Food Enhanced Pharmacokinetics for Clinical Translation of Low Dose Abiraterone Acetate in Metastatic Castration-Resistant Prostate Cancer
Article Information
Meenakshi Meenu1, Ranjit Kumar Sahoo2, Amlesh Seth3, Ujjalkumar Das4, Thirumurthy Velpandian4, Dharamvir Singh Arya1*
1Department of Pharmacology, 4th floor, Teaching Block, All India Institute of Medical Sciences, New Delhi, India
2Department of Medical Oncology, BRAIRCH, All India Institute of Medical Sciences, New Delhi, India
3Department of Urology, 5th floor, Teaching Block, All India Institute of Medical Sciences, New Delhi, India
4Ocular division, Department of Pharmacology, 6th floor, Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India
5Lab No. 4028, Department of Pharmacology, 4th floor, Teaching Block, All India Institute of Medical Sciences, New Delhi, India
*Corresponding Author: Dharamvir Singh Arya, MBBS, MD, PhD, Lab No. 4028, Department of Pharmacology, 4th floor, Teaching Block, All India Institute of Medical Sciences, New Delhi, India
Received: 18 June 2020; Accepted: 01 July 2020; Published: 24 August 2020
Citation: Meenakshi Meenu, Ranjit Kumar Sahoo, Amlesh Seth, Ujjalkumar Das, Thirumurthy Velpandian, Dharamvir Singh Arya. Food Enhanced Pharmacokinetics for Clinical Translation of Low Dose Abiraterone Acetate in Metastatic Castration-Resistant Prostate Cancer. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 314-324.
View / Download Pdf Share at FacebookAbstract
Introduction: Abiraterone acetate (AAc) is prescribed in metastatic castration-resistant prostate cancer (mCRPC) with recommendation to administer in fasted state due to food-drug interaction. This study was designed to compare pharmacokinetics of 250 mg AAc in fed state with 1000 mg in fasted state for use of low dose AAc as a cost-effective alternative.
Patients and Methods: A randomized, open label, nonreplicated crossover clinical study was conducted after ethics approval and trial registration. Eighteen patients were randomized into two groups: 1. Test- 250 mg AAc in fed state (milk, 7% fat); 2. Reference-1000 mg AAc in fasted state. Drug was administered for 6 days and on 7th day, blood samples were withdrawn at predose, 1, 2, 3, 4 and 6 hours. Patients were crossed-over to other groups and process was repeated. Abiraterone levels in plasma were estimated using liquid chromatography–mass spectrometry. Maximum (Cmax) and minimum (Cmin) plasma concentrations, area under curve from 0 to 6 hours (AUC0- 6) were estimated and appropriate statistical tests were applied.
Results: Seventeen mCRPC patients completed the study. Difference in mean Cmax between test (107.86 ng/mL) and reference (88.71 ng/mL) was not statistically significant (p>0.05) so was the difference in mean AUC0-6 (p>0.05). Trough plasma concentrations had statistically significant (p<0.05) difference between test (4.3 ng/mL) and reference (7.5 ng/mL) but were above known half-maximal inhibitory concentration of abiraterone (1.4 ng/mL) in both groups. Conclusion: AAc 250 mg with milk had similar pharmacokinetics with 1000 mg in fasted state and, is a cost-effective alternative.
Keywords
Abiraterone; Low fat diet; Pharmacokinetics; Liquid chromatography–mass spectrometry; Prostate cancer.
Abiraterone articles, Low fat diet articles, Pharmacokinetics articles, Liquid chromatography?mass spectrometry articles, Prostate cancer articles
Abiraterone articles Abiraterone Research articles Abiraterone review articles Abiraterone PubMed articles Abiraterone PubMed Central articles Abiraterone 2023 articles Abiraterone 2024 articles Abiraterone Scopus articles Abiraterone impact factor journals Abiraterone Scopus journals Abiraterone PubMed journals Abiraterone medical journals Abiraterone free journals Abiraterone best journals Abiraterone top journals Abiraterone free medical journals Abiraterone famous journals Abiraterone Google Scholar indexed journals Low fat diet articles Low fat diet Research articles Low fat diet review articles Low fat diet PubMed articles Low fat diet PubMed Central articles Low fat diet 2023 articles Low fat diet 2024 articles Low fat diet Scopus articles Low fat diet impact factor journals Low fat diet Scopus journals Low fat diet PubMed journals Low fat diet medical journals Low fat diet free journals Low fat diet best journals Low fat diet top journals Low fat diet free medical journals Low fat diet famous journals Low fat diet Google Scholar indexed journals Pharmacokinetics articles Pharmacokinetics Research articles Pharmacokinetics review articles Pharmacokinetics PubMed articles Pharmacokinetics PubMed Central articles Pharmacokinetics 2023 articles Pharmacokinetics 2024 articles Pharmacokinetics Scopus articles Pharmacokinetics impact factor journals Pharmacokinetics Scopus journals Pharmacokinetics PubMed journals Pharmacokinetics medical journals Pharmacokinetics free journals Pharmacokinetics best journals Pharmacokinetics top journals Pharmacokinetics free medical journals Pharmacokinetics famous journals Pharmacokinetics Google Scholar indexed journals Liquid chromatography–mass spectrometry articles Liquid chromatography–mass spectrometry Research articles Liquid chromatography–mass spectrometry review articles Liquid chromatography–mass spectrometry PubMed articles Liquid chromatography–mass spectrometry PubMed Central articles Liquid chromatography–mass spectrometry 2023 articles Liquid chromatography–mass spectrometry 2024 articles Liquid chromatography–mass spectrometry Scopus articles Liquid chromatography–mass spectrometry impact factor journals Liquid chromatography–mass spectrometry Scopus journals Liquid chromatography–mass spectrometry PubMed journals Liquid chromatography–mass spectrometry medical journals Liquid chromatography–mass spectrometry free journals Liquid chromatography–mass spectrometry best journals Liquid chromatography–mass spectrometry top journals Liquid chromatography–mass spectrometry free medical journals Liquid chromatography–mass spectrometry famous journals Liquid chromatography–mass spectrometry Google Scholar indexed journals Prostate cancer articles Prostate cancer Research articles Prostate cancer review articles Prostate cancer PubMed articles Prostate cancer PubMed Central articles Prostate cancer 2023 articles Prostate cancer 2024 articles Prostate cancer Scopus articles Prostate cancer impact factor journals Prostate cancer Scopus journals Prostate cancer PubMed journals Prostate cancer medical journals Prostate cancer free journals Prostate cancer best journals Prostate cancer top journals Prostate cancer free medical journals Prostate cancer famous journals Prostate cancer Google Scholar indexed journals Abiraterone acetate articles Abiraterone acetate Research articles Abiraterone acetate review articles Abiraterone acetate PubMed articles Abiraterone acetate PubMed Central articles Abiraterone acetate 2023 articles Abiraterone acetate 2024 articles Abiraterone acetate Scopus articles Abiraterone acetate impact factor journals Abiraterone acetate Scopus journals Abiraterone acetate PubMed journals Abiraterone acetate medical journals Abiraterone acetate free journals Abiraterone acetate best journals Abiraterone acetate top journals Abiraterone acetate free medical journals Abiraterone acetate famous journals Abiraterone acetate Google Scholar indexed journals oral oncologic drugs articles oral oncologic drugs Research articles oral oncologic drugs review articles oral oncologic drugs PubMed articles oral oncologic drugs PubMed Central articles oral oncologic drugs 2023 articles oral oncologic drugs 2024 articles oral oncologic drugs Scopus articles oral oncologic drugs impact factor journals oral oncologic drugs Scopus journals oral oncologic drugs PubMed journals oral oncologic drugs medical journals oral oncologic drugs free journals oral oncologic drugs best journals oral oncologic drugs top journals oral oncologic drugs free medical journals oral oncologic drugs famous journals oral oncologic drugs Google Scholar indexed journals erlotinib articles erlotinib Research articles erlotinib review articles erlotinib PubMed articles erlotinib PubMed Central articles erlotinib 2023 articles erlotinib 2024 articles erlotinib Scopus articles erlotinib impact factor journals erlotinib Scopus journals erlotinib PubMed journals erlotinib medical journals erlotinib free journals erlotinib best journals erlotinib top journals erlotinib free medical journals erlotinib famous journals erlotinib Google Scholar indexed journals nilotinib articles nilotinib Research articles nilotinib review articles nilotinib PubMed articles nilotinib PubMed Central articles nilotinib 2023 articles nilotinib 2024 articles nilotinib Scopus articles nilotinib impact factor journals nilotinib Scopus journals nilotinib PubMed journals nilotinib medical journals nilotinib free journals nilotinib best journals nilotinib top journals nilotinib free medical journals nilotinib famous journals nilotinib Google Scholar indexed journals lapatinib articles lapatinib Research articles lapatinib review articles lapatinib PubMed articles lapatinib PubMed Central articles lapatinib 2023 articles lapatinib 2024 articles lapatinib Scopus articles lapatinib impact factor journals lapatinib Scopus journals lapatinib PubMed journals lapatinib medical journals lapatinib free journals lapatinib best journals lapatinib top journals lapatinib free medical journals lapatinib famous journals lapatinib Google Scholar indexed journals
Article Details
1. Introduction
Metastatic castration-resistant prostate cancer (mCRPC) is a therapeutically challenging stage in the evolution of prostate cancer and significantly increases economic burden for the patients [1,2]. Abiraterone acetate with prednisone had been approved for mCRPC due to survival benefit in both chemotherapy naïve and resistant patients in India [3]. In Feb 2018, United States Food and Drug Administration (U.S. FDA) approved it for metastatic high-risk castration-sensitive prostate cancer [4]. Abiraterone acetate plus prednisone administration had been evaluated in fasted state in clinical research and Phase III trials, which formed the basis of regulatory approval [5,6,7]. It had been recommended that abiraterone acetate, 1000 mg, once daily, “must be taken in an empty stomach”, with no food to be consumed for at least 2 h before or 1 h after drug administration (i.e., modified fasted state) due to its increased absorption in the presence of food [8].
Recent reports apparently diverge with general recommendation for administering abiraterone acetate in fasted state wherein some of these studies suggest better patient outcome while others insist upon insignificant differences between fed and fasted state [9,10,11]. In a randomized, open-label, single-dose, crossover study, it was found that mean values of maximum plasma concentration (Cmax) and area under the curve (AUC0-∞, from 0 to infinity) changed by 7- and 5-folds, respectively with low-fat ( ∼ 7%) meal, and 17- and 10- folds respectively with high-fat meal ( ∼ 56%) in healthy human volunteers. However, in mCRPC patients, AUC0-∞ and safety profiles did not differ significantly between fed (low fat) and modified fasted states [12]. This raised an important question on rationality of drug dosages and consequent pharmacoeconomic impact of low dosing recommendation. Clearly, a better understanding of food-abiraterone interaction is needed. Moreover, a retrospective study on the food labeling patterns among 104 oral drugs approved between 2000 and 2009, concluded that it was more likely for anticancer drugs (e.g., erlotinib, nilotinib, lapatinib) than for other drugs to be labeled for administration in the fasted state on account of having notable food effects [13]. In the instance of abiraterone acetate, fat content of food increases its absorption in a dose dependent manner after oral administration. There are considerable clinical interests in understanding the effect of food content on bioavailability of oral oncologic drugs [12,14].
The present study compared the pharmacokinetics (PK) of 250 mg of abiraterone acetate in fed state (milk, 7% fat) with 1000 mg in fasted state in mCRPC patients using limited sampling technique. This project was primarily undertaken to generate evidence for a cost-effective alternative dose of abiraterone acetate in prostate cancer patients.
2. Materials and Methods
2.1 Materials
Following chemicals were procured- Abiraterone acetate tablets (MyTeraTM, batch No- NT8079A, manufacturing date- 08/2018, expiry date- 07/2020), abiraterone (Cayman chemical Co., USA), formic acid, methanol and acetonitrile of Liquid Chromatography-Mass Spectrometry (LC-MS) grade. LC-MS experiments were performed using a triple quadrupole tandem mass spectrometer- 4000 QTrap, AB Sciex, Foster City, California, United States of America, (USA) coupled with high performance liquid chromatography system (HPLC, Agilent Technologies, 1260 Infinity, Santa Clara, California, USA) consisted of quaternary pump (G1311C), multisampler (G7167A), thermostatted column compartment (G1316A) with variable wavelength UV detector (G1314F) and online degasser. All the parameters of tandem mass spectrometer and HPLC were controlled by Analyst software, version 1.5.2 (AB Sciex, Foster City, California, USA) and OpenLAB control panel software (Agilent Technologies, 1260 Infinity, Santa Clara, California, USA), respectively.
2.2 Study design
It was a randomized, open label, non-replicated, crossover clinical study conducted between July 2018 and September 2019. The study was approved by Institutional Ethics Committee (IEC-224/04.05.2018) and prospectively registered in Clinical Trial Registry of India (CTRI/2018/07/014859). The study followed the guidelines established by Declaration of Helsinki (Code of Ethics of the World Medical Association) regarding human experiments and good clinical practice of International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH).
2.3 Patients
Eighteen patients were recruited in the study between July 2018 and September 2019. It satisfied the minimum regulatory requirement of a sample size of 12 subjects [15]. Patients of age ≥18 years, histologically confirmed adenocarcinoma of prostate, metastatic disease based on a positive bone scan or imaging on 68Ga PSMA-CT scan were included. Castration resistance was diagnosed as disease progression after surgical castration or <50 ng/dL testosterone levels. Patients, who did not provide signed informed consent, uncontrolled DM or hypertension, hepatic or renal dysfunction, serum potassium less than 3.5 mEq/dL, use of CYP3A4 inhibitors or substrates of CYP2D6, autoimmune disease requiring corticosteroid therapy, active infection or other medical condition that would contraindicate the use of prednisone (corticosteroid), clinically significant heart disease, administration of an investigational therapeutic agent within 30 days of screening, prior radiation therapy completed <4 weeks prior to enrollment, prior chemotherapy (<1 year) for CRPC, any "currently active" second malignancy, active psychiatric illness, thyrotoxicosis, known brain metastasis, history of pituitary or adrenal dysfunction, obesity, lactose intolerance, hypersensitivity to abiraterone acetate or its excipients, or allergy to milk protein were excluded.
Screening of mCRPC patients included medical and surgical history, physical examination, routine blood chemistry, hematology tests, urinalysis and routine staging work-up for cancer. These were conducted in Oncology and Urology departments. Evaluations of metastasis and castration resistance were based on clinical features, biochemical and radiological examinations and were done as part of routine staging work-up. It provided serum prostate-specific antigen (PSA) values and tumor metastases burden for every patient. Patients, fulfilling the eligibility criteria, were recruited in the study after obtaining signed informed consents. Simple randomization technique was used to assign patients into two groups: Test group (T)- abiraterone acetate 250 mg once daily in fed state (200 mL of milk, 7% fat) and Reference group (R)- abiraterone acetate 1000 mg once daily in modified fasted state. Prednisone was to be continued as prescribed. To achieve steady state of abiraterone in the blood, drugs were administered for 6 days with 200 mL water (fasted state) or 200 mL of milk (fed state). Single standardized warm meal was provided 2 hrs after drug administration. Water was allowed ad libitum after 2 hrs of drug intake. Blood samples were collected on 7th day on site in clinical pharmacology unit (division of Department of Pharmacology). Subjects were crossed-over to the other group from next day. 2 mL blood was withdrawn in EDTA blood collection tubes from peripheral veins at pre dose, 1 hour (hr), 2 hrs, 3 hrs, 4 hrs and 6 hrs (limited sampling technique) [16]. Samples were centrifuged at 2500 rpm for 15 minutes (min) at 4°C within 5 min of withdrawal of blood to obtain plasma. After centrifugation, plasma was placed into a properly labeled Eppendorf tube using clean pipette and immediately stored at –80°C.
2.4 Precautions
Each abiraterone tablet package was clearly identified by its lot number, manufacture, and expiry dates. Alcohol, caffeine or related xanthene containing food or beverage and black pepper containing foods were not allowed during study period.
2.5 Estimation of abiraterone using LC-MS
At the time of estimation, frozen samples were thawed and subjected to LC-MS to determine plasma levels of abiraterone.
2.6 Calibration standards and quality control
Calibration standards and quality control samples were prepared using finasteride. Calibration standards for abiraterone were prepared in blank human plasma in the range of 0.39-400 ng/mL. Quality control samples were also prepared at three concentration levels: LQC (1 ng/mL), MQC (75 ng/mL) and HQC (300 ng/mL) using plasma as a matrix. Simple extraction protocol using single step direct protein precipitation followed by high speed centrifugation was performed for optimum recovery of abiraterone and finasteride from plasma matrix according to reported extraction protocol [17]. Extraction solvent consisted of pure acetonitrile containing finasteride at a concentration of 5 ng/mL. An aliquot of 5 μL from resulting clear supernatant was subjected to LC-MS analysis. Samples that exceeded the calibration range, were appropriately diluted and extracted.
2.7 Optimized LC-MS conditions
A gradient separation was achieved in Phenomenex RP Hydro (150x2.1 mm, 4 µm; Phenomenex, USA) analytical column using triphasic mobile phase combinations consisting of solvent A (18.2 m? milliQ with 0.1% formic acid), solvent B (methanol with 0.1% formic acid) and solvent C (acetonitrile with 0.1% formic acid) pumped at a flow rate of 0.2 mL/min. Analytes were eluted using optimized gradient method started at ratio of 30:45:25 of A:B:C followed by linear increase to 20:70:10% of A:B:C from 0-1.5 min which was maintained till 4 min and returned back to original conditions at 4.5-6 min. Total run time of the method was 6 min with post-run equilibration time of 2 min between each run. The temperatures of autosampler tray and the column oven were maintained at 10±1°C and 50±1°C, respectively. Electrospray ionization (ESI) in positive ion mode was optimized using Turbo Ion Spray source (AB Sciex, Foster City, USA). Source and compound dependent parameters were optimized using the inbuilt algorithm to attain maximum ion intensity of analytes (abiraterone and internal standard- finasteride). Multiple reaction monitoring mode transitions of mass/charge 350.2/156.2 and 373.3/305.3 were used for abiraterone and internal standard (finasteride), respectively.
3. Data Analysis
Data of baseline demographics and disease characteristics were represented as mean± standard deviation or SD and percentage. PK parameters estimated were time to reach maximum plasma concentration (Tmax), minimum/ trough plasma concentration (Cmin), Cmax, and AUC0-6 (AUC from 0 to 6 hrs) at steady state. The data were log-transformed. Least square means and geometric least square means with their ratios and 90% confidence intervals (CI) for Cmax, and AUC0-6 were estimated. Inter and intra-individual variability were estimated using analysis of variance and expressed as coefficient of variation (CV%). A mixed effect model was applied with period, sequence and treatment (non-replicated study design) as fixed effects and subject within sequence as random effect on log-transformed data using Phoenix WINNONLIN version 8.2.0.4383 (core version 30/01/2014) [15]. Statistical tests (Two one-sided T test, Anderson-Hauck Procedure and Pitman- Morgan’s Adjusted F test) were applied using the same software. For statistical significance, p<0.05 was considered.
4. Results
4.1 Baseline demographic and disease characteristics
Eighteen patients were recruited in the study. One patient did not follow the protocol during the test period and data for 17 subjects were included in the final analysis (CONSORT diagram in figure 1). Mean age was 62.1 years, median weight and BMI were 68.1 kg and 24.7 respectively. Demographic details were documented along with relevant biochemical and biopsy data of all patients, and represented as mean (SD) and percentage (Table 1).
|
Variable |
Mean (SD) |
|
Age (years) |
62.1 (9.25) |
|
Height (cm) |
165.8 (5.94) |
|
Weight (kg) |
68.1 (8.81) |
|
BMI |
24.7 (2.45) |
|
sPSA (ng/mL) |
14.77 (20.167) |
|
Gleason Score |
8 (2) |
|
Disease Burden (N) |
|
|
Visceral metastasis |
17 |
|
Non-regional lymph node metastasis |
17 |
|
Regional lymph node metastasis |
17 |
|
Bone metastasis |
17 |
|
Other Base Line Characteristics (%) |
|
|
Smoking |
0 |
|
Alcohol |
0 |
|
Tobacco (Oral) |
0 |
|
Family h/o prostate cancer |
0 |
|
Hypertension |
29% |
|
Diabetes |
41% |
aSD, standard deviation; sPSA, serum prostate specific antigen; N, number of patients; h/o, history of; BMI, body mass index; mCRPC, metastatic castration-resistant prostate cancer
Table 1: Baseline characteristics of mCRPC patients (N=17).
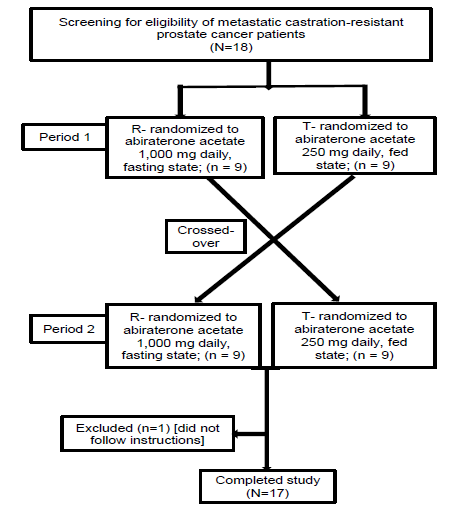
figure 1: CONSORT diagram.
4.2 Pharmacokinetics
Tmax had a similar range (1-3 hrs) in test and reference groups. Mean (arithmetic) Cmax were 107.86 (CV%- 70.4) and 88.71 (CV%- 66.8) ng/mL in test and reference groups respectively (figure 2). Geometric mean ratios for Cmax and AUC0-6 in test and reference groups were 94 (90% CI: 72-124) and 80 (90% CI: 64-99) respectively (Table 2). Least square means and geometric least square means of log- transformed Cmax and AUC0-6 in both groups were also presented in Table 2. Intra-individual variations for Cmax and AUC0-6 were 37.5% and 48.8% respectively, and inter-individual variations for Cmax and AUC0-6 were 53.1% and 68.2% respectively. Mixed effect model with statistical tests (Two one-sided T test, Anderson-Hauck Procedure and Pitman- Morgan’s Adjusted F test) did not find any significant difference between the test and reference groups for Cmax, AUC0-6 and intra-subject variability (p>0.05). The difference in mean Cmin between test group (4.3 ng/mL, CV%- 46.6) and reference group (7.5 ng/mL, CV%- 54.5) was statistically significant (p<0.05).
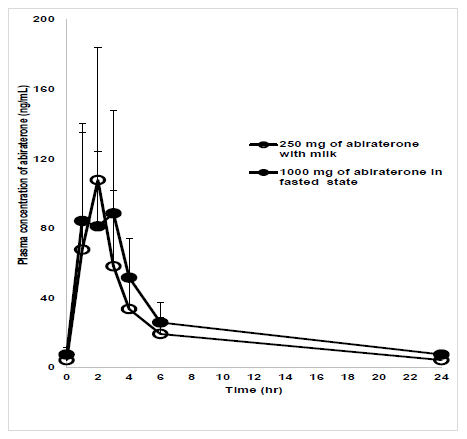
figure 2: Plasma concentration-time plot of abiraterone.
Plasma concentration-time plot of abiraterone in reference and test groups. X-axis: Time in hr; Y-axis: Mean concentration of abiraterone in plasma (ng/mL); Test group (T): 250 mg of abiraterone acetate with milk; Reference group (R): 1000 mg of abiraterone acetate with water.
|
Pharmacokinetic Parameter |
Ref LSM |
Test LSM |
Ref GLSM |
Test GLSM |
GMR % Ref |
90% CI |
Statistical Analysis |
|
Cmax (ln) |
4.6 |
4.5 |
97 |
91 |
94 |
72-124 |
Not significant |
|
AUC0-6 (ln) |
5.8 |
5.5 |
321 |
256 |
80 |
64-99 |
Not significant |
bCmax: maximum plasma concentration; AUC0-6: area under curve between time 0 to t where t= 6 hrs; hrs, hours; ln, natural logarithm; Ref: Reference group; LSM: least square mean; GLSM: geometric least square mean; GMR: Geometric mean ratio; p<0.05 for statistical significance.
Table 2: Pharmacokinetic data of abiraterone for test and reference groups.
5. Discussion
In this project, we selectively studied pharmacokinetics of abiraterone from 0 to 6 hrs using limited sampling technique in fed and fasted states [16]. For fed state, instead of low fat diet, milk was used because of its consistent composition and common usage. This significantly reduced any dietary restrictions otherwise required for mCRPC patients. In addition, timings of drug and milk (fat %) administration to patients were carefully controlled. Absence of any statistically significant differences in PK parameters between standard dose in fasted state and low dose abiraterone with fatty food confirmed that fat enhanced its absorption to a sizeable extent. It increases solubility and absorption of abiraterone in intestine [18]. However, it does not affect metabolic activation of abiraterone acetate into abiraterone, its Tmax, and terminal half-life; hence, a full pharmacokinetics study was not required [12]. Due to high inter-patient variability in PK of abiraterone in both fasted and fed states in our study, we inferred that its bioavailability is equally unpredictable in both conditions. Similar findings were observed in a latest study but, they differed from initial studies which formed the basis of regulatory recommendation of administration of abiraterone in fasted state [5,8,14,19,20]. We found that trough plasma concentration was higher in reference group but maximum plasma concentration was higher in test group. Though maximum plasma concentration can predict therapeutic efficacy, importance of trough plasma concentration for predicting clinical response could not be firmly established from literature. While one study emphasized its significant correlation with clinical response, a larger clinical trial in 2018 did not report any correlation of trough plasma concentration with change in PSA, which is a commonly used progression marker for prostate cancer [20,21]. We also looked into half-maximal inhibitory concentration (IC50) values of abiraterone from literature. As per literature, the mechanism of action of abiraterone is inhibition of two enzymes-17-alpha hydroxylase (known IC50- 4 nM or 1.4 ng/mL) and 17,20- lyase (known IC50- 2.9 nM or 1.01 ng/mL) [22]. Despite a significant difference between two groups, trough plasma concentrations were higher than known IC50 of abiraterone in both test and reference groups. Furthermore, a recent clinical trial established non-inferiority of low dose abiraterone with low fat diet compared to standard dosing in fasted state in CRPC patients, however, this study required dietary restriction for patients, who were administered with 250 mg of abiraterone [21]. Milk ( ∼ 7% fat) in our study presented an appealing alternative of low fat diet in contrast to previous studies and, we can safely anticipate better patient compliance. For other oncologic drugs, there is an ongoing debate on rational dosing recommendation particularly in context of food-drug interactions. A recent example is concurrent intake of erlotinib with a proton pump inhibitor, which resulted in a clinically relevant decrease of bioavailability of erlotinib. In a randomized, cross-over, pharmacokinetic study, non-small-cell lung cancer patients who were co-administered erlotinib and esomeprazole with cola had clinically relevant and statistically significant increase in the bioavailability of erlotinib during esomeprazole coadministration [23,24].
Though regulatory recommendations have mandated fasting state for administration of abiraterone acetate in mCRPC patients, results of this study posed a compelling argument in favor of use of low dose abiraterone acetate in patients of poor socioeconomic status.
6. Conclusion
Pharmacokinetics of 250 mg dose of abiraterone acetate with milk was similar to 1000 mg dose in fasted state in mCRPC patients. findings of this study had been translated into our clinical set-up.
Acknowledgement
The authors acknowledge the contribution of Dr. Moksha Kaul, PhD toward assistance during this project.
Funding
This work was supported by All India Institute of Medical Sciences, New Delhi, India [Institute Collaborative Research Grant (AC001)]. The funding source has no role in the study design, collection, analysis, interpretation of data, writing of report and decision to submit the article for publication.
Conflict of Interests
There is no actual or perceived conflict of interest amongst any of the authors involved in the study.
References
- Hariharan K, Padmanabha V. Demography and disease characteristics of prostate cancer in India. Indian journal of urology: IJU: Journal of the Urological Society of India 32 (2016): 103.
- Trends over time for all sites and on selected sites of cancer & projection of burden of cancer. http://www.icmr.nic.in/ncrp/pbcr_2012-14/all_content/pdf_Printed_Version/Chapter10_Printed.pdf (2018).
- https://cdscoonline.gov.in/CDSCO/Drugs (2019).
- Research C for DE and. Approved Drugs - FDA approves abiraterone acetate in combination with prednisone for high-risk metastatic castration-sensitive prostate cancer. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm596015.htm (2018).
- fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology 13 (2012): 983-992.
- Ryan CJ, Smith MR, fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology 16 (2015): 152-160.
- De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. New England Journal of Medicine 364 (2011): 1995-2005.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202379s024lbl.pdf (2018).
- Chien C, Smith M, De Porre P. Effect of food on abiraterone pharmacokinetics: a review. International Journal of Pharmacokinetics 2 (2017): 183-193.
- Espinosa M, Falcon A, Gutierrez-Valencia A, et al. Pharmacokinetic food-effect study of abiraterone acetate (AA) in patients with metastatic castration resistant prostate cancer (mCRPC): The ABIFOOD trial (EudraCt number: 2012-003226-25).
- Leibowitz-Amit R, Atenafu EG, Seah JA, et al. Low-dose abiraterone (abi) with food in men with metastatic castration-resistant prostate cancer (mCRPC): The Princess Margaret Cancer Centre experience.
- Chi KN, Spratlin J, Kollmannsberger C, et al. Food effects on abiraterone pharmacokinetics in healthy subjects and patients with metastatic castration-resistant prostate cancer. The Journal of Clinical Pharmacology 55 (2015): 1406-1414.
- Kang SP, Ratain MJ. Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products. Clinical Cancer Research 16 (2010): 4446-4451.
- Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. Journal of Clinical Oncology 28 (2010): 1481.
- Guidance for Industry. Statistical approaches to establishing bioequivalence. https://www.fda.gov/media/70958/download (2020).
- Jaiswal J, Gupta SK, Dash SC, et al. Neoral monitoring by limited sampling area under the concentration time curve in stable Indian renal transplant recipients. InTransplantation Proceedings 35 (2003): 1298-1299.
- Benoist GE, van der Meulen E, Lubberman FJ, et al. Analytical challenges in quantifying abiraterone with LC–MS/MS in human plasma. Biomedical Chromatography 31 (2017): e3986.
- Geboers S, Stappaerts J, Mols R, et al. The effect of food on the intraluminal behavior of abiraterone acetate in man. Journal of Pharmaceutical Sciences 105 (2016): 2974-2981.
- Ratain MJ. Importance of food effects for oral oncology drugs. Clinical advances in hematology & oncology: H&O 10 (2012): 397.
- Carton E, Noe G, Huillard O, et al. Relation between plasma trough concentration of abiraterone and prostate-specific antigen response in metastatic castration-resistant prostate cancer patients. European Journal of Cancer 72 (2017): 54-61.
- Szmulewitz RZ, Ibraheem AF, Peer CJ, et al. A prospective international randomized phase II study evaluating the food effect on the pharmacokinetics (PK) and pharmacodynamics (PD) of abiraterone acetate (AA) in men with castration-resistant prostate cancer (CRPC).
- Potter GA, Barrie SE, Jarman M, et al. Novel steroidal inhibitors of human cytochrome P45017. alpha.-Hydroxylase-C17, 20-lyase): potential agents for the treatment of prostatic cancer. Journal of Medicinal Chemistry 38 (1995): 2463-2471.
- Ohgami M, Kaburagi T, Kurosawa A, et al. Effects of Proton Pump Inhibitor Coadministration on the Plasma Concentration of Erlotinib in Patients With Non–Small Cell Lung Cancer. Therapeutic Drug Monitoring 40 (2018): 699-704.
- van Leeuwen RW, Peric R, Hussaarts KG, et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 34 (2016): 1309-1314.
