Galanin receptor agonists limit osteoclast number in in vitro mouse models
Article Information
Heath W McGowan1*, Deanna M Horvath1,2, Amy E Larsen1, Aaron C McDonald1
1Department of Microbiology, Anatomy, Physiology and Pharmacology, La Trobe University, Bundoora, Australia
2School of Allied Health, Human Services and Sport, La Trobe University, Bundoora, Australia
*Corresponding Author: Heath McGowan, Department of Microbiology, Anatomy, Physiology and Pharmacology,
La Trobe University, Bundoora, VIC, Australia, 3086.
Received: 19 March 2025; Accepted: 27 March 2025; Published: 31 March 2025.
Citation: Heath W McGowan, Deanna M Horvath, Amy E Larsen, Aaron C McDonald. Galanin receptor agonists limit osteoclast number in in vitro mouse models. Fortune Journal of Rheumatology 7 (2025): 07-19.
View / Download Pdf Share at FacebookAbstract
Despite evidence that galanin-like compounds are potential regulators of bone activity, little research has been undertaken to investigate the role of these compounds on osseous cells. The aim of this study was to elucidate the mechanisms by which the galanin receptor agonist’s galanin and galnon regulate osteoclasts. Osteoclasts were cultured from cells isolated from femurs of C57BL/6 mice and grown to 80% confluence, then differentiated via the application of mCSF and RANKL. Cultures were analyzed for osteoclast number via TRAP staining, qPCR and immunocytochemistry. A moderate dose of both galanin-like compounds decreased osteoclast count in vitro . The moderate dose of galanin also reduced expression of inflammatory markers. These results indicate that both galanin and galnon can act to reduce osteoclast count, although they have led to contradictory responses with respect to inflammatory expression. These results may help to better treatment for bone-resorptive diseases such as osteoporosis.
Keywords
Bone, Galanin, Galnon, Osteoclast, Osteoporosis.
Bone articles; Galanin articles; Galnon articles; Osteoclast articles; Osteoporosis articles.
Article Details
1. Introduction
Galanin (GAL) is a naturally occurring 3162.60 Dalton (Da) neuropeptide [1] that consists of twenty-nine amino acids (30 for humans), with the first fifteen amino acids being homologous in most species studied [2]. It is widely distributed, having been found in brain, spinal cord, gastrointestinal tract, lungs, skin, pancreas, urogenital tract, adrenal medulla and bone tissues [3-5]. It is capable of regulating cell proliferation and apotosis in cells including neuroblastoma cells [6]. Mice with a GAL-KO genotype show decreased nerve regeneration rates [7] and GAL is expressed by, and has mitogenic effects on, various types of cancer [8]. GAL has many biological effects including nociception, learning and memory, inhibition of insulin, acetylcholine and gastrin release and stimulation of prolactin [9-11], regulating the release of several neurotransmitters in the central nervous system [9, 12] and growth hormone release [13]. Galanin has been shown to act as a mediator of inflammatory pain [14], and also has anti-inflammatory properties [15]. Injury to other tissues, such as bone, also cause an increase in tissue GAL concentrations [4, 16]. While little research has focussed on the effects of GAL on bone, there have been enough studies to indicate that GAL is a potential regulator of bone cell activity, especially during injury or diseased states [4, 16-18]. In addition to osteoblasts (OBs) and osteocytes increasing activity during injury or disease, osteoclast (OCl) activity must also be highly controlled to assist in bone homeostasis [19, 20]. For example, excessive bone resorption, via increased OCl number or activity, results in loss of bone volume and bone diseases such as osteoporosis and osteopaenia [21, 22]; in contrast, decreases in OCl number and activity lead to diseases such as osteopetrosis [23]. Immunolabeling has shown that GAL is present in OCls, and GAL can influence interleukin-1β (1L-1β) and tumor necrosis factor α (TNF-α) expression [4, 24, 25], resulting in an increase [26, 27] or decrease [4, 28, 29] of inflammation. These inflammatory cytokines also cause a significant increase in the expression of GAL mRNA [30] while GAL, in turn, can inhibit the production of TNF-α [31] and 1L-1β [4]. This is extremely relevant to bone homeostasis, as inflammatory states decrease bone formation, while increasing bone resorption by OCls [22, 32, 33]. Therefore, our research investigates the potential of GAL to regulate OCl actions, thereby controlling bone resorption and, potentially, treating bone fragility.
Osteoclasts are multinucleated cells of the haematopoietic cell line formed from the fusion of mononucleated precursors [34]. The only cells known to be able to resorb bone, OCls have between three and 100 nuclei and are highly motile [35, 36]. Osteoclastogenesis is regulated by two molecules secreted by OBs: Receptor activator of nuclear factor kappa-Β (RANK) ligand (RANKL), a tumour necrosis factor family cytokine, and osteoprotegerin (OPG) [37]. RANKL binding to RANK on OCls promotes OCl differentiation, while OPG is a decoy receptor and binds to RANKL to prevent OCl differentiation [37]. Nuclear factor kappa-Β (NF-κB) activity is one of the first indicators of differentiation following RANKL binding and is considered essential for the survival and differentiation of the OCl [38, 39]. Activation of NF-κB leads to activation of genes required for OCl function [38, 39]. Both TNF-α and RANKL are both important in the life-cycle of the OCl and can, like OBs, stimulate transcription [40] or apoptosis [41], with transcription and apoptotic actions both regulated through NF-κB [39].
There are two methods by which NF-κB can be translocated to the nucleus, known as the canonical and non-canonical NF-κB signalling pathways [42, 43]. The canonical pathway involves activation of specific receptors by various substances including RANKL, TNF-α and IL-1β [43, 44]. Through a process involving Sharpin, the result of this canonical pathway allows NF-κB to enter the nucleus and cause transcription [45, 46]. The non-canonical pathway, conversely, is activated by other TNF-superfamily receptors, such as RANK [42, 44]. Following stimulation with one of these ligands (e.g. RANKL), NF-κB also leads to transcription [44, 47]. The diverse results of NF-κB transcription include the regulation of bone turnover. This role of NF-κB was discovered when NF-κB1/2 double knockout (dKO) mice developed osteopetrosis due to a lack of OCls [48, 49]. This defective osteoclastogenesis mirrored symptoms in receptor activator of NF-κB (RANK) and RANKL knockout mice, in which OCl precursors halt their differentiation at a stage where they express RANK but fail to express CTHSK [50]. While these dKO mice have increased numbers of OCl progenitor cells, addition of cytokines such as IL-1β, TNF-α and RANKL failed to stimulate OCl differentiation [50]. Differentiation of OCls in non-GMO mice is highly regulated by these cytokines through NF-κB, and both NF-κB and inflammation have been shown to influence, and be influenced by, GAL [4, 28, 29, 51]. Therefore, GAL is of interest as an agent of bone turnover.
Previous research has suggested that GAL may have a therapeutic role in fracture healing and cartilage formation [24]. This is due to the presence of GAL receptors on osseous cells, suggesting an in vivo influence on bone turnover. All three known GAL receptors can also bind the GAL receptor agonist, galnon (galn), with an affinity in the micromolar range [52]. Galnon is a non-selective agonist in the micromolar range for GALR1-2 but is submicromolar for GALR3 [53]. As previously mentioned, GAL has shown a direct effect on inflammatory cytokines TNF-α and IL-1β [4, 54], which act to stimulate osteoclastogenesis [55], and GAL mRNA has been found in osteoclasts [17]. As such, it is hypothesised that galn will also have some direct effect on OCls. These experiments aim to investigate any effects of GAL and galn on OCls grown in vitro. Utilising OCls grown in vitro and treated with three concentrations of GAL or three concentrations of galn, this paper aims to determine the dose-dependent effects of GAL receptor agonists on: OCl count, as measured by TRAP staining; the expression of OCl markers and related apoptotic and inflammatory markers through analysis of gene expression and, finally; the presence of GAL receptors stained via immunocytochemistry.
2. Materials and Methods
2.1 Cell culture
Cultures of hematopoietic stem cells (HSCs) were obtained from the left and right femora of scavenged C57BL/6 male mice, approximately six months of age, bred and housed in the La Trobe University Animal Research & Training Facility. Animal tissues were collected from animals killed for other purposes (scavenging) and exempt from Animal Ethics approval. Marrow was flushed using 10x antibiotic medium consisted of Dulbecco’s Modified Eagle Medium (DMEM; Gibco Life Technology), antibiotics (1000 units/mL penicillin, 1000 μg/mL streptomycin; Gibco Life Technology) and 25 μg/mL fungizone (Sigma-Aldrich). The antibiotic medium containing bone marrow was aspirated and added to medium containing DMEM, 10% fetal bovine serum (FBS) (Sigma-Aldrich), 4 mM L-glutamine (Sigma-Aldrich). Four biological replicates (n=4) were cultured for each experiment, each with three technical replicates. Collection of HSCs for OCl culture was performed the same way for both GAL and galn treatment. Cells were counted using a TC10TM Automated Cell Counter (Bio-Rad) and diluted to 1x105 cells/mL with cell-free media. This was plated at 100 µL into 96-well culture dishes or at 400 µL into 24-well culture dishes in standard medium and incubated at 37°C in 5% CO2 humidified air. This medium was 10% FBS, 4 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2.5 μg/mL fungizone in DMEM, along with addition of 25 ng/mL macrophage colony stimulating factor (m-CSF) (BioLegend). This propagation media was changed three times per week until 80% confluence was reached.
2.2 Osteoclast Differentiation and Treatment
Once cells were 80% confluent (72 hours), RANKL (Oriental Yeast Co.) was added at 100 ng/mL with 25 ng/mL mCSF to drive differentiation into OCls. As per established protocol, OCls were defined as being TRAP-positive cells with 3-or-greater nuclei 63. Final differentiation media was changed three times per week, and treatment groups containing different concentrations of GAL; at 0 nM (OCl CTRL), 10 nM (OCl GAL 10), 100 nM (OCl GAL 100) or 1 µM (OCl GAL 1000) were tested. Treatment was for three days, and treatment groups contained different concentrations of galn; 0 nM (OCl galn CTRL), 1 µM (OCl galn 1), 10 µM (OCl galn 10) or 100 µM (OCl galn 100). All galn treatments included 0.5% (w/v) DMSO, a solvent for galn.
2.3 Tartrate-Resistant Acid Phosphatase Staining
Tartrate-resistant acid phosphatase (TRAP) staining is used to identify mature OCls [56]. After three days of treatment, cells were stained using a leukocyte acid phosphatase kit from Sigma-Aldrich as per the manufacturer’s instructions. TRAP-positive multinucleated BMSCs were visualised using a Leica DMBRE research microscope; digital images were acquired at x200 and x400 with a Leica DFC420C camera lens with IM50 software (Version 5). Mature OCls were determined as being TRAP-positive and multinucleated [57].
2.4 Analysis of Gene Expression
RNA was extracted from OCl cell cultures and the expression of a number of genes was measured using RT-qPCR. Following aspiration of media, RNA was extracted from cells via homogenisation with PureZOLTM RNA isolation agent from Bio-Rad, as per manufacturer’s instructions. The purity of RNA was assessed through A260/A280 ratios using the NanoDropTM 2000 Spectrophotometer [58]. Exactly 25 ng of RNA was then reverse-transcribed to cDNA using iScriptTM cDNA Synthesis Kit (Bio-Rad) as per the manufacturer’s instructions, utilising the CFX ManagerTM Version 3.0 (Bio-Rad). Primer sequences, shown in Table 1, were prepared commercially by GeneWorks Pty Ltd. Where primer sequences have been previously used in peer-reviewed manuscripts, references have been provided. Primers were designed using the Beacon Designer 2.0 Software (Biosoft International). β-actin was used as an internal reference for each sample. Gene expression was normalised to the β-actin mRNA level and presented as a relative expression. Genes of interest were replicated using the CFX ManagerTM Version 3.0 (Bio-Rad) programmed to perform the real-time PCR protocol over 55 cycles using Ssofast Evagreen (Bio-Rad). The volume of reagents in each well-plate consisted of: SsoFast™ EvaGreen® Supermix (17.5 µL), working solution of forward primer (2.5 µL) and reverse primer (2.5 µL), RNase-free H2O (8.5 µL) and a 1:20 dilution of cDNA (8.5 µL). This mixture was then divided into technical triplicates with a final volume of 11.5 µL per well. A melt curve analysis was performed post-cycling to assist in establishing specificity of DNA products.
Table 1: Primer sequences used for OCl qPCR analysis
|
Primer |
Sense |
Antisense |
|
GALR1 4 |
CGTCCTGGTGGTCGTTGTAG |
AGGCAATGGGCGGTGATTC |
|
GALR2 4 |
GCACAGTTGACCCAGTAG |
CCACACGCAGAGGATAAG |
|
GALR3 4 |
ACCACCACCGCCTTCATC |
ACAGGGTTAGTCTAGTCTCTCC |
|
IL-1β 4 |
TCAGGCAGGCAGTATCAC |
GGATGGGCTCTTCTTCAAAG |
|
TNF-α 4 |
CACCACGCTCTTCTGTCTAC |
GGCTACAGGCTTGTCACTC |
|
CSP3 59 |
TACCCTGAAATGGGCTTGTGT |
GTTAACACGAGTGAGGATGTG |
|
β-actin 60 |
GATTACTGCTCTGGCTCCTAG |
CATCTGCTGGAAGGTGGAC |
|
CTHSK 61 |
CCATATGTGGGCCAGGATG |
AGGAATCTCTCTGTACCCTCTGCA |
|
RANK 62 |
GGACGGTGTTGCAGCAGAT |
GCAGTCTGAGTTCCAGTGGTA |
|
SHARPIN |
ACAGTTGAAGACGCCACATC |
CTGAGAACACCTGATCCTGAAG |
2.5 Immunocytochemistry of Osteoclast Cultures
After a 72-hour treatment, OCls on coverslips were fixed with 4% paraformaldehyde. Following fixation, cells were permeabilized and blocked with 5% normal goat serum (Thermo-Fisher) in 10 mM phosphate-buffered saline (PBS; pH 7.2) with 0.1% Tween 20 for 1 hour at room temperature. Primary antibodies (GALR1, GALR2, or GALR3) at a 4 µg/mL dilution in 10 mM PBS (pH 7.2) with 0.1% Tween 20 were applied and incubated overnight at 40C. After washing with PBS, a 1/200 dilution of Cy3-conjugated goat anti-rabbit antiserum was added for 1 hour at room temperature. Following another round of PBS washes, DAPI (0.1 µg/ml) (Sigma-Aldrich) was applied for 5 minutes to stain nuclei blue. After washing and air-drying, coverslips were mounted with Fluoro-Gel water-based mounting medium (ProSciTech) on microscope slides. Imaging was performed using a Leica DMBRE research microscope with appropriate filters for Cy3 and DAPI. High-resolution digital images were acquired with a Leica DFC420C camera at x200 and x400 magnification, and DAPI and Cy3 images were overlaid using IM50 software (Leica Microsystems). Image analysis and quantification was performed by a third-party, in a double-blind fashion, with slides randomly coded to eliminate bias.
2.6 Statistical Analysis
Statistical analysis was performed on TRAP stain, gene expression and OCl count to determine the existence of any significant differences between groups. Normality of data was analysed via Anderson-Darling testing. A one-way analysis of variance (ANOVA) was performed between all groups for each experiment, followed by post-hoc protected Fisher’s LSD testing to determine any significant difference (p<0.05) between treatments. All values have been expressed as mean ± standard error of the mean (SEM). Statistics were analysed using GraphPad PRISM 10.0 software (GraphPad Software, Inc, La Jolla, CA, USA).
3. Results
3.1 Galanin-treated Osteoclasts: Cell Culture Results
Untreated BMSCs grown with m-CSF (OCl NEG) were differentiated into OCls (OCl CTRL) by culturing in the presence of RANKL for 72 hours. These were further treated with 10 µM GAL (OCl GAL 10), 100 µM GAL (OCl GAL 100) and 1000 µM GAL (OCl GAL 1000). OCls grown with these predetermined concentrations of GAL were counted following TRAP staining and gene expression analysed using qPCR, as well as protein localisation of GALR1-R3 via immunocytochemistry. The results are presented below.
3.2 Tartrate-Resistant Acid Phosphatase (TRAP) count
Undifferentiated BMSCs grown in 25 ng/mL m-CSF (OCl NEG) were differentiated to become OCls by the addition of 100 ng/mL RANKL (OCl CTRL). TRAP staining was performed on cell cultures, with cells counted and OCls defined as being TRAP-positive cells with 3-or-greater nuclei [63]. Representative photos of OCls grown in GAL are below (Figure 1), showing a concentration-specific effect of GAL on OCl size and number. Osteoclasts treated with 100 nM of GAL showed a 75% decrease in OCl number compared to OCl CTRL (**p<0.01) (Figure 2), as well as fewer osteoclasts than GAL 10, and GAL 1000 treatments (Figure 2A) (**p<0.01, ***p<0.005, *p<0.05 respectively). Similar results were also found for OCl diameter (Figure 2B), with treatments of GAL 100 showing decreased diameter compared with CTRL, GAL 10 and GAL 1000 treatments (*p<0.05, **p<0.01, *p<0.05 respectively) (n=4, mean±SEM).
A shows OCl controls (OCl CTRL), while B, C & D show the development of OCls in the presence of increasing concentrations of GAL (10 µM, 100 µM and 1000 µM respectively) (original magnification. x200).
(A) OCl GAL 100 showed a significant decrease in OCl count compared to controls (OCl CTRL), while OCl GAL 100 and OCl GAL 1000 showed significantly fewer mature osteoclasts than OCl GAL 10 (**p<0.01, ***p<0.005 and *p<0.05 respectively). (B) OCl diameter was also significantly decreased in OCl GAL 100 compared to all other groups (*p<0.05; **p<0.01; mean ± SEM)
3.3 Analysis of Gene Expression
Gene expression was determined via qPCR for GAL-treated OCl culture extracts, normalised to the housekeeping gene β-actin, and compared with the normalised expression of the control group, OCl CTRL. Expression of RANK (Figure 3A) was significantly increased in OCl GAL 100 treatment compared with untreated control (***p<0.005), with similar increases in expression compared with OCl GAL 10 and OCl GAL 1000 (**p<0.01 for both) (Figure 3B), again indicating a concentration-specific influence of GAL on OCls.
A) OCL GAL 100 expresses more RANK than control OCl (OCl CTRL) and OCL GAL 10 (**p 0.01) B) GAL 100 showed increased Cathepsin K (CTHSK) expression compared to OCl CTRL (***p<0.005), GAL 10 (**p<0.01) and GAL 1000 groups (**p<0.01, n=4, mean ± SEM).
Treatment with GAL also altered expression of inflammatory cytokines in OCls, with OCl GAL 100 increasing IL-1β by ~300% vs OCl CTRL and OCl GAL 1000 (Figure 4A) (***p<0.005). OCl GAL 10 increased expression of IL-1β compared with OCl CTRL (**p<0.01) and OCl GAL 1000 (*p<0.05). TNF-α expression was also increased ~five-fold by OCl GAL 100-treatment versus OCl CTRL (**p<0.01), OCl GAL 10 (*p<0.05) and OCl GAL 1000 (**p<0.01) (Figure 4B). Expression of Sharpin, a protein essential for canonical NF-κB transcription and anti-apoptosis (Figure 4C), followed a similar trend and increased five-fold in OCl GAL 100 compared to CTRL (**p<0.01); OCl GAL 100 also increased Sharpin expression compared to OCl GAL 10 (*p<0.05), and OCl GAL 1000 (**p<0.01). Increased levels of TNF-α directly lead to increased levels of Sharpin, resulting in transcription and increased numbers of osteoclasts 45. Expression of CSP3 (Figure 4D), a marker of apoptosis, remained unchanged between all treatment groups.
A – B. IL-1β expression increased in OCl GAL 10 (**p<0.01) and OCl GAL 100 (***p<0.005) vs untreated OCls (OCl CTRL). OCl GAL 1000 decreased IL-1β expression vs. OCl GAL 10 (*p<0.05) and OCl GAL 100 (***p<0.005). B) TNF-α expression increased in OCl GAL 100 vs. OCl CTRL (**p<0.01), OCl GAL 10 (*p<0.05) and OCl GAL 100 (**p<0.01; n=4). C) Sharpin expression increased in OCl GAL 100 treatment vs. all other treatment groups (n=4, *p<0.05, **p<0.01). D) CSP3 expression remained unchanged. (mean ± SEM).
Relative expression of GAL receptor subtypes was determined by qPCR. Expression of GALR1 (Figure 5A) increased thirty-fold with OCl GAL 100 treatment (29.16 ± 4.5) compared with OCl CTRL (1.19 ± 0.48) and OCl GAL 1000 treatment (2.30 ± 0.60) (****p<0.0005, ***p<0.005) as well as increasing three-fold compared with OCl GAL 10 treatment (12.25 ± 2.97) (*p<0.05). GALR1 expression increased ten-fold relative to OCl CTRL (*p<0.05). GALR2 expression (Figure 5B) was also upregulated in OCl GAL 100 treatments (6.23 ± 0.81), showing a six-fold increase compared with OCl CTRL (1.39 ± 0.74) (**p<0.005), as well as significantly increasing compared with OCl GAL 10 (2.8 ± 0.25) (*p<0.01) and OCl GAL 1000 (2.06 ± 0.05) (**p<0.005). GALR3 expression was unable to be quantified through qPCR, possibly due to extremely low expression levels.
A) OCl GAL 100 showed significantly higher GALR1 expression than control OCl and OCl GAL 1000 groups (***p<0.005) and OCl GAL 10 (*p<0.05). B) OCl GAL 100 had a significantly higher GALR2 expression than OCl controls, OCl GAL 1000 (**p<0.01) and OCl GAL 10 (*p<0.05; n=4, mean ± SEM).
3.4 Immunocytochemistry
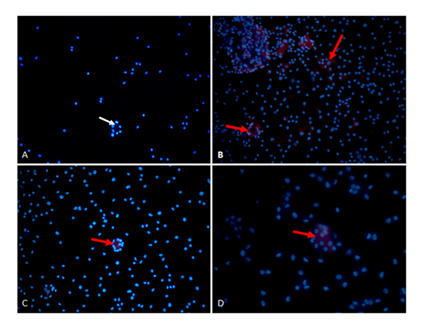
Immunocytochemistry was also performed on OCls to identify the protein expression of GALR1, GALR2 and GALR3 (Figure 6). The presence of GALR1 was shown (Figure 6B) on multinucleated OCls, as well as the presence of GALR2 (Figure 6C) and GALR3 (Figure 7D).
A) Negative control multinucleated OCl nuclei stained for DAPI (blue stain, indicated by white arrow). B-D) OCl cells show immunoreactivity (red staining, indicated by red arrows) for (B) GALR1, (C) GALR2 and (D) GALR3 antibodies. (original magnification X200)
3.5 Galnon-treated Osteoclasts: Cell Culture Results
Untreated BMSCs grown with 25 ng/mL m-CSF (OCl NEG) were differentiated into OCls (OCl galn CTRL) by culturing in the presence of 100 ng/mL RANKL and 0.5% DMSO, a solvent for galn. These were further treated with 1 mM galn (OCl galn 1), 10 mM galn (OCl galn 10) and 100 mM galn (OCl galn 100). OCls grown with these predetermined concentrations of galn were counted via double-blinded TRAP staining and analysed for RNA expression, as well as the presence of GALR1-R3 via immunocytochemistry, the results of which will be presented below.
3.6 Tartrate-Resistant Acid Phosphatase Staining (TRAP Stain)
Undifferentiated BMSCs grown in 25 ng/mL m-CSF (OCl NEG) were differentiated to become OCls by the addition of 100 ng/mL RANKL with 0.5% DMSO (OCl galn CTRL). TRAP staining was performed on cell cultures, with cells counted and OCls defined as being TRAP-positive cells with 3-or-greater nuclei. As seen previously, this treatment regimen stimulates specific BMSCs to differentiate into multinucleated OCls. Representative photos of galn 1, 10 and 100 treatments can be seen in Figure 7 A-D. Treatment with galn decreased OCl number at all concentrations (9 ± 3.5, 11.25 ± 1.7, 7.75 ± 2.8 increasing concentrations respectively) vs OCl galn CTRL (21.5 ± 1.85) (Figure 7 E).
Treatments were A) OCl galn CTRL; B) OCl galn 1; C) OCl galn 10; D) OCl galn 100. Original magnification x200. E) OCls treated with galn 1, galn 10 and galn 100 showed decreased osteoclastogenesis compared to control groups (*p<0.05, **p<0.01; n=4, mean ± SEM).
3.7 Analysis of Gene Expression
Gene expression was determined via qPCR for galn-treated OCl culture extracts, normalised to the housekeeping gene β-actin, and compared with the normalised expression of the control group, OCl galn CTRL. Expression of RANK (Figure 8A) was unchanged between controls and all galn treatments. Cathepsin K, a marker of osteoclastogenesis, decreased in OCl galn 1 (0.34 ± 0.03) and OCl galn 10 (0.29 ± 0.13) compared with OCl galn CTRL (1.28 ± 0.55) (Figure 8B).
A) RANK expression was unchanged with galn treatment. B) OCls treated with galn 1 and galn 10 showed decreased expression of the osteoclast marker CTHSK compared to control groups (*p<0.05; n=4, mean ± SEM).
Expression of the inflammatory cytokine IL-1β (Figure 9 A) was significantly reduced in OCl galn 1 (0.3 ± 0.02) compared with the OCl galn CTRL group (1.65 ± 0.72). Expression of Sharpin, a protein essential for canonical NFκB transcription and whose absence leads to apoptosis (Figure 9C), was decreased in OCl galn 1 (0.60 ± 0.24) compared to CTRL (2.5 ± 0.61); OCl galn 10 also decreased Sharpin expression (3.1 ± 0.28), as did OCl galn 100 (0.68 ± 0.07) (**p<0.01). Expression of Caspase 3 (Figure 9D), a marker of apoptosis, was decreased in OCl galn 1 (0.25 ± 0.03) and OCl galn 100 (0.32 ± 0.15) compared with OCl galn CTRL (1.50 ± 0.46) (*p<0.05). There was no significant change in TNF-α expression.
A) IL-1β expression was decreased in OCl galn 1 compared with OCl galn CTRL, while other groups showed no change. B) TNF expression was unaffected by galn treatment. C) OCls treated with all concentrations of galn showed decreased relative Sharpin expression compared to control groups. D) Expression of the apoptosis molecule, CSP3, was decreased in OCl galn 1 and OCl galn 100 vs OCl galn CTRL (*p < 0.05, **p<0.01; n=4, mean ± SEM).
Expression of GAL receptor subtypes was quantified by qPCR. GALR1 expression (Figure 10 A) almost completely ceased in OCl galn 1 treatment (0.02 ± 0.02), OCl galn 10 (0.0001 ± 8.3 x 10-6) and OCl galn 100 (0.0004 ± 7.6 x 10-5) compared to OCl galn CTRL (6.35 ± 3.1) (*p<0.05). Expression of GALR2 (Figure 10B) was unchanged between all treatment groups, while GALR3 expression was unable to be quantified through qPCR, possibly due to low expression levels in the skeletal tissue.
A) Expression of GALR1 was negligible in all galn treatments (*p<0.05). B) No significant difference was found in GALR2 expression between any treatment groups (n=4, mean ± SEM).
3.8 Immunocytochemistry
Immunocytochemistry was also performed on OCls to identify the protein expression of GALR1, GALR2 and GALR3. The presence of GALR1 was seen (Figure 11B) on multinucleated OCls, as well as the presence of GALR2 (Figure 11C) and GALR3 (Figure 11D). It is interesting to note that the presence of any concentration of galn completely eliminated any GALR1 protein, while GALR2 was present as expected (Figure 11C).
A) Osteoclasts incubated in the absence of secondary antibody showed no background staining; B) Osteoclasts show no immunoreactivity for GALR1 when treated with any concentration of galn (OCl galn 10 shown), nuclei have been counterstained with DAPI (white arrows); C-D) OCls cells show immunoreactivity (red staining, indicated by red arrows) for (C) GALR2 and (D) GALR3 antibodies (original magnification X 400).
4. Discussion
There has been minimal research investigating the potential link between GAL, GAL receptors and OCl function. A previous finding noted that OCls lining unfractured trabecular bone stain moderately-to-intensely for GALR1-like immunoreactivity (GALR1-LI), and that the thickened periosteal fibrous matrix surrounding OCls in fracture calluses stain moderately-to-intensely for GAL-like immunoreactivity (GAL-LI) [24]. Similarly, GAL labelling is increased in OCls cultured from arthritic rats [15]. The use of flavonoid compounds containing GAL, such as the resin propolis, have been shown to suppress OCl activity [64], however it needs to be determined whether it was specifically GAL, or another substance, which led to the suppression of OCl activity in this study. The above research led to our hypothesis that GAL and its non-peptide agonist, galn, may be able to influence OCl activity, and therefore be of benefit in the treatment of bone disease. As GAL has also been shown to function in a dose-dependent manner [4, 65], the experiments in this manuscript utilised a series of three, exponentially-increasing concentrations of either GAL, or its GAL receptor agonist, galn, to determine the effects of these compounds on OCls in vitro . In experiments conducted in this paper, the middle, moderate dose (100 ng) of GAL led to significantly fewer TRAP-positive, multinucleated cells than the OCl CTRL group. As only this one concentration of GAL led to this decrease in OCl number, GAL seems to act in a dose-dependent manner to regulate OCl proliferation. Additionally, galn treatment also showed fewer TRAP-positive, multinucleated OCls than the control group. This occurred in all galn doses and indicates that galn may be a more potent inhibitor of OCl formation, regardless of dosage. There may also be other avenues through which galn may inhibit bone loss; the effect of galn on OBs should be elucidated, as OBs can directly control OCl proliferation and maturity [66]. While experiments in this manuscript utilised co-cultures of undifferentiated MSCs and HSCs which were differentiated to become multi-nucleated, TRAP-positive OCls, it is now important to investigate the actions of GAL and galn in co-cultures of differentiated OBs and OCls to see if there is an even stronger inhibition of OCl formation, both directly on OCls and also indirectly through a decreased RANKL: OPG ratio from OBs.
The dose-dependent effect of GAL continues to be evident, with OCl number affected by only the moderate, middle dose of GAL. Expression of RANK, a receptor found on the surface of OCls, is increased significantly in cells treated with the moderate dose of GAL, as is CTHSK, a bone resorptive molecule. Both of these markers could indicate an increase in OCl differentiation [67, 68]. These findings are, however, in contrast with the OCl count and size measurements, and were unexpected. To help explain this anomalous finding, GAL has previously been shown to either promote [15] or inhibit [69] cell migration, and although gene expression may have increased in OCl progenitor cells, migration and fusion of these cells into mature OCls may have been affected. This is an important finding which may lead to alternate approaches to OCl regulation. No change was seen in RANK expression for any galn concentration treatment, while both the lowest (OCl galn 1) and moderate (OCl galn 10) groups significantly decreased CTHSK expression. Once again, this shows that low-to-moderate galn-treatment is capable of decreasing OCl count and gene expression, giving us a strong indication that treatment of OCl-induced bone diseases with galn may be a new area of research. As OCl proliferation and activity is increased in the presence of inflammation [70-72], we next investigated the effects of our GAL receptor ligands on inflammation. Previous research has shown that GAL can decrease the effects of inflammation with both treatment [4, 73] and GAL knock-out experiments [74]. Interpretation of these findings suggests GAL as a potential treatment for inflammation-induced bone resorption. In the experiments performed in this experiment, expression of IL-1β was significantly increased in low and moderate GAL-treated groups, while the moderate dose also showed increased expression of the inflammatory cytokine TNF-α. As increased inflammation is a potent driver of OCl differentiation and bone resorption [75], an increase in expression of TNF-α and IL-1β may explain the changes in RANK and CTHSK expression seen in these GAL-treated cultures. For galn treatments, only the lowest galn treatment showed any significant change, with a decrease in IL-1β expression. This is a particularly important finding, as a decrease in inflammatory cytokine expression is particularly important in treating bone disease [22, 76]; of interest, recent research has identified rutin, a naturally occurring pigment containing GAL, as being of particular interest as a treatment for osteoporosis due to its ability to limit inflammatory cytokines [77, 78]; once again, however, there is no way identify which component of rutin elicited this change in inflammation. Our findings are extremely important, as they show that galn and GAL have opposite effects on inflammation and that galn, in particular, may be of use in combating bone disease.
As inflammatory cytokines such as TNF-α, IL-1β and RANKL can act via the Sharpin pathway to cause either transcription [79] or apoptosis [80], the expression of Sharpin mRNA was also of interest. Increases in Sharpin have been shown to lead to increased osteoclastogenesis [81], while decreases in Sharpin lead to apoptosis [82]. In our study, Sharpin expression was significantly higher in cells treated with a moderate dose of GAL, and as there was no change in the expression of the apoptotic gene, CSP3, the decrease in OCl number does not seem to be related to programmed cell death. This upregulation of Sharpin expression implies that a moderate dose of GAL may increase inflammation, which increases osteoclastogenesis. This was not reflected in the TRAP-staining results, however, and may indicate that OCl activity, rather than OCl number, was increased. Future studies should utilise resorption pit assay to analyse the resorptive capacity of these OCls. Contrarily, concentrations of galn decreased expression of Sharpin. Experiments by Wang and colleagues have demonstrated that decreased Sharpin leads to a decrease in OCl number with an accompanying increase in OCl apoptosis [82]; our findings, however, did not show any change to OCl number or apoptosis. It needs to be acknowledged, however, that gene expression via mRNA does not always correlate with protein translation [83] although mRNA expression has been shown to correlate quite strongly with protein concentration in many systems [84-86]. Furthermore, although our findings do not support the findings of Wang and team, we have shown significant changes in OCls treated with galn. Therefore, further investigation is warranted to elucidate the actions of galn on Sharpin and CSP3 in vitro. Overall, the differing actions of GAL and galn on inflammation and Sharpin expression show that, as seen in other experiments [52, 87, 88], GAL and galn may have different actions. These conflicting actions may occur as a result of activation of different receptors. As such, we next analysed the expression, and protein localisation, as measured via qPCR and immunocytochemistry, respectively, of GALR1-3.
The expression of GALR1 was significantly increased for cells treated with a low and moderate dose of GAL, and expression of GALR2 was also increased in the moderate GAL-dosed OCls. Both GALR1 [26] and GALR2 [27] have been shown to act, in certain circumstances, to increase inflammation and thus, presumably, increase activity of their downstream regulator, Sharpin. Results of the experiments in this experiment show that GAL-treated OCls show localised expression of GALR1, GALR2 and GALR3 protein. Therefore, GAL most likely acts to increase inflammation through either GALR1 or GALR2 rather than GALR3, as GALR3 has been shown to have strictly anti-inflammatory actions [28, 29, 74]. Our results indicate that, whether via activation of GALR1 or GALR2, GAL increases inflammation in a dose-dependent manner in OCls cultured in vitro. Inflammation has been proven to increase OCls numbers [72], although a decrease in mature, multinucleated OCls was observed. As GALR2 has previously been shown to influence non-osseus cell and tissue motility [89, 90] we propose that GAL, possibly via GALR2, limits motility and fusion of OCl precursors in the same manner, thus leading to decreased numbers of mature OCls. Treatment with all doses of galn, however, showed no change in GALR2 expression, while virtually eliminating any expression of GALR1; galn treatment also eliminated any localisation of GALR1 protein. Therefore, the different actions of GAL and galn discovered in this laboratory may be due to the differing availability of GALR1 as an OCl binding site.
In conclusion, while both GAL and galn decreased OCl number in vitro, galn seems to show greater potential as a negative regulator of OCl proliferation and activity via its action on OCl markers and precursors. As treatment with GAL generally showed an increase in GALR1 expression, and treatment with galn eliminated GALR1 expression, the differing abilities of these GAL-receptor agonists to regulate OCl activity may be due to GALR1 binding. Further studies should look into resorption assays to quantify OCl activity, while GALR1-KO or GALR1-antagonist studies would be expected to verify the findings of galn as a potent inhibitor of bone resorption. Therefore, this leads us to hypothesise that galn may be of benefit as a treatment for osteoporotic bone disease.
Acknowledgements
The authors would like to acknowledge the support of the Department of Microbiology, Anatomy, Physiology and Pharmacology in the award of a department grant.
Conflict of interest
The authors report no conflicts of interest.
Funding statement
This project was supported by a grant from the Department of Microbiology, Anatomy, Physiology and Pharmacology, La Trobe University.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: Heath McGowan and Aaron McDonald; data collection: Heath McGowan; analysis and interpretation of results: Heath McGowan, Aaron McDonald, Deanna Horvath, Amy Larsen; draft manuscript preparation: Heath McGowan; supervision: Aaron McDonald, Deanna Horvath, Amy Larsen. All authors reviewed the results and approved the final version of the manuscript.
Animal Ethics
Animal tissues were collected from animals killed for other purposes (scavenging) and exempt from Animal Ethics approval.
References
- California Peptide Research Inc.
- Langel U, Bartfai T. Chemistry and molecular biology of galanin receptor ligands. Ann N Y Acad Sci 863 (1998): 86-93.
- Xu Y, Rokaeus A, Johansson O. Distribution and chromatographic analysis of galanin message-associated peptide (GMAP)-like immunoreactivity in the rat. Regul Pept 51 (1994): 1-16.
- McDonald AC, Schuijers JA, Gundlach AL, et al. Galanin treatment offsets the inhibition of bone formation and downregulates the increase in mouse calvarial expression of TNFalpha and GalR2 mRNA induced by chronic daily injections of an injurious vehicle. Research Support, Non-U.S. Gov't. Bone 40 (2007): 895-903.
- Tatemoto K, Rokaeus A, Jornvall H, et al. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett 164 (1983): 124-128.
- Berger A, Lang R, Moritz K, et al. Galanin Receptor Subtype GalR2 Mediates Apoptosis in SH-SY5Y Neuroblastoma Cells. Endocrinology 145 (2004): 500-507.
- Mahoney SA, Hosking R, Farrant S, et al. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J Neurosci 23 (2003): 416-421.
- Iismaa T, Fathi Z, Hort YJ, et al. Structural organization and chromosomal localization of three human galanin receptor genes. Ann N Y Acad Sci 863 (1998): 56-63.
- Bartfai T, Hökfelt T, Langel U. Galanin - a neuroendocrine peptide [Review]. Crit Rev Neurobiol 7 (1993): 229-274.
- Hohmann J, Krasnow SM, Teklemichael DN, et al. Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology 77 (2003): 354-366.
- Tarasov KV, Tarasova YS, Crider DG, et al. Galanin and galanin receptors in embryonic stem cells: accidental or essential? Neuropeptides 36 (2002): 239-245.
- Vrontakis ME. Galanin: a biologically active peptide. Current Drug Targets - Cns & Neurological Disorders 1 (2002.): 531-541.
- Holst J, Bersani M, Hvidberg A, et al. On the effects of human galanin in man. Diabetologia 36 (1993): 653-657.
- Kaplan L, Spindel E, Isselbacher K, et al. Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A 85 (1988): 1065-1069.
- Qinyang W, Hultenby K, Adlan E, et al. Galanin in adjuvant arthritis in the rat. The Journal of Rheumatology 31 (2004): 302-307.
- McDonald A, Schuijers JA, Shen P-J, et al. Expression of galanin and galanin receptor-1 in normal bone and during fracture repair in the rat. Comparative Study Research Support, Non-U.S. Gov't. Bone 33 (2003): 788-797.
- Xu ZQ, Shi TJ, Hokfelt T. Expression of galanin and a galanin receptor in several sensory systems and bone anlage of rat embryos. Proc Natl Acad Sci U S A 93 (1996): 14901-14905.
- Qinyang W, Lindgren UJ, Hultenby K. Distribution of galanin in bone and joint tissues. Anat Embryol (Berl) 209 (2005): 227-231.
- Zhao X, Patil S, Xu F, et al. Role of Biomolecules in Osteoclasts and Their Therapeutic Potential for Osteoporosis. Biomolecules 11 (2021): 747.
- Kenkre J, Bassett J. The bone remodelling cycle. Ann Clin Biochem 55 (2018): 308-327.
- Cheng C, Chen L-R, Chen K-H. Osteoporosis due to hormone imbalance: an overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int J Mol Sci 23 (2022): 1376.
- Briot K, Geusens P, Em Bultink I, et al. Inflammatory diseases and bone fragility. Osteoporos Int 28 (2017): 3301-3314.
- Penna S, Villa A, Capo V. Autosomal recessive osteopetrosis: mechanisms and treatments. Dis Model Mech 14 (2021): 048940.
- McDonald A, Schuijers JA, Shen PJ, et al. Expression of galanin and galanin receptor-1 in normal bone and during fracture repair in the rat. Bone 33 (2003): 788-797.
- Azin F, Khazali H. Neuropeptide galanin and its effects on metabolic and reproductive disturbances in female rats with estradiol valerate (EV)-induced polycystic ovary syndrome (PCOS). Neuropeptides 80 (2020): 102026.
- Anselmi L, Cavalli I, Sternini C. CHAPTER 142 - Galanin in the Gastrointestinal Tract: Distribution and Function (2006): 1037-1042.
- DeMorrow S, Williams E, An SY, et al. Targeting Galanin Receptor Signaling as a Novel Therapeutic Strategy for the Treatment of Fatty Liver Disease. The FASEB Journal 36 (2022): 216.
- Brunner SM, Reichmann F, Leitner J, et al. Galanin receptor 3 attenuates inflammation and influences the gut microbiota in an experimental murine colitis model. Sci Rep 11 (2021): 1-15.
- Botz B, Kemény Á, Brunner SM, et al. Lack of Galanin 3 Receptor Aggravates Murine Autoimmune Arthritis. J Mol Neurosci 59 (2016): 260-269.
- Turquier V, Vaudry H, Yon L, et al. Proinflammatory cytokines TNF-alpha and IL-1alpha stimulate neuropeptide gene expression in adrenochromaffin cells. Ann N Y Acad Sci 971 (2002): 45-48.
- Su Y, Ganea D, Peng X, et al. Galanin down-regulates microglial tumor necrosis factor-alpha production by a post-transcriptional mechanism. J Neuroimmunol 134 (2003): 52-60.
- Epsley S, Tadros S, Farid A, et al. The effect of inflammation on bone. Front Physiol 11 (2021): 1695.
- Jimi E, Takakura N, Hiura F, et al. The Role of NF-kappaB in Physiological Bone Development and Inflammatory Bone Diseases: Is NF-kappaB Inhibition "Killing Two Birds with One Stone"? Cells 8 (2019): 1636.
- Arnett TR. Osteoclast biology. Marcus and Feldman's Osteoporosis. Elsevier (2021): 99-110.
- Galson DL, Roodman GD, Joseph L, et al. 2 - Origins of Osteoclasts. Osteoimmunology. Academic Press (2011): 7-41.
- Isojima T, Sims NA. Cortical bone development, maintenance and porosity: genetic alterations in humans and mice influencing chondrocytes, osteoclasts, osteoblasts and osteocytes. Cell Mol Life Sci 78 (2021): 5755-5773.
- Udagawa N, Koide M, Nakamura M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39 (2021): 19-26.
- Novack DV, Faccio R. Osteoclast motility: putting the brakes on bone resorption. Ageing Res Rev 10 (2009): 54-61.
- Kim I, Kim JH, Kim K, et al. IRF2 enhances RANKL-induced osteoclast differentiation via regulating NF-kappaB/NFATc1 signaling. BMB Rep 54 (2021): 482-487.
- Kusnadi A, Park SH, Yuan R, et al. The cytokine TNF promotes transcription factor SREBP activity and binding to inflammatory genes to activate macrophages and limit tissue repair. Immunity 51 (2019): 241-257.
- Chen T, Zhang X, Zhu G, et al. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 99 (2020): e22241.
- Uehara S, Udagawa N, Kobayashi Y. Non-canonical Wnt signals regulate cytoskeletal remodeling in osteoclasts. Cell Mol Life Sci 75 (2018): 3683-3692.
- Amarasekara D, Yun H, Kim S, et al. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw 18 (2018): e8.
- Tokunaga F, Iwai K. LUBAC, a novel ubiquitin ligase for linear ubiquitination, is crucial for inflammation and immune responses. Microbes Infect 14 (2012): 563-572.
- Xia T, Liang Y, Ma J, et al. Loss-of-function of SHARPIN causes an osteopenic phenotype in mice. Endocrine 39 (2011): 104-112.
- Sundberg JP, Pratt CH, Goodwin LP, et al. Keratinocyte-specific deletion of SHARPIN induces atopic dermatitis-like inflammation in mice. PLoS One 15 (2020): e0235295.
- Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res 21 (2011): 71-85.
- Franzoso G, Carlson L, Xing L, et al. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev 11 (1997): 3482-3496.
- Iotsova V, Caamano J, Loy J, et al. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med 3 (1997): 1285-1289.
- Xing L, Bushnell TP, Carlson L, et al. NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis. J Bone Miner Res 17 (2002): 1200-1210.
- Brzozowska M, Całka J. occurrence and distribution of galanin in the physiological and inflammatory states in the mammalian gastrointestinal tract. Front Immunol 11 (2021): 602070.
- Sollenberg U, Bartfai T, Langel U. Galnon--a low-molecular weight ligand of the galanin receptors. Neuropeptides 39 (2005): 161-163.
- Bartfai T, Lu X, Badie-Mahdavi H, et al. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci U S A 101 (2004): 10470-10475.
- Talero E, Sanchez-Fidalgo S, Calvo JR, et al. Chronic administration of galanin attenuates the TNBS-induced colitis in rats. Regul Pept 141 (2007): 96-104.
- Watanabe Y, Namba A, Aida Y, et al. IL-1beta suppresses the formation of osteoclasts by increasing OPG production via an autocrine mechanism involving celecoxib-related prostaglandins in chondrocytes. Mediators Inflamm (2009): 308596.
- Qiao J-H, Mishra V, Fishbein MC, et al. Multinucleated giant cells in atherosclerotic plaques of human carotid arteries: Identification of osteoclast-like cells and their specific proteins in artery wall. Exp Mol Pathol 99 (2015): 654-662.
- Bonnelye E, Chabadel A, Saltel F, et al. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 42 (2008): 129-138.
- Gill S, von Hippel P. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182 (1989): 319-326.
- Kermer P, Klocker N, Labes M, et al. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 In vivo. J Neurosci 20 (2000): 2-8.
- Higashi Y, Aratake T, Shimizu S, et al. Influence of extracellular zinc on M1 microglial activation. Sci Rep 7 (2017): 43778.
- Mishina Y, Starbuck MW, Gentile MA, et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem 279 (2004): 27560-27566.
- Ohishi M, Ono W, Ono N, et al. A novel population of cells expressing both hematopoietic and mesenchymal markers is present in the normal adult bone marrow and is augmented in a murine model of marrow fibrosis. Am J Pathol 180 (2012): 811-818.
- Tasca A, Astleford K, Lederman A, et al. Regulation of Osteoclast Differentiation by Myosin X. Sci Rep 7 (2017): 7603.
- Juwita DA, Almahdy A, Abdillah R, et al. The bone strengthening effects of propolis in ovariectomized female white rats as models for postmenopause. Current Issues in Pharmacy and Medical Sciences 34 (2021): 119-122.
- Fonseca-Rodrigues D, Almeida A, Pinto-Ribeiro F. A New Gal in Town: A Systematic Review of the Role of Galanin and Its Receptors in Experimental Pain. Cells 11 (2022): 839.
- Udagawa N, Koide M, Nakamura M, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab 39 (2021): 19-26.
- Rocho FR, Bonatto V, Lameiro RF, et al. A patent review on cathepsin K inhibitors to treat osteoporosis (2011–2021). Expert Opin Ther Pat 32 (2022): 561-573.
- Fu C, Shi R. Osteoclast biology in bone resorption: a review. STEMedicine 1 (2020): e57-e57.
- Levison SW. Hierarchical interactions among cytokines establish the functional state of microglia. Journal of Neurochemistry 81 (2002): 71-71.
- Baum R, Gravallese EM. Impact of Inflammation on the Osteoblast in Rheumatic Diseases. journal article. Current Osteoporosis Reports 12 (2014): 9-16.
- Cafiero C, Gigante M, Brunetti G, et al. Inflammation induces osteoclast differentiation from peripheral mononuclear cells in chronic kidney disease patients: crosstalk between the immune and bone systems. Nephrology Dialysis Transplantation (2017): 65-75.
- Tong X, Yu G, Fu X, et al. A Review of Signaling Transduction Mechanisms in Osteoclastogenesis Regulation by Autophagy, Inflammation, and Immunity. Int J Mol Sci 23 (2022): 9846.
- Lang R, Kofler B. The galanin peptide family in inflammation. Neuropeptides 45 (2011): 1-8.
- Schmidhuber SM, Santic R, Tam CW, et al. Galanin-Like Peptides Exert Potent Vasoactive Functions In Vivo. J Invest Dermatol 127 (2006): 716-721.
- Espirito Santo A, Ersek A, Freidin A, et al. Selective inhibition of TNFR1 reduces osteoclast numbers and is differentiated from anti-TNF in a LPS-driven model of inflammatory bone loss. Biochem Biophys Res Commun 464 (2015): 1145-1150.
- Tseng H-W, Samuel SG, Schroder K, Lévesque J-P, Alexander KA. Inflammasomes and the IL-1 Family in Bone Homeostasis and Disease. Current Osteoporosis Reports (2022): 1-16.
- Gera S, Pooladanda V, Godugu C, et al. Rutin nanosuspension for potential management of osteoporosis: Effect of particle size reduction on oral bioavailability, in vitro and in vivo activity. Pharm Dev Technol 25 (2020): 971-988.
- Lee H-H, Jang J-W, Lee J-K, et al. Rutin improves bone histomorphometric values by reduction of osteoclastic activity in osteoporosis mouse model induced by bilateral ovariectomy. Journal of Korean Neurosurgical Society 63 (2020): 433-443.
- Barrow M. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmunity Reviews 20 (2021): 102741.
- Alsaedi II, Taqi ZJ, Hussien AMA, et al. Graphene nanoparticles induces apoptosis in MCF-7 cells through mitochondrial damage and NF-KB pathway. Materials research express 6 (2019): 095413.
- Xia T, Liang Y, Ma J, et al. Loss-of-function of SHARPIN causes an osteopenic phenotype in mice. Endocrine 39 (2011): 104-12.
- Wang Z, Potter CS, Sundberg JP, et al. SHARPIN is a key regulator of immune and inflammatory responses. J Cell Mol Med 16 (2012): 2271-2279.
- Nie L, Wu G, Zhang W. Correlation of mRNA expression and protein abundance affected by multiple sequence features related to translational efficiency in Desulfovibrio vulgaris: a quantitative analysis. Genetics 174 (2006): 2229-2243.
- Willcocks LC, Lyons PA, Clatworthy MR, et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. The Journal of experimental medicine 205 (2008): 1573-1582.
- Teymouri M, Mollazadeh S, Mortazavi H, et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathology - Research and Practice 221 (2021): 153443.
- Ståhlberg A, Thomsen C, Ruff D. Quantitative PCR Analysis of DNA, RNAs, and Proteins in the Same Single Cell. Clin Chem 58 (2012): 1682-1691.
- Quynh NT, Islam MS, Floren A, et al. Effects of galnon, a non-peptide galanin-receptor agonist, on insulin release from rat pancreatic islets. Biochem Biophys Res Commun 328 (2005): 213-220.
- Abramov U, Floren A, Echevarria DJ, et al. Regulation of feeding by galnon. Neuropeptides 38 (2004): 55-61.
- Lin C-y, Zhang M, Huang T, et al. Spexin enhances bowel movement through activating L-type voltage-dependent calcium channel via galanin receptor 2 in mice. Sci Rep 5 (2015): 12095.
- Godlewski J, Kmiec Z. Colorectal cancer invasion and atrophy of the enteric nervous system: potential feedback and impact on cancer progression. Int J Mol Sci21 (2020): 3391.