Genetic Polymorphism in Porphyria: Resolution of A Case of Tetraplegia by Administration of Hemin During a Porphyric Crisis and Subsequent Therapeutic Procedure with the use of Givosiran
Article Information
Guida Claudio Carmine1, Nardella Maria1, Perez Ys Aurora1, Andreaserena Recchia2, Tonti Maria Pia2, Savino Maria3, Crisetti Annalisa1, Napolitano Francesco1, Ferrara Gaetano1, Aucella Francesco1, Manuali Aldo2, Aucella Filippo1 ORCID ID 0000-0003-1027-7049.
1Department of Medical Sciences, Division of Nephrology - Interregional Reference Center for Porphyria, Fondazione IRCCS “Casa Sollievo della Sofferenza”, 71013 San Giovanni Rotondo, Italy
2Intensive Care Unit, Fondazione IRCCS “Casa Sollievo della Sofferenza”, 71013 San Giovanni Rotondo, Italy
3Immunohematology and Transfusion Medicine Service, Fondazione IRCCS “Casa Sollievo della Sofferenza”, 71013 San Giovanni Rotondo, Italy
*Corresponding author: Claudio Carmine Guida, Department of Medical Sciences - Division of Nephrology and Dialysis- Interregional Reference Center for Porphyria, Fondazione IRCCS “Casa Sollievo della Sofferenza”, 71013 San Giovanni Rotondo, Italy.
Received: 05 June 2023; Accepted: 12 July 2023; Published: 25 August 2023
Citation: Guida CC, Nardella M, Perez Ys A, Recchia A, Tonti MP, Savino M., Crisetti A, Napolitano F, Ferrara G, Aucella F, Manuali A., Aucella F. ORCID ID 0000-0003-1027-7049. Genetic Polymorphism in Porphyria: Resolution of A Case of Tetraplegia by Administration of Hemin During a Porphyric Crisis and Subsequent Therapeutic Procedure with the use of Givosiran. Journal of Biotechnology and Biomedicine 6 (2023): 331-335.
View / Download Pdf Share at FacebookAbstract
Porphyria is a very rare genetic pathology due to congenital defects of heme metabolism with deficiency of one of the enzymes that determine specific forms of porphyria. Acute hepatic porphyrias (AEP) includes four different clinical forms: a) from ALA D deficiency, b) acute intermittent porphyria, c) hereditary coproporphyria and d) variegate porphyria. The forms of porphyria are characterized by elevated levels of delta aminolevulinic acid (ALA) and porphobilinogen (PBG). Acute intermittent porphyria (AIP) is caused by a genetic mutation of hydroxymethylbilane synthetase (HMBS), which in a good percentage (about 20% of cases) does not show a positivity for hepatic porphyria on biomolecular examination, so that diagnosis with relative therapy is determined by the clinical condition. Our study describes a 54-year-old woman who, due to a porphyria crisis, presented serious symptoms with progressive aggravation that lead her to be hospitalized in an intensive care unit where she was intubated due to tetraplegia and severe respiratory failure. Treatment with human emin first and Givosiran subsequently allowed progressive amelioration of the clinical conditions.
genetic pathology articles genetic pathology Research articles genetic pathology review articles genetic pathology PubMed articles genetic pathology PubMed Central articles genetic pathology 2023 articles genetic pathology 2024 articles genetic pathology Scopus articles genetic pathology impact factor journals genetic pathology Scopus journals genetic pathology PubMed journals genetic pathology medical journals genetic pathology free journals genetic pathology best journals genetic pathology top journals genetic pathology free medical journals genetic pathology famous journals genetic pathology Google Scholar indexed journals delta aminolevulinic acid articles delta aminolevulinic acid Research articles delta aminolevulinic acid review articles delta aminolevulinic acid PubMed articles delta aminolevulinic acid PubMed Central articles delta aminolevulinic acid 2023 articles delta aminolevulinic acid 2024 articles delta aminolevulinic acid Scopus articles delta aminolevulinic acid impact factor journals delta aminolevulinic acid Scopus journals delta aminolevulinic acid PubMed journals delta aminolevulinic acid medical journals delta aminolevulinic acid free journals delta aminolevulinic acid best journals delta aminolevulinic acid top journals delta aminolevulinic acid free medical journals delta aminolevulinic acid famous journals delta aminolevulinic acid Google Scholar indexed journals Acute hepatic porphyrias articles Acute hepatic porphyrias Research articles Acute hepatic porphyrias review articles Acute hepatic porphyrias PubMed articles Acute hepatic porphyrias PubMed Central articles Acute hepatic porphyrias 2023 articles Acute hepatic porphyrias 2024 articles Acute hepatic porphyrias Scopus articles Acute hepatic porphyrias impact factor journals Acute hepatic porphyrias Scopus journals Acute hepatic porphyrias PubMed journals Acute hepatic porphyrias medical journals Acute hepatic porphyrias free journals Acute hepatic porphyrias best journals Acute hepatic porphyrias top journals Acute hepatic porphyrias free medical journals Acute hepatic porphyrias famous journals Acute hepatic porphyrias Google Scholar indexed journals clinical condition articles clinical condition Research articles clinical condition review articles clinical condition PubMed articles clinical condition PubMed Central articles clinical condition 2023 articles clinical condition 2024 articles clinical condition Scopus articles clinical condition impact factor journals clinical condition Scopus journals clinical condition PubMed journals clinical condition medical journals clinical condition free journals clinical condition best journals clinical condition top journals clinical condition free medical journals clinical condition famous journals clinical condition Google Scholar indexed journals tetrapyrrolic articles tetrapyrrolic Research articles tetrapyrrolic review articles tetrapyrrolic PubMed articles tetrapyrrolic PubMed Central articles tetrapyrrolic 2023 articles tetrapyrrolic 2024 articles tetrapyrrolic Scopus articles tetrapyrrolic impact factor journals tetrapyrrolic Scopus journals tetrapyrrolic PubMed journals tetrapyrrolic medical journals tetrapyrrolic free journals tetrapyrrolic best journals tetrapyrrolic top journals tetrapyrrolic free medical journals tetrapyrrolic famous journals tetrapyrrolic Google Scholar indexed journals uroporphyrins articles uroporphyrins Research articles uroporphyrins review articles uroporphyrins PubMed articles uroporphyrins PubMed Central articles uroporphyrins 2023 articles uroporphyrins 2024 articles uroporphyrins Scopus articles uroporphyrins impact factor journals uroporphyrins Scopus journals uroporphyrins PubMed journals uroporphyrins medical journals uroporphyrins free journals uroporphyrins best journals uroporphyrins top journals uroporphyrins free medical journals uroporphyrins famous journals uroporphyrins Google Scholar indexed journals coproporphyrins articles coproporphyrins Research articles coproporphyrins review articles coproporphyrins PubMed articles coproporphyrins PubMed Central articles coproporphyrins 2023 articles coproporphyrins 2024 articles coproporphyrins Scopus articles coproporphyrins impact factor journals coproporphyrins Scopus journals coproporphyrins PubMed journals coproporphyrins medical journals coproporphyrins free journals coproporphyrins best journals coproporphyrins top journals coproporphyrins free medical journals coproporphyrins famous journals coproporphyrins Google Scholar indexed journals protoporphyrins articles protoporphyrins Research articles protoporphyrins review articles protoporphyrins PubMed articles protoporphyrins PubMed Central articles protoporphyrins 2023 articles protoporphyrins 2024 articles protoporphyrins Scopus articles protoporphyrins impact factor journals protoporphyrins Scopus journals protoporphyrins PubMed journals protoporphyrins medical journals protoporphyrins free journals protoporphyrins best journals protoporphyrins top journals protoporphyrins free medical journals protoporphyrins famous journals protoporphyrins Google Scholar indexed journals hematoporphyrins articles hematoporphyrins Research articles hematoporphyrins review articles hematoporphyrins PubMed articles hematoporphyrins PubMed Central articles hematoporphyrins 2023 articles hematoporphyrins 2024 articles hematoporphyrins Scopus articles hematoporphyrins impact factor journals hematoporphyrins Scopus journals hematoporphyrins PubMed journals hematoporphyrins medical journals hematoporphyrins free journals hematoporphyrins best journals hematoporphyrins top journals hematoporphyrins free medical journals hematoporphyrins famous journals hematoporphyrins Google Scholar indexed journals hyponatremia articles hyponatremia Research articles hyponatremia review articles hyponatremia PubMed articles hyponatremia PubMed Central articles hyponatremia 2023 articles hyponatremia 2024 articles hyponatremia Scopus articles hyponatremia impact factor journals hyponatremia Scopus journals hyponatremia PubMed journals hyponatremia medical journals hyponatremia free journals hyponatremia best journals hyponatremia top journals hyponatremia free medical journals hyponatremia famous journals hyponatremia Google Scholar indexed journals
Article Details
Introduction
Porphyrins are tetrapyrrolic compounds present in plant and animal species (Figure 1) defined as the pigments of life by Hans Fisher in 1930 because they are precursors of the final molecules which give the green color to grass (chlorophyll), red to blood (haemoglobin), orange to vitamins (vitamin B12), and yellow to some bacteria (coenzyme F430 in Methanobacterium thermoautotrophicum). The final product (prosthetic group) which will then be conveyed by a support apo-protein, can bind a metal between the tips of the four pyrrolic nuclei: magnesium in the plant species (chlorophyll) and iron in the animal species, where the compound assumes the name of heme (such as in hemoglobin, where the nitrogen atoms of each pyrrole ring are bonded to a metal atom of iron). Porphyrins can be classified into five categories: uroporphyrins, coproporphyrins, ethioporphyrins, protoporphyrins, hematoporphyrins. Their synthesis occurs in the liver and in the cells of the hematopoietic tissue. The incorrect or excessive production of porphyrins leads to pathologies called porphyrias. Porphyrias are the result of partial defects in one or more of the eight enzymes involved in heme biosynthesis.
Porphyrias are inherited morbid conditions caused by defects in heme biosynthesis and in the metabolism of porphyrins, a group of pigments composed of four pyrrole nuclei connected to each other by methine bridges (Figure 2) [1-4]. Neuro-visceral attacks in acute intermittent porphyria (AIP) are characterized by abdominal pain, neurological disorders and psychiatric disorders and, in the most severe cases, can lead to respiratory paralysis and coma [5-8]. In some cases the metabolic alteration can be acquired [9-11]. Porphyrias are rare in children and after the fifth decade and have a mortality of 20-25% within the first five years after the first attack. The causes of attacks can be different: drugs, alcohol, stress, fasting, menstrual cycle, infections. The prevalence of acute porphyria is 10.1/100000 (according to Orphanet, November 2022).
Figure 1: Chemical structure of Porphyrins.
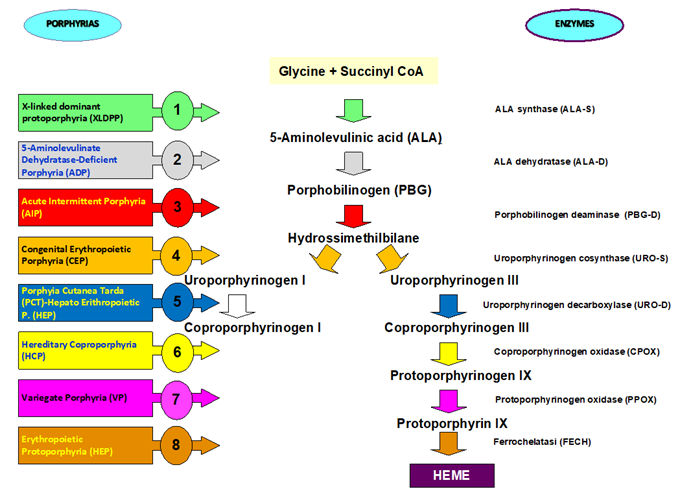
Figure 2: Biosynthesis and metabolism pathway of porphyrins with related inherited genetic conditions.
Renal involvement in acute porphyrias includes hyponatremia, urinary retention, tubulointerstitial nephropathy, hypertension, and chronic kidney disease. Most of porphyria patients suffer from renal colic associated with pallor, nausea, vomiting, fever, acute urine retention and dark urine [12-15]. An acute attack may be preceded by a period of varying degrees of behavioral changes such as anxiety, irritability, restlessness and insomnia and may rapidly progress to symptoms of severe autonomic neuropathy and acute sensory and motor neuropathy (similar to Guillan-Barre syndrome). It can progress to general paralysis leading to severe respiratory failure up to and including death from cardiorespiratory arrest [16-20].
Case Report
Here, we report the case of a 54-year-old woman with parents who died of intestinal neoplasia at 40 years (mother) and stroke at 70 years (father), penultimate of eleven siblings. She was allergic to various foods. Referred irregular bowel movements and voiding difficulties in physiological anamnesis. At the age of 35 she underwent cholecystectomy for lithiasis and various surgical operations for the removal of interphalangeal joint neo-formations of the hands, carpal tunnel and heel spur. At the age of 45 years, she had a recurrence of pericarditis undergoing steroid therapy. She was on beta-blocker therapy for sinus tachycardia. She also reported poly-arthrosis and fibromyalgia. Abdominal pain symptoms began to be frequently represented at the age of 35 years, with always negative gastroenterological evaluations and was subsequently accompanied by extension of pain to the osteoarticular system with burning pain in the limbs. At age 50, persistent micturition difficulty and evidence of severe microcytic anemia treated with iron infusion were referred. She practiced neurophysiological tests that highlighted marked suffering of the neuro-vegetative system with subsequent hospitalization at the Division of Neurology at our hospital due to sensory neuropathy and to undertake a cycle of motor re-education to reduce painful symptoms and improve autonomy in Activity of Daily Living (ADLs). On hospital discharge, she was diagnosed with painful neuropathy. The following year, due to the persistent discomfort, the patient performs genetic analysis of the transthyretin (TTR) gene for suspected familial amyloid polyneuropathy (FAP), but with negative results. In 2020, when she was 52 years old, the patient underwnt a genetic test for porphyria with buccal mucosa brushing, in order to detect a form of hepatic porphyria (due to ALAD deficiency, acute intermittent porphyria, hereditary coproporphyria and variegate porphyria). The genetic test for hepatic porphyria was negative as well. At the age of 54 years, on January 13rd, 2022 (at 01:56 hour) the patient was admitted to our Emergency Department due to hyperpyrexia, widespread arthralgias, signs and symptoms of small fiber neuropathy. A therapeutic attempt to relieve the painful symptoms with an infusion of intravenous tramadol and paracetamol were unsuccessful. She was the transferred to the Division of Internal for worsening arthralgia with functional impotence, bed rest necessity and impossibility to perform movements and finding of severe swallowing difficulty, evidence of right upper limb paresis and tetra-hyposthenia with lower limb pain. Lively patellar tendon reflex on the right and positive pronator drift (Mingazzini maneuver) of lower limbs were shown.
She was transferred to the Sub-Intensive Unit of the Division of Neurology for marked neck flexor muscle deficit with failure to lift the head off the bed, marked and severe tetraparesis prevailing on the right side of the body with dyspnea, dysphagia. Spinacentesis was performed and analysis of cefalo-rachidian liquid showed the presence of increased cellularity given by lympho-monocyte elements. The patient was transfer to the Intensive Care Unit of our hospital, where she arrives with acute respiratory failure on a neurological basis in a picture of Guillain-Barré syndrome, for which she was treated with administration of high-dose intravenous immunoglobulins. The clinical condition deteriorated progressively with worsening dysphonia and impaired physical mobility up to ataxia, absence of movements of the shoulder girdle with abdominal breathing. The patient was sedated and subjected to orotracheal intubation with an appropriate sized endotracheal tube, i.e., 7.5 mm ID, and subjected to pressure and volume controlled ventilation. Persistent hypertension and tachycardia were present despite pain sedation. The patient was treated with percutaneous tracheostomy and positioning of Twist Plus tracheostomy tube with counter cannula. Ror every day of hospitalization in the intensive Care Unit she was subjected to dosage of delta-aminolevulinic acid (dALA) and porphobilinogen (PBG) on extemporaneous urine specimens not exposed to light. The ALA and PBG urinary values were normal, contrary to elevated levels of urinary ALA and PBG found in the two sons, completely asymptomatic, who also underwent specific urinary investigations for porphyria (ALA 7.30-6.95 and PBG 3.47-3.66 in the son and ALA 8.72-5.88 and PBG 2.33-2.26 in the daughter; normal values range is 0.00-5.00 for ALA and 0.00-2.00 for PBG). Eight days after hospitalization, on January 21st, 2022, the finding of altered urinary data in her two children led us to commence infusion therapy with hemin (Normosang®) at a dosage of 3 mg/kg/24h via central venous catheter in the right internal jugular for the first four consecutive days and then twice a week for the following four weeks. Since the second administration of hemin, the patient was awake and conscious and minimal voluntary movements of the lower limbs and shoulder girdle were appreciated. On the third day the patient presented small closing movements of both hands in addition to the closing movements of the girdles and feet while on the fourth day of administration of hemin she was able to scanty move her hands and lower limbs. Ten days after the first administration of hemin, the patient was awake and conscious, moved her lower limbs spontaneously and with reduced muscle tone also her upper limbs. On the fifteenth day the patient was abel to feed spontaneously. During hospitalization, the patient and her two children underwent a biomolecular examination for the diagnosis of porphyria, which detected the same polymorphism causative for acute intermittent porphyria, precisely deletion of exon 11: c613-31 A>G (position: intron 10 – 613-31 / mutation: cac > cgc / sequence change: SA (of exon 11) ). This polymorphism was found to be causative of deletion of exon 11 in a 1997 paper (21). This sequencing method (Sanger method) allows to identify point mutations in the coding region of the gene and in the splicing junctions, but has some limitations: it does not allow to detect dose imbalances of the analyzed genes (deletions and duplications of single exons, or multiexonics and of the entire gene), epimutations and mosaics. Analyzing the allelic frequency databases, the above-mentioned variant has a frequency of 14% (1000 Genomes) in the reference population and it was always considered benign according to all the consulted databases (Franklin, Ensamble genome broweser, Clin Var, Gnomad, SIFT, Polyphen, Mutation transfer and others). Therefore it shouldn’t be considered pathogenetic. According to the current knowledge, in addition to monitoring ALA and PBG levels, these data require periodic updates in order to identify the genetic defect responsible for the Porphyria case described in this paper. On the 24th day spontaneous breathing was present and the patient was subsequently transferre to Rehabilitation Unit, where she was subjected to a rehabilitation project and program that lead her to hospital discharge in a good clinical condition. She was vigilant, collaborative, oriented, with autonomous breathing in room air, with good muscular trophism, good coordination and autonomous walking, with only avoidance of the load on the right for midfoot pain. Starting from September 2022, the patient is on monthly therapy with Givosiran (Givlaari®), a drug based on the mechanism of action of RNA interference (RNAi) with a specific target for aminolevulinic acid synthase 1 (ALAS 1). This natural mechanism is based on blocking the production of proteins that cause the porphyritic pathology. Monthly subcutaneous administration of Givosiran has the potential to significantly lower induced hepatic levels of ALAS1 in a sustained manner and thereby reduce the neurotoxic intermediates of heme, aminolevulinic acid (ALA) and porphobilinogen (PBG), towards normal levels. By reducing the accumulation of these intermediates, Givosiran reduces the occurrence of severe and life-threatening neuro-visceral attacks, controls chronic symptoms, and reduces the disease burden. The administration is subcutaneous and after the first four months of hospital administration the patient currently practices the vial at home with great recovery of the quality of life. The variant found in the patient and her two children is frequently expressed in the reference population. Nevertheless, it could have a dose effect on the production of the enzyme (PBGd), increasing its pathogenetic effect, together with a genetic defect not yet identified, as already described in Erythropoietic protoporphyria (22). The clinical history and the final results have shown that an excessively rigid evaluation of the data can be fallacious and lead to giving up important treatment decisions for the patient’s life. The diagnostic-therapeutic path allowed the patient to return to a good quality of life, removing forever a real risk of death.
References
- Young JW, Conte ET. Porphyrias and porphyrins. Int J Dermatol 30 (1991): 399-406.
- Herbert L Bonkovsky , Jun-Tao Guo, Weihong Hou, Ting Li, Tarun Narang, Manish Thapar Porphyrin and heme metabolism and the porphyrias. Compr Physiol . 2013 Jan;3(1):365-401.
- Woods JS. Regulation of porphyrin and heme metabolism in the kidney. Semin Hematol 25: 336-348.
- I nefrologi e le porfirie - Canavese C. Gabrielli D., Guida C.C., Cappellini M.D. - Giornale Italiano di Nefrologia, anno 19 (1988): 93-412
- Ventura P, Cappellini MD, Rocchi E. The acute porphyrias: a diagnostic and therapeutic challenge in internal and emergency medicine. Intern Emerg Med 4 (2009): 297-308.
- Puy H, Gouya L, Deybach JC. Porphyrias. Lancet 365 (2010): 924-37.
- Kauppinen R. Porphyrias. Lancet 365 (2005): 241-252.
- Thunell S. Porphyrins, porphirin metabolism and porphyrias. Update. Scand J Clin Lab Invest 60 (2000): 509-540.
- Ventura P. Le Porfirie, In Brunetti P, Santeusanio F (Eds) Trattato di Medicina Interna. Volume Malattie delle Ghiandole Endocrine, del Metabolismo e della Nutrizione. Padova: Piccin Nuova Libraria (2011): 931-952
- Ventura E, Rocchi E, Le Porfirie, In: Teodori 2000 Trattato di Medicina Interna - Guarini G, Fiorelli G, Malliani A, Violi E, Volpe M (Eds). Società Editrice Universo: Italy 2 (2001): 2301-2334.
- Elder GH, Hift RJ, Meissner PN. The acute porphyrias. Lancet 349 (1997): 1613-1617.
- Mydlík M, Derzsiová K. Kidney damage in acute intermittent porphyria. Przegl Lek 68 (2011): 610-613.
- Andersson C, Wikberg A, Stegmayr B, et al. Renal symptomatology in patients with acute intermittent porphyria. A population-based study. J Intern Med 248 (2000): 319-325.
- Marsden JT, Chowdhury P, Wang J, et al. Acute intermittent porphyria and chronic renal failure. Clin Nephrol 69 (2008): 339-346.
- O'Mahoney D, Wathen CG. Hypertension in porphyria--an understated problem. QJM 89 (1996): 161-162.
- Solinas C, Vajda FJ. Neurological complications of porphyria. J Clin Neurosci 15 (2008): 263-268.
- Pischik E, Kauppinen R. Neurological manifestations of acute intermittent porphyria. Cell Mol Biol (Noisy-le-grand) 55 (2009): 72-83.
- Meyer UA, Schuurmans MM, Lindberg RL. Acute porphyrias: pathogenesis of neurological - manifestations. Semin Liver Dis 18 (1998): 43-52.
- Andrea Ricci, Claudio Carmine Guida, Paola Manzini, et al. Kidney Involvement in Acute Hepatic Porphyrias: Pathophysiology and Diagnostic Implications - Diagnostics 11 (2021): 2324.
- Paolo Ventura, Maria Domenica Cappellini, Claudio Carmine Guida, et al. A challenging diagnosis for potential fatal diseases: Recommendations for diagnosing acute porphyrias. European Journal of Internal Medicine 25 (2014): 497-505.
- HPuy, JC Deybach, J Lamoril, et al. Molecular Epidemiology and Diagnosis of PBG Deaminase Gene Defects in Acute Intermittent Porphyria - J. Hum. Genet. 60 (1997): 1373-1383.
- Laurent Gouya, Caroline Martin-Schmitt, Anne-Marie Robreau, et al. Contribution of a common single-nucleotide polymorphism to the genetic predisposition for erythropoietic protoporphyria - Am J Hum Genet 78 (2006): 2-14
