incidentally, at the T12 level and coinciding with the distal tip of the intrathecal catheter, an 8 mm arachnoid collection/loculation is noted (white arrow).
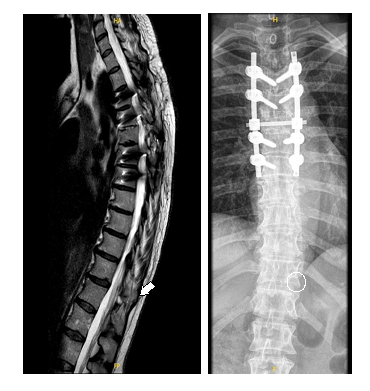
The case was discussed jointly with the Pain Unit and it was decided to replace the entire infusion system, as the pump battery was nearing depletion. The Synchromed™ II 20ml intrathecal pump and Ascenda™ catheter were replaced, with the new catheter positioned up to the vertebral hemibody of T11 (Figure E). The only postoperative complication was a hemicranial headache, which resolved with conventional analgesia. Following replacement, baclofen infusion was started at a dose of 180 µg/day, resulting in complete resolution of pain and disappearance of both spasticity and spasms.
3. Discussion
When symptom control cannot be adequately achieved with oral baclofen, or when effective doses produce poorly tolerated side effects, direct intrathecal administration becomes an effective approach to control spasticity. This method allows for sufficient drug concentrations near the target receptors in the central nervous system, while significantly decreasing the total baclofen dose required[1, 8, 9]. Several complications associated with this technique have been described, most of them related to the catheter (rupture, migration or displacement from the intrathecal space, disconnection, occlusion...) [10]. However, a notable and diagnostically challenging complication is the development of an inflammatory mass (granuloma) at the catheter tip.
The development of granulomas at the tip of the intrathecal infusion catheter is a known but infrequent complication, reported mainly with opioid infusion, with an estimated incidence of less than 3% at two years after therapy initiation of therapy [1, 3]. However, some cases of granuloma formation related to intrathecal baclofen infusion have also been reported. This complication not only compromises the efficacy of the drug delivery system but may also result in severe neurological sequelae.
It was in 1991 that the first case of an inflammatory mass associated with the tip of an intrathecal catheter in a patient receiving morphine therapy was reported [11]. Since then, several cases of catheter-associated masses have been described. The factors that contribute to the formation of such inflammatory masses, as well as the underlying mechanisms remain unclear.
Several hypotheses have been proposed in the literature regarding their etiology, including physicochemical properties of the drug solution, infection with indolent organisms, hypersensitivity to catheter materials, and trauma during catheter implantation [1, 12]. Additional contributing factors may include catheter-related variables such as tip design, trauma during insertion, final tip positioning, and material hypersensitivity. In line with the findings of Murphy et al. [1] regarding catheter design, our patient received a fenestrated-tip catheter. Older intrathecal systems used end-hole catheters, but current models are only available with closed lateral fenestrations, a design previously considered less likely to induce granuloma formation.
Consistent with the present case, it has been suggested by Murphy et al. and North et al. [1, 11] that syringomyelia or myelomalacia secondary to spinal cord trauma may be associated with altered CSF flow dynamics. In our patient, myelomalacia was identified on MRI as a sequela of the injury. It is possible that significant CSF flow disruption could lead to localized drug accumulation, thereby increasing the risk of granuloma formation.
Due to the slow-growing nature of this lesion, it is difficult to estimate how long it will take for the inflammatory mass to develop. This combined with the tendency to attribute increased spasticity and clinical deterioration to recurrent UTIs presents a significant diagnostic challenge in clinical practice. Some experimental animal (canine) studies [13] found no granuloma formation with intrathecal therapy at 2mg/mL over a 28-day period, although this does not exclude the possibility of this complication with prolonged infusions. Deer et al [12] reported a median time to granuloma formation of 26 months. Anderson et al. [15] estimated a mean duration of 24.5 months in patients receiving morphine or hydromorphone, whereas Kratzsch et al. [16] proposed an average onset time of 6.9 years.
In our case, the reported symptoms (radiating girdle-like pain and spasms) began shortly after implantation, and over the following months were highly variable and fluctuating, overlapping with symptoms of organic conditions such as UTIs and intestinal disorders. Therefore, the possibility of catheter-related trauma at the time of implantation must also be considered. Moreover, according to several authors, there is a direct correlation between the treatment intensity and symptoms severity, with higher drug doses increasing the likelihood of granuloma formation [17]. In our patient, the same dose was maintained continuously for over four years (195 µg/day). Standard imaging studies, such as T2-weighted MRI and/or contrast-enhanced CT, are essential for identifying the mass [14, 18-20]. MRI is preferred as the imaging modality of choice, as it not only enables visualization of the lesion, but also provides optimal assessment of spinal cord and nerve roots involvement. However, intrathecal catheters may be difficult to visualize on MRI alone [14].
In summary, this complication associated with intrathecal baclofen infusion represents a challenging diagnostic problem, with a non-specific clinical presentation that, in our case, overlapped with other symptoms and complications secondary to the spinal cord injury itself—such as transient neuropathic pain, symptoms related to urinary tract infections, and concurrent abdominal complaints. Diagnosis relies on imaging studies, which may be difficult both to perform due to interference from the implanted electronic device, and to interpret.
4. Conclusion
Granuloma formation associated with intrathecal therapy is a complication most commonly described in the context of chronic opioid administration. As a result, current consensus recommendations primarily focus on this class of drugs. When occurring in association with intrathecal baclofen infusion, this complication is rare, presents a diagnostic challenge due to its non-specific clinical manifestations, and its underlying pathophysiology remains unclear. MRI is the diagnostic modality of choice, and in our case, replacement of the intrathecal catheter system proved effective in resolving the patient's symptoms.
Funding
This article has received funding from Fundación Pública Galega de Investigación Biomédica INIBIC for the article processing charges.
References
- Murphy PM, Skouvaklis DE, Amadeo RJ, et al. Intrathecal catheter granuloma associated with isolated baclofen infusion. Anesth Analg 102 (2006): 848-852.
- De Sousa N, Santos D, Monteiro S, et al. Role of Baclofen in Modulating Spasticity and Neuroprotection in Spinal Cord Injury. J Neurotrauma 39 (2022): 249-258.
- Haering M, Saleh C, Jaszczuk P, et al. Intrathecal pump catheter-tip granuloma recurrence with associated myelomalacia - How safe is intrathecal analgesic infusion therapy? A case report. Surg Neurol Int 24 (2019): 62.
- Coffey RJ, Burchiel K. Inflammatory mass lesions associated with intrathecal drug infusion catheters: report and observations on 41 patients. Neurosurgery 50 (2002): 78-86.
- Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation 15 (2012): 436-464.
- Duarte R, Raphael J, Eldabe S. Intrathecal drug delivery for the management of pain and spasticity in adults: an executive summary of the British Pain Society's recommendations for best clinical practice Br J Pain 10 (2016): 67-69.
- Rupp R, Biering-Sørensen F, Burns SP, et al. International Standards for Neurological Classification of Spinal Cord Injury: Revised 2019. Top Spinal Cord Inj Rehabil 27 (2021): 1-22.
- Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320 (1989): 1517-1521.
- Koulousakis A, Kuchta J. Intrathecal antispastic drug application with implantable pumps: results of a 10-year follow-up study. Acta Neurochir Suppl 97 (2007): 181-184.
- Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage 19 (2000): 209-215.
- North RB, Cutchis PN, Epstein JA, et al. Spinal cord compression complicating subarachnoid infusion of morphine: case report and laboratory experience. Neurosurgery 29 (1991): 778-784.
- Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): Recommendations for Intrathecal Drug Delivery: Guidance for Improving Safety and Mitigating Risks. Neuromodulation 20 (2017): 155-176.
- Yaksh TL, Horais KA, Tozier NA, et al. Chronically infused intrathecal morphine in dogs. Anesthesiology 99 (2003): 174-187.
- Deer TR, Raso LJ, Garten TG. Inflammatory mass of an intrathecal catheter in patients receiving baclofen as a sole agent: a report of two cases and a review of the identification and treatment of the complication. Pain Med 8 (2007): 259-262.
- Anderson SR, Orbegozo M, Racz G, et al. Intrathecal granuloma in patients receiving high-dose intrathecal morphine therapy: a report of two cases. Pain Pract 1 (2001): 61-67.
- Kratzsch T, Stienen MN, Reck T, et al. Catheter-tip Granulomas Associated with Intrathecal Drug Delivery--A Two-Center Experience Identifying 13 Cases. Pain Physician 18 (2015): E831-840.
- Yaksh TL, Hassenbusch S, Burchiel K, et al. Inflammatory masses associated with intrathecal drug infusion: a review of preclinical evidence and human data. Pain Med 3 (2002): 300-312.
- Phillips JA, Escott EJ, Moossy JJ, et al. Imaging appearance of intrathecal catheter tip granulomas: Report of three cases and review of the literature. AJR Am J Roentgenol 189 (2007): W375-381.
- Deer TR, Raso LJ, Coffey RJ, et al. Intrathecal baclofen and catheter tip inflammatory mass lesions (granulomas): a reevaluation of case reports and imaging findings in light of experimental, clinicopathological, and radiological evidence. Pain Med 9 (2008): 391-395.
- Arnold PM, Harsh V, Oliphant SM. Spinal cord compression secondary to intrathecal catheter-induced granuloma: a report of four cases. Evid Based Spine Care J 2 (2011): 57-62.