Global Estimate of the Total Number of Human Strongyloidiasis Cases: A Systematic Review
Article Information
Heron Gezahegn*
School of Global Health and Bioethics, Euclid University, Banjul, Gambia
*Corresponding Author: Heron Gezahegn Gebretsadik, School of Global Health and Bioethics, Euclid University, Banjul, Gambia.
Received: 29 March 2023; Accepted: 05 April 2023; Published: 19 April 2023
Citation:
Heron Gezahegn. Sensitivity of Diagnostic Methods for Detecting S. Stercoralis Infection and Comparison of the Total Global Number of Strongyloidiasis Cases with Other Recognized NTDs: A Systematic Review. Journal of Orthopedics and Sports Medicine. 5 (2023): 192-198.
View / Download Pdf Share at FacebookAbstract
Background: The soil-dwelling nematode Strongyloides stercoralis is one of the most underestimated and neglected parasites in the Neglected Tropical Diseases (NTDs) group. All available data were systematically reviewed and summarized to better understand the problem's magnitude and estimate the total number of Strongyloidiasis cases in humans at best and worst.
Methods: A systematic electronic literature search was performed in the PubMed, WHOLIS, and ISI Web of Science databases for articles published between January 1990 and May 2017. Reports with quantitative data on prevalence, incidence, duration of infection, remission/cure, and mortality in humans were considered. After obtaining the raw data from the systematic review, correction for diagnostic accuracy, selection of the reference population, and adjustments for age and reference year 2017 were made as a prerequisite for estimating the total number of strongyloidiasis cases in humans worldwide.
Findings: A total of 166 community-based studies from 60 countries and all continents except Antarctica were identified to estimate the total number of infections worldwide. Overall, it was estimated that 159 542 655 people are infected with S. stercoralis worldwide in the best-case scenario and 260 710 055 in the worst-case scenario, with Asia and Europe having the highest and lowest numbers of strongyloidiasis cases, respectively.
Conclusions: This study revealed a high number of infections in both scenarios. Although there is still a need for more sufficient data on the epidemiology of strongyloidiasis, the current information emphasizes that S. stercoralis should not be neglected. Furthermore, the findings of this study can be used as a basis for Burden of Disease (BoD) calculation.
Keywords
Strongyloidiasis; Infection; Prevalence; S. stercoralis; Systematic; Estimate
Strongyloidiasis articles Strongyloidiasis Research articles Strongyloidiasis review articles Strongyloidiasis PubMed articles Strongyloidiasis PubMed Central articles Strongyloidiasis 2023 articles Strongyloidiasis 2024 articles Strongyloidiasis Scopus articles Strongyloidiasis impact factor journals Strongyloidiasis Scopus journals Strongyloidiasis PubMed journals Strongyloidiasis medical journals Strongyloidiasis free journals Strongyloidiasis best journals Strongyloidiasis top journals Strongyloidiasis free medical journals Strongyloidiasis famous journals Strongyloidiasis Google Scholar indexed journals Cochin-China diarrhea articles Cochin-China diarrhea Research articles Cochin-China diarrhea review articles Cochin-China diarrhea PubMed articles Cochin-China diarrhea PubMed Central articles Cochin-China diarrhea 2023 articles Cochin-China diarrhea 2024 articles Cochin-China diarrhea Scopus articles Cochin-China diarrhea impact factor journals Cochin-China diarrhea Scopus journals Cochin-China diarrhea PubMed journals Cochin-China diarrhea medical journals Cochin-China diarrhea free journals Cochin-China diarrhea best journals Cochin-China diarrhea top journals Cochin-China diarrhea free medical journals Cochin-China diarrhea famous journals Cochin-China diarrhea Google Scholar indexed journals Gastrointestina articles Gastrointestina Research articles Gastrointestina review articles Gastrointestina PubMed articles Gastrointestina PubMed Central articles Gastrointestina 2023 articles Gastrointestina 2024 articles Gastrointestina Scopus articles Gastrointestina impact factor journals Gastrointestina Scopus journals Gastrointestina PubMed journals Gastrointestina medical journals Gastrointestina free journals Gastrointestina best journals Gastrointestina top journals Gastrointestina free medical journals Gastrointestina famous journals Gastrointestina Google Scholar indexed journals Epigastric pain articles Epigastric pain Research articles Epigastric pain review articles Epigastric pain PubMed articles Epigastric pain PubMed Central articles Epigastric pain 2023 articles Epigastric pain 2024 articles Epigastric pain Scopus articles Epigastric pain impact factor journals Epigastric pain Scopus journals Epigastric pain PubMed journals Epigastric pain medical journals Epigastric pain free journals Epigastric pain best journals Epigastric pain top journals Epigastric pain free medical journals Epigastric pain famous journals Epigastric pain Google Scholar indexed journals Roundworms articles Roundworms Research articles Roundworms review articles Roundworms PubMed articles Roundworms PubMed Central articles Roundworms 2023 articles Roundworms 2024 articles Roundworms Scopus articles Roundworms impact factor journals Roundworms Scopus journals Roundworms PubMed journals Roundworms medical journals Roundworms free journals Roundworms best journals Roundworms top journals Roundworms free medical journals Roundworms famous journals Roundworms Google Scholar indexed journals Reptiles articles Reptiles Research articles Reptiles review articles Reptiles PubMed articles Reptiles PubMed Central articles Reptiles 2023 articles Reptiles 2024 articles Reptiles Scopus articles Reptiles impact factor journals Reptiles Scopus journals Reptiles PubMed journals Reptiles medical journals Reptiles free journals Reptiles best journals Reptiles top journals Reptiles free medical journals Reptiles famous journals Reptiles Google Scholar indexed journals Amphibians articles Amphibians Research articles Amphibians review articles Amphibians PubMed articles Amphibians PubMed Central articles Amphibians 2023 articles Amphibians 2024 articles Amphibians Scopus articles Amphibians impact factor journals Amphibians Scopus journals Amphibians PubMed journals Amphibians medical journals Amphibians free journals Amphibians best journals Amphibians top journals Amphibians free medical journals Amphibians famous journals Amphibians Google Scholar indexed journals Livestock articles Livestock Research articles Livestock review articles Livestock PubMed articles Livestock PubMed Central articles Livestock 2023 articles Livestock 2024 articles Livestock Scopus articles Livestock impact factor journals Livestock Scopus journals Livestock PubMed journals Livestock medical journals Livestock free journals Livestock best journals Livestock top journals Livestock free medical journals Livestock famous journals Livestock Google Scholar indexed journals Deoxyribonucleic Acid articles Deoxyribonucleic Acid Research articles Deoxyribonucleic Acid review articles Deoxyribonucleic Acid PubMed articles Deoxyribonucleic Acid PubMed Central articles Deoxyribonucleic Acid 2023 articles Deoxyribonucleic Acid 2024 articles Deoxyribonucleic Acid Scopus articles Deoxyribonucleic Acid impact factor journals Deoxyribonucleic Acid Scopus journals Deoxyribonucleic Acid PubMed journals Deoxyribonucleic Acid medical journals Deoxyribonucleic Acid free journals Deoxyribonucleic Acid best journals Deoxyribonucleic Acid top journals Deoxyribonucleic Acid free medical journals Deoxyribonucleic Acid famous journals Deoxyribonucleic Acid Google Scholar indexed journals Polymerase Chain Reaction articles Polymerase Chain Reaction Research articles Polymerase Chain Reaction review articles Polymerase Chain Reaction PubMed articles Polymerase Chain Reaction PubMed Central articles Polymerase Chain Reaction 2023 articles Polymerase Chain Reaction 2024 articles Polymerase Chain Reaction Scopus articles Polymerase Chain Reaction impact factor journals Polymerase Chain Reaction Scopus journals Polymerase Chain Reaction PubMed journals Polymerase Chain Reaction medical journals Polymerase Chain Reaction free journals Polymerase Chain Reaction best journals Polymerase Chain Reaction top journals Polymerase Chain Reaction free medical journals Polymerase Chain Reaction famous journals Polymerase Chain Reaction Google Scholar indexed journals Immunoassays articles Immunoassays Research articles Immunoassays review articles Immunoassays PubMed articles Immunoassays PubMed Central articles Immunoassays 2023 articles Immunoassays 2024 articles Immunoassays Scopus articles Immunoassays impact factor journals Immunoassays Scopus journals Immunoassays PubMed journals Immunoassays medical journals Immunoassays free journals Immunoassays best journals Immunoassays top journals Immunoassays free medical journals Immunoassays famous journals Immunoassays Google Scholar indexed journals Antibody test articles Antibody test Research articles Antibody test review articles Antibody test PubMed articles Antibody test PubMed Central articles Antibody test 2023 articles Antibody test 2024 articles Antibody test Scopus articles Antibody test impact factor journals Antibody test Scopus journals Antibody test PubMed journals Antibody test medical journals Antibody test free journals Antibody test best journals Antibody test top journals Antibody test free medical journals Antibody test famous journals Antibody test Google Scholar indexed journals
Article Details
Abbreviations:
BoD: Burden of Disease; WHO: World Health; NTDs: Neglected Tropical Diseases; MeSH: Medical Subject Headings; PubMed: U.S. National Library of Medicine, National Institute of Health; WHOLIS: World Health Organization Library Information System; ISI: Institute for Scientific Information; URL: Uniform Resource Locator; UNSD: United Nations Statistics Division; DNA: Desoxyribonucleic Acid; PCR: Polymerase Chain Reaction; ELISA: Enzyme-Linked Immunosorbent Assays; IFAT: Immunofluorescence Antibody Tests; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses
1. Introduction
Strongyloidiasis is a parasitic disease caused by nematodes of the genus Strongyloides and is one of the so-called Neglected Tropical Diseases (NTDs) [1-3]. After first reported in 1876 from the feces of French soldiers returning from ancient Indochina with diarrhea, the disease was known for many years as "Cochin-China diarrhea" [1]. This name also indicates this parasitic infection's most common gastrointestinal symptoms, namely epigastric pain and watery diarrhea [2]. After its initial discovery, it took more than a century to largely decipher the basic biology of this parasite and its extraordinary ability to disseminate within host tissues, which can lead to a variety of clinical complications [1].
The genus Strongyloides belongs to the roundworms, which are members of the order Rhabditida [4,5]. Most of the 52 species are soil-dwelling, microbivorous nematodes that do not infect humans [6]. Within this genus, there are over 40 species that can infect birds, reptiles, amphibians, livestock, and other primates [7-9]. Strongyloides stercoralis is the most common and clinically relevant species that infect humans (10-13). The only other, less common Strongyloides species that infect humans, S. fülleborni, occasionally occurs in Africa and Papua New Guinea, where it infects mainly chimpanzees and baboons [5,14,15].
stercoralis can survive and reproduce in a host for decades while causing minimal or no symptoms (in individuals with a healthy immune system). However, it can also lead to life-threatening conditions [16]. Typically, although not exclusively in immunocompromised patients, S. stercoralis can lead to systemic disease because of its potential for autoinfection [17]. Autoinfection with increasing worm burden can spread parasites throughout the host's body and hyperinfection. People with HIV infection or those taking medications that suppress the immune system are particularly at risk. Spreading strongyloidiasis can lead to pulmonary involvement, septicemia (secondary gram-negative sepsis), shock, emaciation, and death [10]. High mortality rates of 60-85% due to S. stercoralis hyperinfection syndrome and disseminated disease may occur [16-19].
Strongyloides are found on all continents except Antarctica. However, the tropics, subtropics, and warm-temperate regions are the most common endemic areas [20]. At the local level, infections are common in rural areas, institutions, and lower socioeconomic groups [21,22]. Although the global prevalence of strongyloidiasis is unknown, estimates range from 30 to 100 million infected people worldwide [23,24]. A study conducted in Northeast Asia found that the prevalence of strongyloidiasis was higher in men and the elderly than in women and the young [1,25]. In many tropical and subtropical regions with poor sanitation and warm, humid climates, intestinal nematode infection may affect 10% and 40% of the population [11]. However, all these facts may vary from region to region. For example, studies on the prevalence of S. stercoralis have shown that schoolchildren and adults over 45 years of age in rural and semi-rural areas of Cambodia are highly affected [24,26,27]. A meta-analysis of community and hospital surveys showed that prevalence rates of 15.1-19.6% and 12.1-16.0%, respectively, were estimated for Cambodia. Another example is Ethiopia, where high prevalence rates of S. stercoralis ranging from 14.1% to 17.9% (community-based data) and 23.6% to 40% (health facility data) were predicted nationwide [28]. The prevalence rate of Strongyloides infection in HIV-positive individuals is estimated to be 43% in Ethiopia [29]. In certain areas, such as Argentina, the prevalence rate of Strongyloidiasis may also be as high as 50% [29,30]. Nevertheless, there are insufficient global epidemiological data on S. stercoralis infection. This systematic review estimated the total number of strongyloidiasis cases in humans worldwide.
2. Methodology
2.1 Systematic review: search strategy
An electronic search was performed on May 23, 2017, in the PubMed (U.S. National Library of Medicine, National Institute of Health), WHOLIS (World Health Organization Library Information System), and ISI Web of Science (Thomson Reuters) databases. The keywords used were "Strongyloides" and "strongyloidiasis" combined with the Boolean operator "OR." For searches in the PubMed and WHOLIS databases, the terms were used as Medical Subject Headings (MeSH). Literature published between January 1990 and May 2017 was considered. The entire systematic review was conducted according to PRISMA guidelines.
2.2 Systematic review: extraction of data
Relevant data such as study population, sample size, data collection period, sex and age of the studied population, and key epidemiologic parameters were extracted and recorded in a simple Microsoft Excel (Microsoft Excel version 2010, Microsoft Corp., Redmond, WA, United States of America) data extraction template.
2.3 Data analysis
The extraction template was designed to include critical epidemiological parameters such as prevalence, incidence, mortality, and remission/cure rate. However, in this analysis, only the prevalence raw data were used to determine the total number of Strongyloides stercoralis infections. These raw prevalence data were adjusted for diagnostic accuracy, reference population selection, and population growth, as described below. The total number of infections was then calculated. Based on these data, the global distribution of strongyloidiasis cases was also determined.
Correction for the sensitivity of different diagnostic techniques
To estimate the total number of individuals infected with Strongyloides spp. Worldwide, it was essential to understand and correct, where possible, limitations due to differences in the sensitivity of the diagnostic tools used. Considering the potential sensitivity deficits of each diagnostic method reported in the literature reviewed, sensitivity correction factors were estimated to obtain a fair assumption about the actual number of infected individuals. Raw prevalence data obtained from the systematic review were multiplied by the appropriate correction factor to correct for the insensitivity of the diagnostic technique used. The correction factors for the different diagnostic techniques were as follows:
- for diagnostic methods with low sensitivity (i.e., less reliable conventional fecal-based methods such as direct microscopy, Formalin-Ethyl Acetate Concentration Technique (FECT), etc.): 5.88 (5.56-6.67)
- for moderately sensitive diagnostic techniques (i.e., more reliable conventional fecal-based methods such as Kato-Katz, Bearmann, Koga agar plate culture method, etc.): 1.19 (1.11-1.30)
- for highly sensitive diagnostic techniques (i.e., molecular tests for biological markers and mainly Deoxyribonucleic Acid (DNA) such as Polymerase Chain Reaction (PCR, COPRO-DNA) and serological tests and immunoassays for antigen detection such as various types of enzyme-linked immunosorbent assays (ELISA), Immunofluorescence Antibody Tests (IFAT), and Luciferase Immunoprecipitation Systems (NIE-LIPS), etc.): 1.05 (1.02-1.14)
Selection of the reference population
The corrected prevalence data were combined with plausible reference populations. National census reports and United Nations Statistics Division (UNSD) databases were searched to finally determine the total population count for each reference population.
Correction of population growth to adjust to the reference year 2017
The year 2017 was chosen as the reference year for estimating the total number of individuals infected with Strongyloides spp. Therefore, it was essential to adjust the pre-2017 reference population estimates to 2017. The most recent national population growth figures that could be obtained were used for these adjustments. The adjusted strongyloidiasis prevalence rates were not adjusted, so it was implicitly assumed that they had not changed since the respective studies.
Calculation of the total number of infected individuals at the national and global levels
Multiplying the sensitivity-corrected strongyloidiasis prevalence by the number of the reference population adjusted for population growth and age groups yielded the total number of infected persons at the national and global levels. When more than one raw prevalence data point was available from overlapping locations, only the estimate at the higher administrative level was used to avoid double or even multiple counting/estimation of cases. In addition, to avoid double or multiple counting/estimation, only the most recent prevalence data point was used for further projections when more than one raw prevalence data point was available that could be associated with the same reference population (i.e., the same geographic location and also the same administrative level).
The total number of infected persons was calculated under two scenarios: a lower or best-case scenario and an upper or worst-case scenario. For the lower scenario, the more conservative values of the different variables were always used, i.e., the lower bound of the sensitivity correction factors and the more narrowly defined reference population. The upper values of the different variables were always used for the upper scenario, i.e., the upper bound of the sensitivity correction factors and the broader defined reference population.
2.4 Ethical Consideration
No ethical review was required for the proposed research.
2.5 Findings
2.5.1 Databases search result
The initial electronic search yielded a total of 8441 hits. 2022 and 6419 articles were found in PubMed and ISI Web of Science using MeSH terms and keywords, respectively. No study was found in WHOLIS. A total of 2410 articles were identified as duplicates and excluded using the EndNote (1678) and Manual (732) search strategies. Of the 6031 articles without duplicates, 545 were classified as potentially relevant based on their title and abstract. In addition, nine articles were identified by searching the bibliographies of the included full texts, personal archives, and recommendations from colleagues and collaborators.
A total of 554 articles were screened for full-text accessibility by EndNote full-text search, URL search, Google search, Swiss Tropical and Public Health Institute, and University of Basel library sources. Approximately 97% (n=536) of the total articles reviewed for full-text accessibility were found, retrieved, and finally evaluated against the inclusion criteria.
After thoroughly reading all available full text, 166 articles containing relevant data for the analysis were finally included. The following flowchart illustrates the search strategy and methodology used in the literature search (Figure 1).
2.5.2 Characteristics of the literature studied
Characteristics related to the type of study design used
Of the 166 articles, 161 (97%) and 3 (1.8%) used Cross-Sectional Studies (CSS) and cohort studies, respectively. One study (0.6%) used a case-control design. Another article (0.6%) was a systematic review (Figure 6). After thoroughly assessing the original underlying studies, the systematic review was included to avoid double-counting articles.
Characteristics related to the study location
The studies included in the review were conducted on all continents except Antarctica. Studies were conducted in 60 countries worldwide; 20 (33.33%) African, 14 (23.33%) Asian, 13 (21.68%) North American, 9 (15%) South American, 2 (3.33%) European, and 2 (3.33%) Oceanic countries were reported. Regarding the number of studies, 51 (30.72%) were conducted in Africa and 50 (30.12%) in South America, followed by 49 (29.52%), 9 (5.42%), 5 (3.01%), and 2 (1.21%) in Asia, North America, Oceania, and Europe, respectively (Table 1). Most studies per country were from Brazil (19), followed by Thailand, Nigeria, Ethiopia, Peru, Argentina, Cambodia, India, and Venezuela (14, 12, 10, 9, 8, 7, 6, and 6, respectively) (Figure 2).
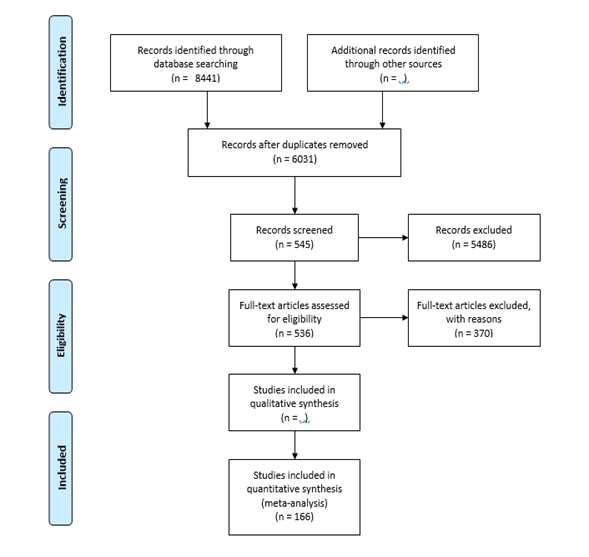
Figure 1: PRISMA flow diagram (2009) illustrating the systematic review process.
|
Continent |
The proportion of the number of countries (%) |
The proportion of the number of studies (%) |
|
|
1 |
Africa |
20 (33.33) |
51 (30.72) |
|
2 |
Asia |
14 (23.33) |
49 (29.52) |
|
3 |
North America |
13 (21.68) |
9 (5.42) |
|
4 |
South America |
9 (15) |
50 (30.12) |
|
5 |
Oceania |
2 (3.33) |
5 (3.01) |
|
6 |
Europe |
2 (3.33) |
2 (1.21) |
|
Total |
60 (100) |
166 (100) |
|
Table 1: Demonstrates proportion by percent of the number of countries and studies in the six continents.
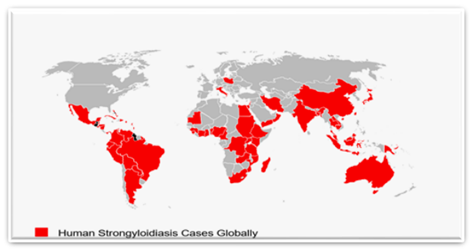
Figure 2: Illustrates human strongyloidiasis cases identified by the review globally on a world map.
Characteristics related to the year of studies performed
The number of studies conducted in the 2010–2014-year group was the highest, 35 (21.1%), followed by 32 (19.3%) and 30 (18.1%) in the 2005-2009- and 1990-1994-year groups, respectively. The lowest number of studies, 22 (13.2%), was conducted in the 1995–1999-year group.
Minimum and maximum number of infections by continent
Asia is the continent most affected by S. stercoralis infections, with 101,835,998 and 125,097,339 cases, respectively, in both the minimum and maximum scenarios. South America is the second most affected continent, with 31,741,414 and 98,319,373 infections in the minimum and maximum scenarios. Africa ranked third in highest and lowest prevalence cases, with 21,939,446 minimum and 33,212,127 maximum infection cases, respectively. Europe was the least affected continent, with 11,337 and 61,535 infections in the best and worst scenarios (Table 2).
|
Continent |
Maximum number of infection |
Minimum number of infection |
|
Asia |
101835998 |
125097339 |
|
South America |
31741414 |
98319373 |
|
Africa |
21939446 |
33212127 |
|
Oceania |
2746752 |
2746752 |
|
North America |
1267708 |
1272929 |
|
Europe |
11337 |
61535 |
|
Total |
159542655 |
260710055 |
Table 2: Demonstrates the minimum and maximum number of infections in each continent.
3. Discussion
Many epidemiologic features of S. stercoralis infection are not well known. Worldwide prevalence rates vary, as do the different types and numbers of studies conducted [29,31]. The prevalence of strongyloidiasis has been estimated at 100 million people worldwide, mainly in tropical regions, where 60 million people are infected. It is an emerging global infection that is underestimated in many areas [17]. The main reasons for this are that not enough studies have been conducted that focused on S. stercoralis, and studies that reported the prevalence of S. stercoralis mostly used diagnostic methods with low sensitivity and analyzed only a single sample [31]. Tropical climate, lack of education, rural conditions, and poor sanitation are the main reasons for the high prevalence of strongyloidiasis [12].
According to available information, 10% and 40% of the population is infected with S. stercoralis in many tropical and subtropical countries. High Strongyloides infection rates of up to 60% have also been estimated in resource-poor countries with environmental and socioeconomic conditions that favor the spread of the parasite [29]. Strongyloidiasis is endemic in Southeast Asia, Latin America, sub-Saharan Africa, and parts of the southeastern United States [1]. A similar global distribution pattern of strongyloidiasis was also found in this study. Asia was the most affected region, with 101,835,998 and 125,097,339 infected persons in the minimum and maximum scenarios, respectively. South America was the second most affected continent, with an estimated 31,741,414 and 98,319,373 cases in the minimum and maximum scenarios, respectively. The number of infected cases in Africa was estimated at 21,939,446 and 33,212,127, and Europe at 11,337 and 61,535 in the low and high scenarios, respectively. Most studies were conducted in Africa 51 (31.5%), South America 50 (31.1%), Asia 49 (29.4%), and North America 9 (5.4%). Few studies were found in Oceania 5 (3%) and Europe 2 (1.2%). The total number of infected individuals was generally calculated to be a minimum of 159 542 655 and a maximum of 260 710 055 worldwide. As described earlier, this review calculated the global estimate of strongyloidiasis cases in humans in two scenarios. The minimum scenario estimate showed that the prevalence of strongyloidiasis is 1.5 times higher than the current maximum estimate of 100 million infections worldwide. Therefore, the previous global estimates may be underestimates. The maximum prevalence estimate from this study was 2.5 times higher than the current maximum estimate of 100 million infections worldwide by the World Health Organization [5]. The global estimate presented here underscores that S. stercoralis should be addressed.
4. Conclusion
Global prevalence rates of strongyloidiasis infection vary widely. The current estimate in this systematic review far exceeded the total number of strongyloidiasis cases worldwide as determined by WHO. Although it is undoubtedly essential to conduct further thorough investigations, the results of this study suggest that S. stercoralis infection needs to be urgently addressed. Therefore, all stakeholders, including WHO, need to pay appropriate attention to the disease. Second, the disease should be classified by WHO as one of the recognized NTDs. These critical steps will allocate budgetary resources to improve the global ivermectin supply and promote activities such as footwear wearing in endemic areas to sustainably prevent the disease from affecting millions of people worldwide.
Acknowledgement
I want to thank Dr. Thomas Fuest and Prof. Peter Odermatt for their excellent supervision throughout the research work. Without their sound scientific support, this work would not have been accomplished. My thanks also go to Dr. Dora Buonfrate for her contribution during the data extraction phase of the project.
Conflict of Interest
The author declares that there are no potential conflicts of interest related to this article's research, authorship, and/or publication.
References
- Puthiyakunnon S, Boddu S, Li Y, et al. Strongyloidiasis—An Insight into Its Global Prevalence and Management. PLOS Negl Trop Dis 8 (2014): e3018.
- Ruankham W, Bunchu N, Koychusakun P. Prevalence of Helminthic Infections and Risk Factors in Villagers of Nanglae Sub-District, Chiang Rai Province, Thailand. J Med Assoc Thai 97 (2014): S29-S35.
- Schaer F, Guo L, Streit A, et al. Strongyloides stercoralis genotypes in humans in Cambodia. Parasitol Int 63 (2014): 533-536.
- Sithithaworn P, Srisawangwong T, Tesana S, et al. Epidemiology of Strongyloides stercoralis in north-east Thailand: application of the agar plate culture technique compared with the enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg 97 (2003): 398-402.
- WHO Strongyloidiasis (2017).
- Mangali A, Sasabone P, Syafruddin, et al. Prevalence of intestinal helminthic infections in Kao District, North Halmahera, Indonesia. Southeast Asian J Trop Med Public Health 25 (1994): 737-744.
- Amor A, Rodriguez E, Saugar JM, et al. High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up. Parasit Vectors (2016).
- Vonghachack Y, Sayasone S, Bouakhasith D, Taisayyavong K, Akkavong K, Odermatt P. Strongyloides stercoralis is highly prevalent on Mekong Islands in Southern Lao PDR. Trop Med Int Health 20 (2015): 315.
- Yelifari L, Bloch P, Magnussen P, et al. Distribution of human Oesophagostomum bifurcum, hookworm and Strongyloides stercoralis infections in northern Ghana. Trans R Soc Trop Med Hyg 99 (2005): 32-38.
- CDC C. Prevention. CDC Responds to Disease Outbreaks 24-7 | About | CDC (2014).
- Schär F, Giardina F, Khieu V, et al. Occurrence of and risk factors for Strongyloides stercoralis infection in Southeast Asia. Acta Trop 159 (2016): 227-238.
- Khieu V, Schär F, Marti H, et al. Prevalence and risk factors of Strongyloides stercoralis in Takeo Province, Cambodia. Parasit Vectors 7 (2014): 221.
- Buonfrate D, Baldissera M, Abrescia F, et al. Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case-control study, February 2013 to July 2014. Euro Surveill 21 (2016).
- Ananthakrishnan S, Nalini P, Pani SP. Intestinal geohelminthiasis in the developing world. Natl Med J India 10 (1997): 67-71.
- Beltramino D, Lura MC, Carrera E. Selective vs. mass treatment with antihelminthic drugs: experience in two hyperendemic communities. Rev Panam Salud Publica 13 (2003): 10-18.
- Kassalik M, Mönkemüller K. Strongyloides stercoralis Hyperinfection Syndrome and Disseminated Disease. J Gastroenterol Hepatol 7 (2011): 766-768.
- Khieu V, Schar F, Marti H, et al. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLOS Negl Trop Dis 7 (2013): e2035.
- Fernandez MC, Verghese S, Bhuvaneswari R, et al. A comparative study of the intestinal parasites prevalent among children living in rural and urban settings in and around Chennai. J Commun Dis 34 (2002): 35-39.
- Glinz D, N'Guessan NA, Utzinger J, et al. High prevalence of Strongyloides stercoralis among school children in rural Cote d'Ivoire. J Parasitol 96 (2010): 431-433.
- Jongsuksuntigul P, Intapan PM, Wongsaroj T, et al. Prevalence of Strongyloides stercoralis infection in Northeastern Thailand (agar plate culture detection). J Med Assoc Thai 86 (2003): 737-741.
- Ketzis JK, Conan A. Estimating occurrence of Strongyloides stercoralis in the Caribbean Island countries: Implications for monitoring and control. Acta Trop 171 (2017): 90-95.
- Khieu V, Schar F, Forrer A, et al. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLOS Negl Trop Dis 8 (2014): e2854.
- Yelifari L, Bloch P, Magnussen P, et al. Distribution of human Oesophagostomum bifurcum, hookworm and Strongyloides stercoralis infections in northern Ghana. Trans R Soc Trop Med Hyg 99 (2005): 32-38.
- Schär F, Inpankaew T, Traub RJ, et al. The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitol Int 63 (2014): 597-603.
- Sanprasert V, Srichaipon N, Bunkasem U, et al. Prevalence of intestinal protozoan infection among children in thailand: a large-scale screening and comparative study of three standards detection methods. Southeast Asian J Trop Med Public Health 47 (2016): 1123-1133.
- Schär F, Guo L, Streit A, et al. Strongyloides stercoralis genotypes in humans in Cambodia. Parasitol Int 63 (2014): 533-536.
- Khieu V, Hattendorf J, Schär F, et al. Strongyloides stercoralis infection and re-infection in a cohort of children in Cambodia. Parasitol Int 63 (2014): 708-712.
- Chhakda T, Muth S, Socheat D, et al. Intestinal parasites in school-aged children in villages bordering Tonle Sap Lake, Cambodia. Southeast Asian J Trop Med Public Health 37 (2006): 859-864.
- Schär F, Trostdorf U, Giardina F, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. PLOS Negl Trop Dis 7 (2013): e2288.
- Khieu V, Schär F, Forrer A, et al. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLOS Negl Trop Dis 8 (2014): e2854.
- Schaer F, Odermatt P, Khieu V, et al. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop 126 (2013): 89-92.
