Giant Filiform Thrombus Anchored at the Outflow of a Percutaneous Aortic Valve Down to the Abdominal Aorta
Article Information
Cianciulli Tomás F MD1,5, Estrada Jorge E MD2, Philippe Eric PhD3, Desaulniers Denis MD3, Zhang Ze PhD3, Zappi Andrea MD4, Guidoin Robert PhD3
1Department of Cardiology, Hospital of the Government of the City of Buenos Aires “Dr. Cosme Argerich”, Buenos Aires, Argentina
2Department of Cardiovascular Surgery, Hospital of the Government of the City of Buenos Aires “Dr. Cosme Argerich”, Buenos Aires, Argentina
3Department of Surgery, Faculty of Medicine, Université Laval and Centre de Recherche du CHU de Québec –Université Laval, Québec, QC, Canada
4Department of Pathology, Hospital of the Government of the City of Buenos Aires “Dr. Cosme Argerich”, Buenos Aires, Argentina
5Researcher of the Ministry of Health of the Government of the City of Buenos Aires, Buenos Aires, Argentina
*Corresponding authors: Tomás F Cianciulli, Echocardiography Laboratory, Department of Cardiology, Hospital of the Government of the City of Buenos Aires “Dr. Cosme Argerich”. Pi y Margall 750 (C1155ADP), Buenos Aires, Argentina.
Robert Guidoin, Department of Surgery, Faculty of Medicine, Université Laval and Centre de Recherche du CHU de Québec – Université Laval, Québec, QC, Canada
Received: 27 February 2024; Accepted: 07 March 2024; Published: 28 March 2024
Citation: Cianciulli Tomás F, Estrada Jorge E, Philippe Eric, Desaulniers Denis, Zhang Ze, Zappi Andrea, Guidoin Robert. Giant Filiform Thrombus Anchored at the Outflow of a Percutaneous Aortic Valve Down to the Abdominal Aorta. Cardiology and Cardiovascular Medicine. 8 (2024): 102-110.
View / Download Pdf Share at FacebookAbstract
A 72-year-old patient was fitted with a CoreValve prosthesis and passed away after 132 days. The valve with the 26 cm filiform thrombus attached at the outflow of the valve was harvested at autopsy.
A processed porcine pericardium and a CoreValve non-implanted were selected as controls. The porcine pericardium showed a thin layered architecture holding interstitial cells. The collagen bundles showed a sinusoidal morphology in most unloaded collagen tissues. The reference valve consisted of a nitinol frame holding three coapting leaflets. The structure of the pericardium was well preserved; however, the sutures were associated with compression, distortion or damage to the collagen, more frequently on the fibrous side.
The floating thrombus was the result of massive thrombosis not only at the outflow of the valve but at the inflow alike. The inflow showed fractured thrombi covering the flow surface. A leaflet was detached at the extraction of the floating thrombus. A second leaflet was rolled up and the third one was still visible. Thrombi were present everywhere and incorporated bacteriemic colonizations. The thrombotic deposits on the remaining leaflet were poorly anchored to its surface; specifically, a fibrous capsule was detached from the leaflet but still fixed to the nitinol frame. Despite major trauma, the sinusoidal structure of the collagen fibers was well preserved. Most of the floating thrombus was reorganized. This lethal issue of percutaneous aortic valve deployment might be more frequent than reported and shall be cautiously investigated, as the risk of thrombotic accumulation cannot be considered as negligible.
Keywords
Floating thrombus; Percutaneous heart valve; Thrombosis; TAVI
Article Details
1. Introduction
The development of a thrombus from the aortic arch down to descending thoracic aorta is a rare but under-recognized source of systemic emboli with potential catastrophic consequences [1-6]. More specifically it is the source of systemic emboli resulting in significant morbidity [7,8]. No clear guidelines exist for management [9]. Treatments are well individualized. Both endovascular [10,11] and open surgical techniques [12-14] have reported satisfactory results. Conservative treatments may be considered in asymptomatic, inoperable and high-risk patients.
The etiology is not yet fully understood. A floating aortic thrombus is often seen in relatively young patients without severe atherosclerosis although an atherosclerotic process may contribute in its pathogenesis [15]. A high prevalence of hematologic disorders may be causative factors for thrombus formation. The presence of cardiovascular implants has not been fully investigated yet [16]. We previously reported the presence of a floating thrombus attached to a woven thoracic graft in the case of a 69-year-old woman, after a surgical repair of a ruptured aneurysm. Together with post-operative complications, the patient developed a septic condition. A computed tomography (CT) scan disclosed a dissection like an image mimicking a genuine aortic dissection. The patient passed away six weeks later of multiple organ failure. He was autopsied. The woven graft was dissected and showed a one-piece floating thrombus that was 9 cm long, anchored at the proximal suture line of the prostheses. The structure of the thrombus was dense without fragmentation and showed a false lumen. The mural thrombus was made of layers of fibrin from granular to dense and laminated. Some sites of high fragility with fractures were observed and represented potentially embolizing structures [17].
The issue of a floating thrombus attached to a valve as observed at the autopsy deserves attention. Transcatheter aortic valve implantation (TAVI) is currently an interventional method for symptomatic patients with severe aortic stenosis who are at intermediate or high operative risk of death or who are deemed inoperable [18]. Major complications related to TAVI, including death, have been published. In such cases, it is not always possible to ascertain the cause of death. Autopsy after TAVI is a new field of interest in cardiovascular pathology. Early analyses of percutaneous valves after autopsy or at surgical conversion revalving showed a good integration of the valve into the annulus and outflow tract while the underlying valve does not interfere with the normal functioning of the prothetic valve [19]. However, there is a time dependent degeneration of percutaneous heart valves consisting of thrombus formation, hyperplasia, fibrosis, and tissue remodeling [20,21]. Valve leaflet thrombosis impairing its motion is the most frequent observation [22,23]. Autopsy of patients who underwent percutaneous valve implantation adds to the clinical diagnosis regarding the cause of death. Cerebral autopsy can be considered extremely helpful in determining the cause of death [24].
We hereby report the examination of the heart of a patient fitted with a CoreValve for 132 days [25]. A 26 cm cylindrical floating thrombus was attached to a leaflet at the outflow of the valve down to the aortic carrefour. A control pericardium was analyzed to serve as reference to in vivo changes. A reference CoreValve was investigated to identify the sites at risk of thrombosis and/or degradation after implantation. It was obtained during a clinical procedure as the patient passed away before its deployment.
2. Materials and Methods
2.1 Case report
A 72-year-old man with a medical history of liver transplantation and chronic kidney disease for more than a decade was admitted with symptomatic severe aortic valve stenosis. Due to his high surgical risk, the patient underwent percutaneous transcatheter aortic valve implantation via transfemoral catheter, using a 26 mm CoreValve prosthesis (Medtronic). The procedure was performed without difficulty, but the final valve position was suboptimal, with the ventricular side of the stent contacting the anterior mitral leaflet [25]. The patient was readmitted at the hospital 45 days after TAVI deployment, due to fever and heart failure. Enterococcus faecalis was retrieved in blood cultures, and hence antibiotic treatment with ceftriaxone-ampicillin was started. Late prosthetic infective endocarditis was suspected and transesophageal echocardiography showed no evidence of endocarditis, but the aortic prosthesis was deeply implanted in the left ventricular outflow tract, with a peak systolic gradient of 30 mmHg and a mean gradient of 14 mmHg, mild to moderate paravalvular aortic regurgitation, and a perforation of the anterior mitral leaflet contiguous to the aortic prosthesis, with severe mitral regurgitation. The patient did not present embolic events. While on antibiotic treatment, 52 days after readmission the patient suffered severe upper gastrointestinal bleeding with hemorrhagic shock and passed away 132 days after valve deployment. An autopsy was completed. The valve and the fusiform thrombus were fixed in a 5% solution of formaldehyde and available for analyses. The study was approved by the ethic committee of the first author’s institution.
2.2 Control devices
A commercial porcine pericardium (Bio-Sud, Buenos Aires, Argentina) and a CoreValve that could not be implanted as the patient passed away immediately prior the deployment were selected as references.
2.3 Non-destructive investigations
The valves, both reference and explanted, were first examined and imaged using a digital camera (Nikon, Tokyo, Japan). The pictures were processed with the Adobe Photoshop CS software (Adobe, San Jose, CA, USA). The micro-CT imaging was performed solely on the control device with a GE eXplore Locus micro-CT scanner (London, ON, Canada) at 45 µm voxel resolution (80 kV, 360 projections, 490 µm, two frames averaging and 400 ms of exposure time).
2.4 Destructive investigations
Selected samples of the explanted device were investigated in scanning electron microscopy (SEM) and light microscopy. Specimens 0.5 × 0.5 cm were cut at various sites of the device to characterize the links of the valve with its support and the sole leaflets. For SEM, the specimens were post fixed in a 2% solution of isotonic buffered glutaraldehyde and then transferred to a 1% osmium tetroxide solution. They were dried by immersion in solution of ethanol of graded concentrations and then in hexamethyldisilazane (HMDS). Then the specimens were coated with a gold palladium and observed with SEM under accelerating voltages ranging from 15 kV to 30 kV. For light microscopy, the specimens were embedded in a polymethylmethacrylate resin (PMMA). After the polymerization was achieved, the specimens were trimmed and sectioned on a precision band saw under coolant. Slices 300 µm in thickness were affixed onto a methyl methacrylate slide with a UV adhesive. Grinding and polishing were done on a digital Exact grinder system to obtain slices 20- 30 µm thick. Toluidine blue (0.5% in a borax solution) and hematoxylin/eosin staining were performed prior to light microscopy observation. The fusiform floating thrombus was embedded in paraffin and slices 5 µm thick were obtained prior hematoxylin/eosin staining. The slides were then ready for observation in light microscopy.
The reference device was fully dehydrated in alcohol of graded concentrations from 30 to 100% prior to embedding in PMMA. Three slices of the device perpendicular to the blood flow direction were sectioned on a precision band saw under coolant: inflow site, middle section and outflow of the valve. The slices were processed as the specimen of the explanted device and hematoxylin eosin stained.
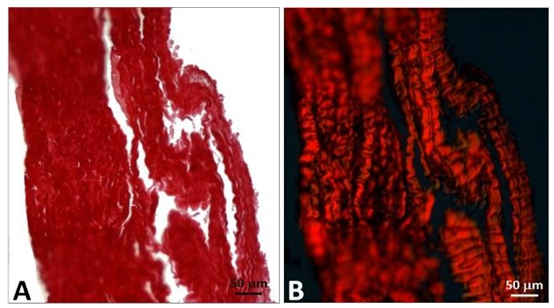
In addition, samples of the leaflets of the explanted CoreValve and the control porcine pericardium were processed for observation in light microscopy, including polarized microscopy after staining with hematoxylin/eosin, Masson’s trichrome, Verhoelf and red picrosirius. The purpose was to evidence the sinusoidal structure of the collagen bundles.
3. Results
3.1 Porcine pericardium
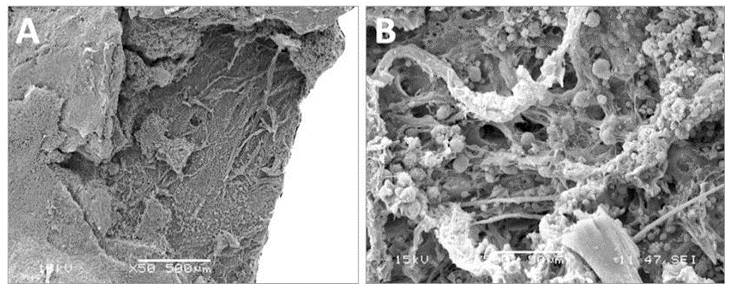
The porcine pericardium showed a layered architecture of collagen bundles presenting some interstitial cells. They displayed wavy crimps typical of an unloaded collagenous tissue. The collagen bundles were not bound together. The mesothelial cells found in vivo on the serous surface with smooth surface were no longer present due to tissue processing (Figure 1). The serous side (epicardium or visceral layer) was more compacted compared to the fibrous side with loose connective tissue. The epicardium serves as the inflow side in valve prosthesis whereas the serous side is deployed at the outflow (Figure 2).
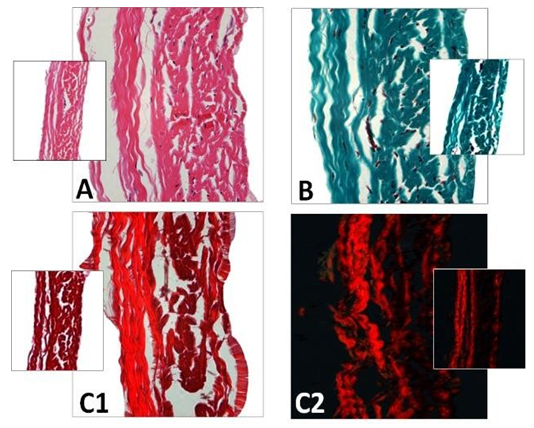
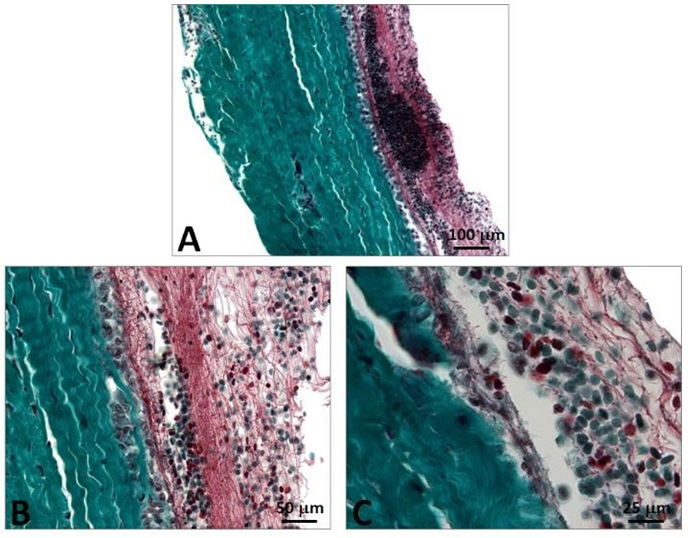
Figure 1: Cross-section of porcine pericardium in light microscopy. The fibrous section is on the left (inflow) and the serous one on the right (outflow) after staining with hematoxylin-eosin (A) Masson’s trichrome (B) and red pinosirius in polarized light microscopy (C, C2). The collagen bundles display a wavy structure comprising some elastic fibers. The serous surface (outflow) is considerably smoother than the fibrous surface (inflow). Some rare cells are still present in the pericardium.
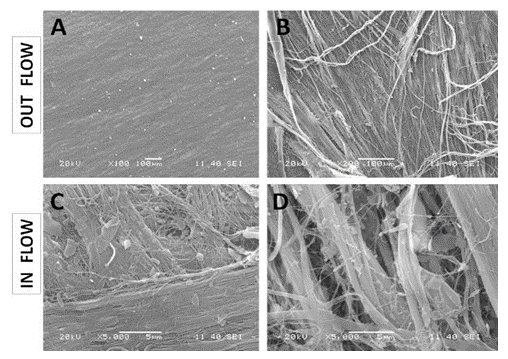
Figure 2: SEM observations of the porcine pericardium. The serous surface (outflow) looks smooth with mild undulations caused by the underneath collagen sinusoidal fibers at low magnification (A). The presence of tiny fibers over this smooth surface is observed at high magnification (B). The fibrous surface (inflow) shows interlacing fibres in various directions at low magnification (C). Bundles of collagen of various morphologies and diameters are visible at higher magnification within an open structure (D).
3.2 Reference device
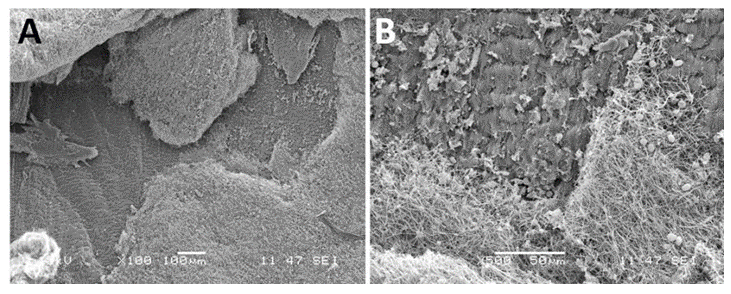
This device was made of three coapting valve leaflets, made from a single layer of porcine pericardium and fixed onto a self-expanding multi-level radiopaque frame made of nitinol (Figure 3).
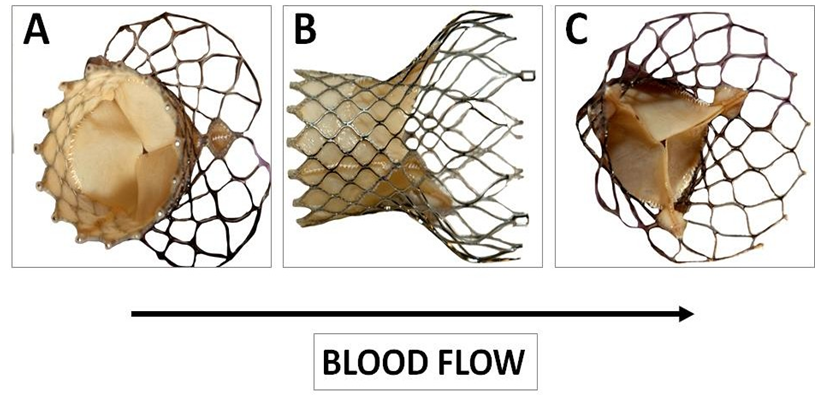
Figure 3: Macroscopy of the CoreValve used as reference. This bioprosthesis is manufactured by suturing three valve leaflets and a skirt made from a single layer of porcine pericardium onto a self-expanding multilevel, radiopaque frame made of nitinol. This support shows a multilevel self-expanding configuration with diamond cell shape. This noncylindrical frame design incorporates three different sections with different diameters and totally different degrees of radial and hoop strength. The lower section (A) exerts a high radial force to secure the device stability. The middle section (B) holds the valve leaflets. The upper section (C) is the largest diameter with lower radial force.
This porcine pericardium was previously processed with alpha-amino oleic acid to prevent mineralization of the valve after deployment. The frame comprises an outflow circumferential structure defining an outflow end of the frame and consisting essentially of a single row of diamond shaded expandable cells. The frame also comprises three V-shaped structures spaced apart from the outflow circumferential structures and three struts longitudinally aligned with the longitudinal axis of the frame. The three struts are configured to facilitate mounting the porcine pericardium leaflets to the frame and interconnect the outflow circumferential structure to the three V-shaped structures. Care was taken to the fibrous side of the pericardium at the inflow and the serous one at the outflow (Figure 4).
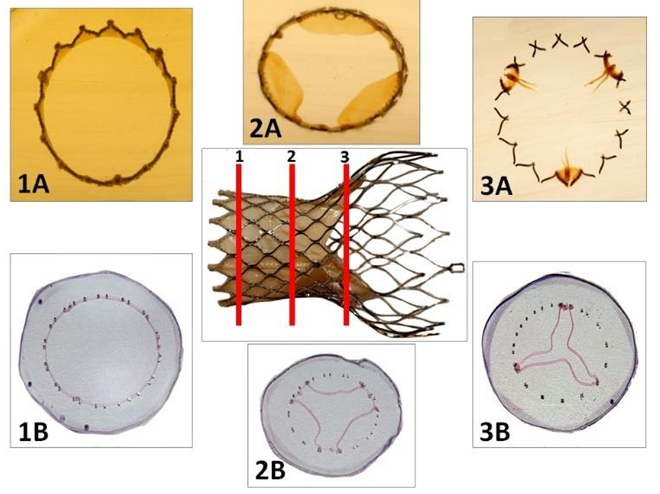
Figure 4: Processing of the CoreValve for histological investigation. The device is embedded in PMMA and semi-thin slides (1, 2, 3) are cut on a precision band saw under coolant (1A, 2A, 3A). These slides 300 µm thick are affixed with a UV adhesive on a methyl methacrylate slide. Guiding and polishing allows to obtain slices 20-30 µm thick. Metaboxcylin eosin staining is performed prior to observation in light microscopy (1B, 2B, 3B).
Figure 5: Histology of the porcine pericardium associated with the reference CoreValve. The pericardium shows an intact structure with well differentiated content consisting of multiple layers of collagen. The sinusoidal structure of the collagen is evidenced in the serous section with its smooth surface. The fibers in the fibrous section are less undulated and the surface is not as smooth (A). At contact with the nitinol frame, the pericardium is compressed by the polytetrafluoroethylene suture, but its structure is well preserved (B). The suture can result in fracture of the pericardium however the serous layer is well preserved (C). The passage of sutures can cause not only compression but restructuration of the pericardium (D).
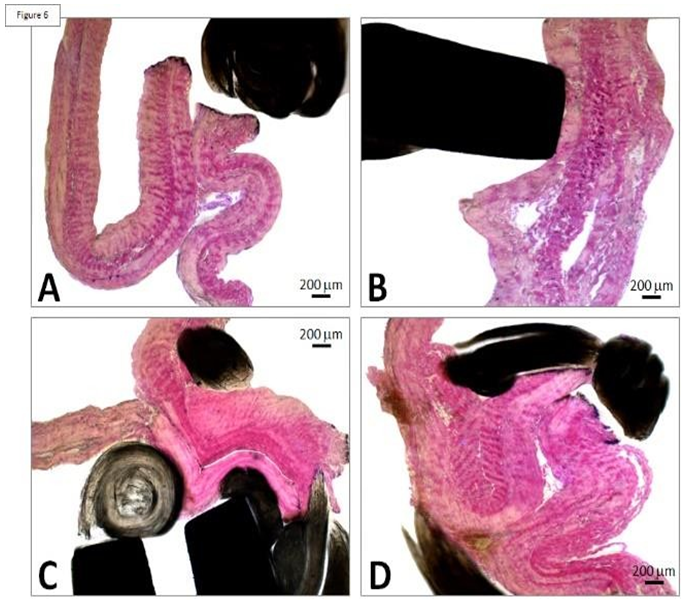
The risk of thrombotic developments is aggravated in case of stasis and thus the serous side of the pericardium is selected at the outflow. Thrombosis is unlikely at the inflow as there is no risk of stasis. The frame also includes three undulating structures interconnecting with the adjacent V-shaped structures. Such a device is deployed within the native valve (Figures 5 and 6).
3.3 Patient’s autopsy and pathological investigation
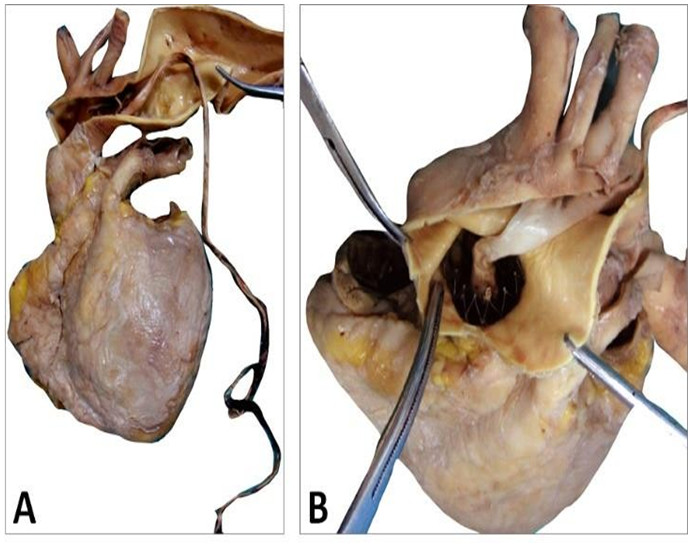
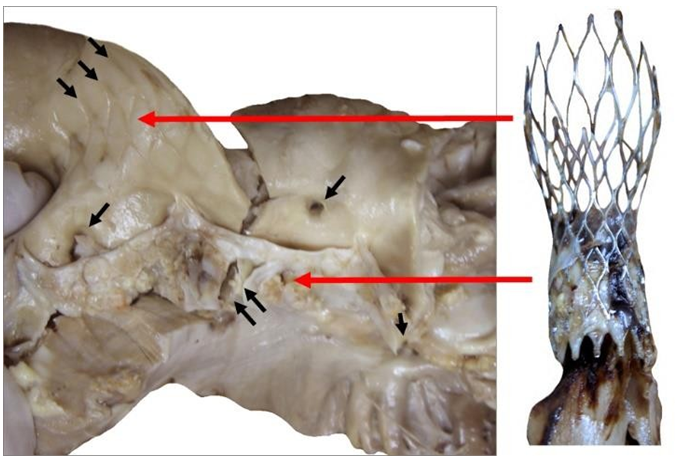
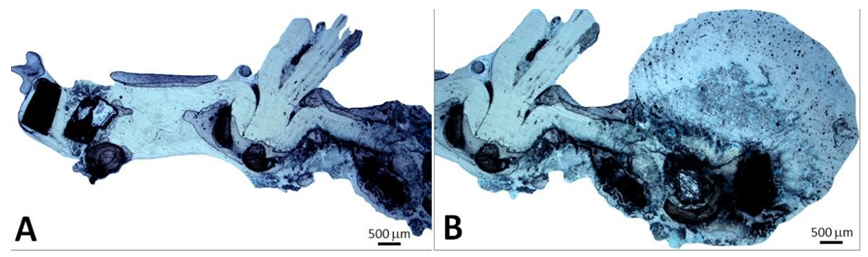
The CoreValve did not show any vegetations. A large and filiform 26 cm long thrombus was observed, attached to the aortic side of one of the prosthetic cusps, and reached the aortic carrefour (Figure 7).
Figure 7: Patient’s heart with this filiform thrombus 26 cm long within the lumen of the aorta and deployed at the outflow of the aortic arch and attached on the aortic side of a cusp (A). This floating thrombus reaches the aortic carrefour. Its coloration is white (B) and its surface is smooth at the outlet of the valve and becomes red and thicker distally with an irregular structure likely to cause embolizations.
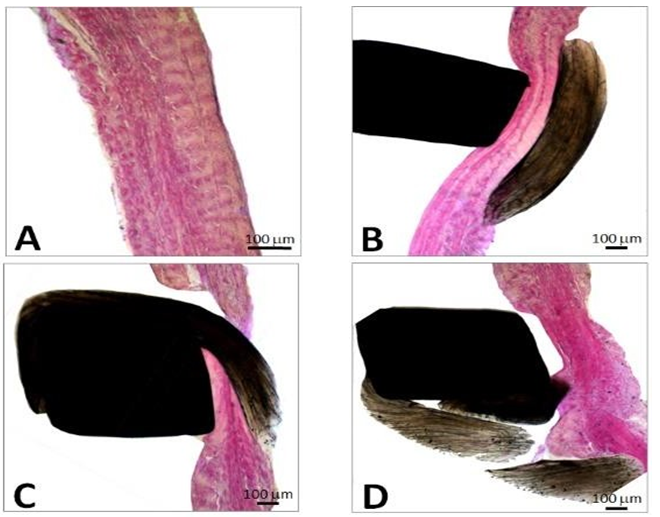
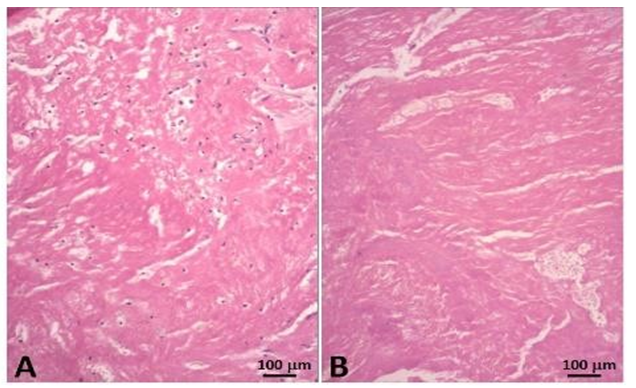
This fusiform thrombus showed a smooth external sheath in its thoracic set-up whereas more distally fragile poorly amalgamated thrombotic fragments were visible (Figures 8-10). Such structures were likely to embolize. The histological examination with hematoxylin eosin shows the base of the thrombus, attached to the aortic side of one of the prosthetic cusps with an organizing thrombus showing histiocytes and fibroblasts, meanwhile the distal sector of the aortic filiform thrombus is composed mainly by platelets and fibrin.
Figure 9: Explanted CoreValve (right) beside the native aortic valve with calcifications at the bottom of the leaflets and fracture along the aortic wall (double arrows, left). The leaflets are highly calcified as seen in the aortic wall. The ostiums of both coronaries were open (single arrow). The marks of the upper section of the device are observed above the original valve (triple arrows).
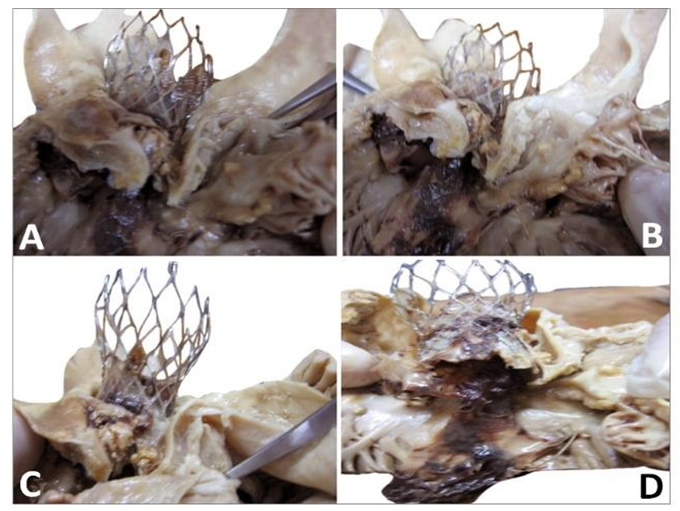
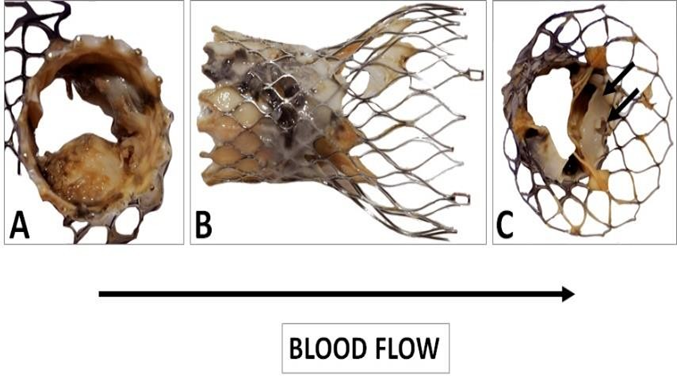
Figure 10: Gross observation of the explanted CoreValve from inflow (A) to outflow (C). A leaflet is missing as it was encroached with the floating thrombus that was pulled out. The remaining leaflets show severe thrombus covering their surface. There is a poor tissue incorporation of the lower and middle section of the valve (B). The observation of the outflow confirms the absence of a leaflet and the severe damage to another one (C). The remaining leaflet offers an interesting morphology as it is twinned with a reorganized thrombotic leaflet originating from the metallic frame (double arrows).
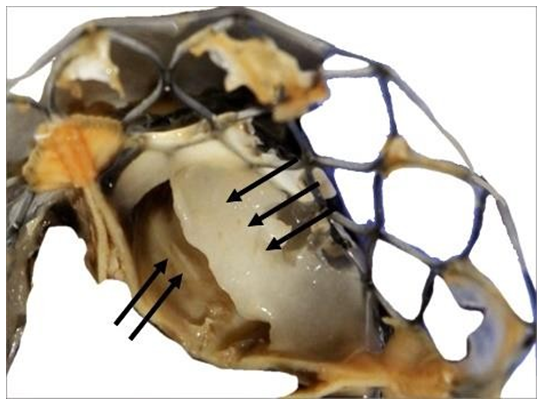
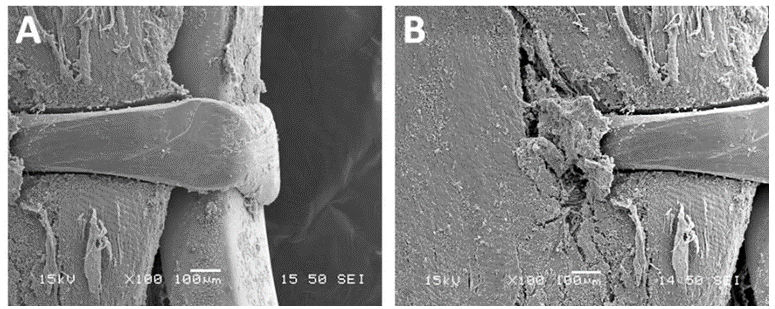
The observation of the CoreValve showed a missing leaflet as it was pulled out with the reorganized thrombi. A great variability in the structure of the thrombus was visible. The most spectacular observation was the development of a reorganized smooth sheath attached to outflow of the device (Figure 11).
Figure 11: Detail of the fibrous structure along the suture line of a pericardium leaflet. This structure is very stiff. It is the result of the reorganization of thrombotic accumulations over the leaflet itself (triple arrows). Such a structure is likely to shrink and thus to split from the leaflet. The motility of the pericardium leaflet can however be severely unpaired (double arrows).
This capacity to develop such a fibrous structure originating from the nitinol frame explained the development of the fusiform thrombus. The remaining cusps were filled with friable thrombi. In the meantime, the leaflets were properly fixed to the stent and did not show any tearing (Figures 12-15).
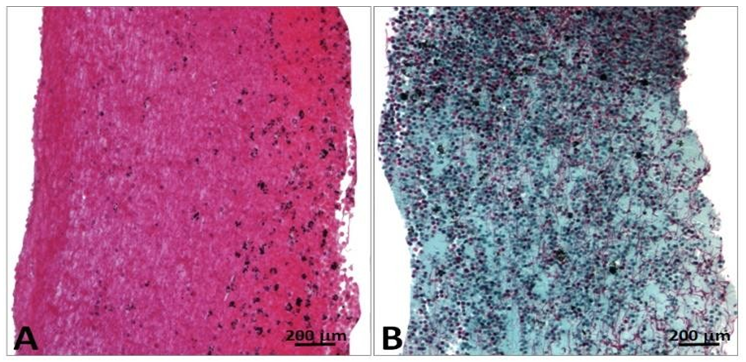
The structure of the leaflets was relatively well preserved despite thickening and distortion (Figures 16 and 17).
More specifically, the collagen bundles preserved its undulated shape despite the presence of deformations (Figure 18).
The morphology of the floating thrombus showed different densities from granular to dense and laminated to fragile structures likely to embolize (Figures 19 and 20).
4. Discussion
To our knowledge, this thrombotic complication of TAVI, by means of a fusiform thrombus, has not been reported yet. It entails a potential mechanism for increased embolic risk. The issue of floating thrombus is poorly understood as is developed within the structure of a percutaneous valve. This is totally unknown. The rationale for prescribing a more potent anti-platelet and anti-thrombotic therapy to prevent this potentially catastrophic complication remains unclear.
Once the diagnosis of prosthetic thrombosis with clinical or incidental manifestations has been established, anticoagulation with acenocoumarol or novel oral anticoagulants is indicated for at least 2 years. After initiating oral anticoagulation, disappearance of the thrombus and normalization of the transvalvular gradient occur in less than three months. In patients undergoing TAVI who did not have an indication for oral anticoagulation, Brouwer et al. [26] demonstrated that bleeding or thromboembolic events at 1 year were significantly less frequent with aspirin than with aspirin plus clopidogrel administrated for 3 months.
Autopsy revealed unexpected findings in 61.1% of patients and resulted in partly or completely different causes of death, as was clinical determined [27]. Most common complications are significant perivalvular regurgitation, prosthetic endocarditis, electrocardiographic conduction abnormalities, and prosthetic thrombosis. In most cases, TAVI thrombosis is asymptomatic, and is usually an incidental finding, with an incidence of 4% to 14% observed in several studies [28]. According to a meta-analysis, subclinical prosthetic thrombosis is not associated with an increased thromboembolic risk. The potential mechanisms of TAVI thrombosis are: excessive catheter manipulation, aggressive post-dilatation or the implantation of small valves (<23 mm). The echocardiographic study may suggest TAVI thrombosis due to the visualization of a thrombus, increased thickness and decreased valve mobility, or an increased transvalvular gradient. However, multidetector computed tomography (MDCT) has greater sensitivity to detect a thrombus in the prosthesis. Due to this silent behavior, it is very important to perform regular checks for early detection and appropriate treatment. Echocardiographic monitoring is recommended at 30 days, 6 months and annually thereafter [29].
The structural valve degradation for the long term was addressed by O’Hair et al. [30] in 2022. Data from CoreValve vs High Risk Pivotal (615 patients) were pooled with the CoreValve Extreme Risk Pivotal trial (485 patients) and the CoreValve continued Access Study (2178 patients). The five-year rate of structural valve deterioration was 4.38% in patients receiving TAVI. Doppler echocardiography revealed structural valve deterioration and was associated with worse clinical outcomes.
Evaluation of the long-term durability of the percutaneous valves focused on the feasibility [31-33] and the durability [34-36] of the devices. Practically, the percutaneous deployment of aortic valves is a well-accepted surgical technique. The durability of the devices is not inferior to surgical valves. The questions that shall be raised are related to adverse events. They are fortunately rare but what to do in case of adverse events as both mechanisms of transcatheter valve degeneration [37] and formation of mural floating thrombus are still largely unknown [38].
Systematic macroscopic and microscopic pathologic analyses of self-expanding transcatheter aortic valves were proposed by Yahagi et al. [20]. The valves were harvested during the CoreValve US Pivotal Trial of extreme and high-risk patients [39]. A total of 21 cases were collected, 19 death and 2 surgical explants. Most of the patients died because of heart failure. No fracture in the nitinol frame was observed. Self-expanding valves demonstrated favorable pathological findings and subclinical leaflet thrombus formation warrants further investigation. The meta-analysis conducted by Bogyi et al. [23] summarize the indication of treatments as follows: oral anticoagulation is associated with lower risk of subclinical leaflet thromboses compared to single anti-platelet therapy and dual anti-platelet therapy. Proper patient selection and clinicians’ experience in the implantation technique are essential to reduce possible complications that may occur during specific steps of the procedure. If positioning is too low, mitral valve dysfunction, heart block, paravalvular regurgitation, or embolization into the left ventricular cavity can occur. Long-term effects of lesser degrees of leaflet contact are unknown; nevertheless, an echocardiographic follow-up of the mitral regurgitation (MR) after TAVI with the CoreValve revealed that post-procedure regurgitation remained unchanged in the majority of patients, but worsened in 33%, owing to the design characteristics and final positioning. TAVI with a self-expanding CoreValve system has a larger part extending into the LV outflow tract compared to the Edwards-SAPIEN system [29]. Should a floating thrombus be detected in patients fitted with a percutaneous heart valve, a surgical approach can probably be recommended [16]. The mechanism of such a thrombus development can be analyzed with reference to the Virchow’s triad: thrombosis is the result of alteration in the blood flow, vascular endothelial injury or alteration in the constitution of the blood. The endothelial injuries are ruled by surface phenomena and with procoagulant surfaces, including bacteria, shards of foreign materials, and biomaterials [27].
5. Study Limitations
We hereby report the investigations carried out on a single case of floating thrombus after TAVI. Such a pathological examination cannot be based upon accepted guidelines because it is extremely rare. One can anticipate that such explants will be defined based upon the ongoing literature. The accessibility to control devices is difficult. More investigations would be appropriate, specifically the encroachment of the different materials.
6. Conclusion
Deployment of percutaneous aortic heart valves is a well-accepted technology and the number of patients treated increases very rapidly as TAVI is no longer restricted to frail and elderly patients. Besides stimulating statistical reports, there are adverse events that are probably underreported. Those features must be paid more attention, and the explant analyses will allow the development of safer devices and provide more precise guidelines.
Acknowledgements
The authors are indebted to Rachid Zegdi for the donation of the control CoreValve and to Jorge Jordana for the porcine pericardium. The collaboration of Véronique Gagné for typing this manuscript was greatly appreciated.
Funding
This research received no external funding.
Author’s contributions
TFC and JEE data extraction, original draft preparation, revision of the clinical data; EP: histological analysis; DD: analysis of the results; ZZ: formal analysis; AZ: autopsy and microscopy observations; RG: project administration, methodology, final edition of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Data access statement
All data have been included in the article.
Ethics statement
This work has the permission of the institutional ethic committee.
References
- Nguyen Q, Ma X, Vervoort D, et al. Management Strategies for Descending Thoracic Aortic Thrombus: A Review of the Literature. Innovations 17 (2022): 283-296.
- Lee S, Cho JW, Kwon OC. Floating Thrombus in the Ascending Aorta of the Patient with Systemic Sclerosis. A Case Report. Korean J Thorac Cardiovasc Surg 44 (2011): 72-75.
- Noh TO, Seo PW. Floating Thrombus in Aortic Arch. Korean J Thorac Cardiovasc Surg 46 (2013): 464-466.
- Kim SD, Hwang JK, Lee JH, et al. Free Floating Thrombus of the Aorta: An Unusual Cause of Peripheral Embolization. J Korean Surg Soc 80 (2011): 204- 211.
- Abe N, Yasumori K, Shimabu Kuro N, et al. Two Cases of Protruding Thrombus in the Ascending Aorta. Ann Vasc Dis 14 (2021): 64-67.
- Yang P, Li Y, Huang Y, et al. A Giant Floating Thrombus in the Ascending Aorta: A Case Report. BMC Surg 20 (2020): 321.
- DePaulis R, Welter L. Can We Quantify the Risk of Embolization for a Free Floating Thrombus? J Thorac Cardiovasc Surg 153 (2017): 804-805.
- Gionis M, Munaki V, Kaiafa G, et al. Thoracic Aortic Mural Thrombus, An Unusual Source of Peripheral Thromboembolism: A Report of Two Cases. J Surg Res 6 (2023): 04-09.
- Vaidya YP, Schaffert TF, Shaw PM, et al. Management of Mobile Thrombus of the Thoracic Aorta. J Vasc Surg Cases Innov Tech 7 (2021): 627-629.
- Luebke T, Aleksic M, Brunkwall J. Endovascular Therapy of Symptomatic Mobile Thrombus of the Thoracic Aorta. Eur J Vasc Endovasc Surg 36 (2008): 550-552.
- Rancic Z, Pfammatter T, Lachat M, et al. Floating Aortic Arch Thrombus Involving the Supraaortic Trunks: Successful Treatment with Supraaortic Debranching and Autograft Implantation. J Vasc Surg 50 (2009): 1177-1180.
- Böckler D, van Tengg-Kobligk H, Schoebniger M, et al. An Unusual Cause of Peripheral Artery Embolism: Floating Thrombus of the Thoracic Aorta Surgically Removed. Vasa 36 (2007): 121-123.
- Weiss S, Bühlmann R, von Allmen RS, et al. Management of Floating Thrombus in the Aortic Arch. J Thorac Cardiovasc Surg 152 (2016): 810-817.
- Li G, Chen Y, Wang H, et al. Case Report: Surgical Strategies of a Giant Thrombus from the Ascending Aorta to Arch. Front Cardiovasc Med (2023): 1091303.
- Laperche T, Laurian C, Rondeau R, et al. Mobile Thromboses of the Aortic without Aortic Debris. A Transoesophageal Echocardiographic Findings Associated with Unexplained Arterial Embolism. The Filiale Echocardiographie de la Société Française de Cardiologie. Circulation 96 (1997): 288-294.
- Yang S, Yu, J, Zeng W, et al. Aortic Floating Thrombus Detected by Computed Tomography Angiography Incidentally: Five Cases and Literature Review. J Thorac Cardiovasc Surg 153 (2017): 791-803.
- Li B, Liu B, Fu Y, et al. A Floating Thrombus Anchored at the Proximal Anastomosis of a Woven Thoracic Dissection. J Long-term Eff Med Implants 25 (2015): 179-200.
- Baumgartner H, Falk V, Bax JJ, et al. ESC Scientific Document group 2017. ESE/EACTS Guidelines for the Management of Valvular Heart Disease. Eur Heart J 38 (2017): 2739-2791.
- Noble S, Asgor A, Cartier R, et al. Anatomo-Pathological Analysis After CoreValve Revalving System Implantation. EuroIntervention 5 (2009): 78-85.
- Yahagi K, Torii S, Ladich E, et al. Pathology of Self-Expanding Transcatheter Aortic Valves: Findings from the CoreValve US Pivotal Trials. Catheter Cardiovasc Interv 91 (2018): 947-955.
- Sellers SL, Turner CT, Sathananthan J, et al. Transcatheter Aortic Heart Valves: Histological Analysis Providing Insight to Leaflet Thickening and Structural Valve Degeneration. JACC Cardiovasc Imaging 12 (2019): 135-145.
- Yanagisawa R, Tanaka M, Yashima F, et al. Early and Late Leaflet Thrombosis after Transcatheter Aortic Valve Replacement. Circulation: Cardiovasc Interv 12 (2019): e007349.
- Bogyi M, Schernhaner R, Loewe C, et al. Subclinical Leaflet Thromboses after Transcatheter Aortic Valve Replacement: A Meta-Analysis. JACC Cardiovasc Interv 14 (2021): 2643-2656.
- van Kesteren F, Wiegerinck EMA, Rizzo S, et al. Autopsy After Transcatheter Aortic Valve Implantation. Virchows Arch 470 (2017): 331-339.
- Cozzarin A, Cianciulli TF, Guidoin R, et al. CoreValve Prosthesis Causes Anterior Mitral Leaflet Perforation Resulting in Severe Mitral Regurgitation. Can J Cardiol 30 (2014): 1108e11-1108e14.
- Brouwer J, Nijenhuis VJ, Delewi R, et al. Aspirin with or without Clopidogrel after Transcatheter Aortic Valve Implantation. N Engl J Med 383 (2020): 1447-1457.
- Raghav V, Midha P, Sharma R, et al. Transcatheter Aortic Valve Thrombosis: A Review of Potential Mechanisms. J R Soc Interface 18 (2021): 20210599.
- Hansson NC, Grove EL, Andersen HR, et al. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing factors and Clinical Implications. J Am Coll Cardiol 68 (2016): 2059-2069.
- Baumgartner H, Falk V, Bax JJ, et al. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur Heart J 38 (2017): 2739-2791.
- O’Hair D, Yakubov SJ, Grubb KJ, et al. Structural Valve Deterioration After Self-expanding Transcatheter or Surgical Aortic Valve Implantations in Patients at Intermediate or High Risk. JAMA Cardiol 8 (2023): 111-119.
- Lamarche Y, Cartier R, Denault AY, et al. Implantation of the CoreValve Percutaneous Aortic Valve. Ann Thorac Surg 83 (2007): 284-287.
- Asgar AW, Bonan R. Transcatheter Aortic Valve Implantation: Experience with the CoreValve Device. Interv Cardiol Clin 1 (2012): 27-36.
- Kovac J, Schuler G, Gerckens U, et al. Four-year Experience with the CoreValve Transcatheter Heart Valve. Eurointervention 12 (2016): e1036- e1046.
- Buellesfeld L, Gerckens U, Schuler G, et al. 2-year Follow- up of Patients Undergoing Transcatheter Aortic Valve Implantation using a Self-expanding Valve Prosthesis. J Am Coll Cardiol 57 (2011): 1650-1657.
- Barbanti M, Petronio AS, Ettori F, et al. 5-years Outcomes after Transcatheter Valve Implantation with CoreValve Prosthesis. JACC Cardiovasc Interv 8 (2015): 1084-1091.
- van Mieghem NM, Deeb GM, Søndergaard L, et al. Self-expanding Transcatheter vs. Surgical Aortic Valve Replacement in Intermediate-risk Patients: 5-year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol 7 (2022): 1000-1008.
- Gill H, Meier D, Li A, et al. Mechanisms of Transcatheter Valve Degeneration. A translational Perspective. Cardiac Interv Today 17 (2023): 44-49.
- Fuknhara S, Brescia AA, Shiomi S, et al. Surgical Explantation of Transcatheter Aortic Bioprostheses: Results and Clinical Implications. J Thorac Cardiovasc Surg 162 (2021): 539-547.
- Popma JJ, Adams DM, Reardon MJ, et al. Transcatheter Aortic Valve Replacement using a Self-expanding Bioprostheses in Patients with Severe Aortic Stenosis and Extreme Risk for Surgery. J Am Coll Cardiol 63 (2014): 1972-1981.