Familial Adenomatous Polyposis in Pediatric Patients: A Case Series
Article Information
Maria Rogalidou1,2*, Konstantinos Katsanos3, Anna Batistatou4, Meropi Tzoufi5, Dimitrios Christodoulou3, Ekaterini Siomou5
1Pediatric-Gastroenterology Unit, Department of Pediatrics, University Hospital of Ioannina, Greece
2Division of Gastroenterology and Hepatology, First Department of Pediatrics, University of Athens, Agia Sofia Children’s Hospital, Athens, Greece
3Division of Gastroenterology, Medical School of Ioannina, Greece
4Division of Pathology, Medical School of Ioannina, Greece
5Division of Pediatrics, Medical School of Ioannina, Greece
*Corresponding author: Maria Rogalidou, Pediatric-Gastroenterology Unit, Department of Pediatrics, University Hospital of Ioannina, Greece.
Received: 12 September 2025; Accepted: 19 September 2025; Published: 26 September 2025
Citation: Maria Rogalidou, Konstantinos Katsanos, Anna Batistatou, Meropi Tzoufi, Dimitrios Christodoulou, Ekaterini Siomou. Familial Adenomatous Polyposis in Pediatric Patients: A Case Series. Archives of Clinical and Biomedical Research. 9 (2025): 388-391.
View / Download Pdf Share at FacebookAbstract
Background: Familial Adenomatous Polyposis (FAP) is a rare inherited disorder characterised by the development of hundreds to thousands of adenomatous polyps in the colon and rectum. It is caused by mutations in the APC (Adenomatous Polyposis Coli) gene located on chromosome 5q21. Without treatment, nearly all individuals with FAP will develop colorectal cancer, typically by the fourth decade of life.
Case Series Presentation: We retrospectively analysed the clinical features of pediatric patients diagnosed with FAP at our centre. Seven patients, aged between 7.5 and 17 years (5 boys and 2 girls), were included in this case series. All patients had a positive family history of FAP. One patient was diagnosed with Turcot syndrome; the remaining six had no additional pathological findings or associated conditions. All patients were asymptomatic at presentation, with normal growth parameters and unremarkable routine blood tests. Genetic testing was performed in five patients, and all underwent endoscopic evaluation with histological examination. Various grades of dysplasia were identified in the colonic polyps. Management strategies were individualised for each patient based on the severity and extent of disease.
Conclusions: Colorectal adenocarcinoma can develop in pediatric patients with FAP, even in the absence of symptoms. Children with a positive family history should undergo early and regular screening. In confirmed cases, close surveillance and timely intervention are essential to prevent malignant transformation.
Keywords
Familial Adenomatous Polyposis; APC gene mutation; Colonic polyps; Ge-netic screening; Endoscopic surveillance; Children
Familial Adenomatous Polyposis articles; APC gene mutation articles; Colonic polyps articles; Ge-netic screening articles; Endoscopic surveillance articles; Children articles
Article Details
1. Introduction
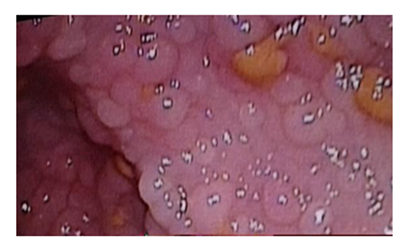
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited condition, characterized by the development of hundreds to thousands of adenomatous polyps in the colon and rectum (Figure 1), caused by a mutation in the adenomatous polyposis gene (APC) occurring in 1 3:10,000 births, with almost 100% penetrance. In 20% to 30% of cases, the condition is caused by a spontaneous mutation with no clinical or genetic evidence of FAP in the parents or family. Hyer et al. [1] reported that the gene responsible for FAP, APC, is located on chromosome 5q21. Attard et al. [2] reported that FAP typically presents in childhood or adolescence, with the youngest reported case of polyps at age 7, with some patients requiring colectomy before age 10, especially those with APC gene mutations at codon 1309. Some studies reported that common symptoms include rectal bleeding, diarrhoea or constipation, abdominal pain, unexplained anaemia, weight loss (in more advanced stages) or completely asymptomatic identified through family screening [3,4]. Attard et al. [5] reported that upper gastrointestinal involvement is common, with fundic gland polyposis and duodenal adenomas frequently observed. Some ttudies showed that common extraintestinal manifestations include osteomas, congenital hypertrophy of the retinal pigment epithelium, and desmoid tumours [6,7]. It can also be presented as Gardner syndrome and Turcot syndrome. Gardner syndrome features extracolonic manifestations like osteomas and dental anomalies, while Turcot syndrome is associated with central nervous system tumours. Galiatsatos et al. [8] reported that these syndromes can occur simultaneously, suggesting they are phenotypic variants of FAP. Cazorla et al. [9] in a recent research identified new cancer types potentially associated with FAP, such as mucoepidermoid carcinoma of the parotid gland.
Hyer et al. [1] showed that without intervention, FAP patients inevitably develop colorectal cancer by their fourth decade. Management typically involves early colectomy, Alkhouri et al. [3] reported that 58% of pediatric patients undergoing this procedure in one study. Munck et al. [4] suggested that regular endoscopic surveillance is crucial, as high-grade dysplasia and even invasive carcinoma have been detected in pediatric patients. Management guidelines recommend genetic testing, colonoscopy, and upper endoscopy for all pediatric FAP patients, regardless of symptoms as some studies supported [3,4]. Munck et al. [4] suggested that genetic counselling and testing play crucial roles in diagnosis and family screening. Early detection and intervention are essential to improve outcomes in pediatric FAP patients.
We report a case series of FAP, an extremely rare condition in paediatrics, its special characteristics, clinical picture, endoscopic, histological and genetic testing findings.
2. Case Series Presentation
We retrospectively analyzed the clinical features of pediatric patients diagnosed with FAP at our centre. Seven patients with Familial adenomatous polyposis, aged 7.5-17 years (mean age 13.8), 5 boys & 2 girls were included. All patients had a positive familial history of FAP. One patient has Turcot syndrome, while the rest of the patients have no other pathological findings or associated conditions. Except for the patient with Turcot syndrome, the rest of the patients were asymptomatic, with normal growth and normal blood tests, without anaemia. All patients who have been genetically tested for mutations in the APC gene were found; two patients refused to have genetic testing for economic reasons. All patients underwent upper and lower gastrointestinal (GI) endoscopy. Only one patient has upper GI involvement, with 2 small polyps found in the stomach. All patients had been found with colonic polyps with various densities. Regarding the histological findings, one patient had no dysplasia, while three patients had low and three moderate dysplasia in colonic biopsies. Interestingly, in the patient with Turcot syndrome Tubular-vilous adenoma (median grade differentiation) with high-grade dysplasia & focal signs of adenocarcinoma was found.
The special characteristics of the patients appear in Table 1.
|
Patients |
Genetic study |
Endoscopy |
Histological findings |
|
B=boy/G=girl y=years |
ND=Not done |
Nr of polyps |
|
|
B /12.5 y |
ND |
~1000- 10.000 |
Adenomatous polyps with moderate grade epithelial dysplasia |
|
B/7.5 y |
P.Ser1213X (c3638 C>A) |
~ 10-100 |
Adenomatous polyps |
|
G /14 y Sdr Turcot |
P.Ser1213X (c3638 C>A) |
~ 100 |
Tubular-vilous adenoma (median grade differentiation) with high grade |
|
dysplasia & focal signs of adenocarcinoma |
|||
|
B /16 y |
ND |
~1000 |
Adenomatous polyps with low to moderate grade epithelial dysplasia |
|
G/16 y |
p.Ser583X (c.1748 G>A) |
~ 10-100 |
Adenomatous polyps with moderate grade epithelial dysplasia |
|
B /17 y |
p.Ser583X (c.1748 G>A) |
~ 100-1000 |
Adenomatous polyps with low grade epithelial dysplasia |
|
B/14 y |
p.Ser583X (c.1748 G>A) |
~ 100-1000 |
Adenomatous polyps with low grade epithelial dysplasia |
Table 1: Patient’s characteristics.
3. Discussion
Barnard [10] reported that familial adenomatous polyposis (FAP) is an autosomal dominant disorder caused by APC gene mutations, leading to nearly inevitable progression to colorectal cancer by mid-adulthood if untreated.
This study presents the clinical and genetic characteristics of seven pediatric patients diagnosed with Familial Adenomatous Polyposis (FAP) at our centre. All patients had a positive family history, reinforcing the importance of early screening in at-risk individuals. Notably, almost all patients were asymptomatic at diagnosis, underscoring the silent progression of the disease in its early stages and highlighting the crucial role of surveillance in genetically predisposed populations. The age at diagnosis ranged from 7.5 to 17 years, consistent with literature reporting variable onset of colonic polyposis in children with FAP. Although most guidelines recommend beginning endoscopic screening in early adolescence, our findings suggest that polyposis may be evident even in younger children, such as the 7.5-year-old boy who already presented with adenomatous polyps.
Genetic testing confirmed APC mutations in five of seven patients, with two declining due to financial constraints. Genetic testing in FAP kindreds achieves over 90% detection of pathogenic mutations and is crucial for risk stratification and family counselling. If a mutation is identified, unaffected relatives can be spared intensive surveillance; conversely, those with confirmed mutations warrant early, regular endoscopic monitoring from ages 10–12 as some studies suggested [1,10].
In our cohort, genetic analysis identified two distinct profiles:
p.Ser1213X (c.3638C>A) – identified in two patients, one of whom had Turcot syndrome with high-grade dysplasia and focal adenocarcinoma, suggesting a potentially more aggressive phenotype.
p.Ser583X (c.1748G>A) – found in three patients, all with moderate polyp burden and low to moderate dysplasia.
The correlation between mutation location and clinical phenotype has been previously reported, with mutations in the 5′ and 3′ ends of the APC gene often associated with attenuated FAP, and mutations in the central region (particularly between codons 1250–1464) linked to classic FAP with dense polyposis. The p.Ser1213X variant lies within this central region, which may explain the more severe phenotype seen in our Turcot syndrome patient. In contrast, the p.Ser583X mutation, located outside this region, was associated with milder polyp burden and lower-grade dysplasia in our series.
Histological examination confirmed the presence of adenomatous polyps in all patients, with varying grades of dysplasia. One patient already showed focal adenocarcinoma at the time of diagnosis, emphasising that malignant transformation can occur even during adolescence in high- risk individuals.
The presence of Turcot syndrome in one patient demonstrates the potential for FAP to manifest as part of a broader syndromic picture involving CNS tumours, further supporting the need for multidisciplinary evaluation and ongoing neurological monitoring in certain cases.
4. Endoscopic Findings and Upper GI Involvement
Our cohort’s single case of gastric involvement—with two small stomach polyps—is consistent with the relatively low prevalence of significant upper gastrointestinal (GI) lesions in children. While gastric polyps are common in FAP, progression to malignancy is rare. In your pediatric cohort, the early and limited gastric involvement aligns with findings such as Stone et al. [11]., reported that 71% of children had gastric polyps by age 14, with low-grade dysplasia only in two cases and no invasive carcinoma observed. This reinforces the importance of early upper GI endoscopy—even before the standard age of 20–25 years in selected high-risk children as Barnard [10] reported.
5. Colonic Histology and Management Implications
All seven patients had colonic polyps, with varying dysplasia. Three showed low-grade, three moderate, and one (Turcot variant) high-grade dysplasia with focal adenocarcinoma. This spectrum highlights the importance of early surveillance to guide timely prophylactic colectomy, typically recommended in adolescence or before dysplasia progresses to carcinoma. Hyer et al. [1] and Attard et al. [2] reported that particularly, APC codon 1309 mutations are associated with aggressive disease and may warrant earlier surgical intervention.
Overall, our findings underscore the heterogeneity of FAP in pediatric patients and the importance of tailored surveillance strategies based on genetic findings, family history, and endoscopic phenotype. Genetic counselling and regular endoscopic follow-up remain central to early detection and cancer prevention in this high-risk group.
6. Limitations
This study is limited by the small sample size and single-centre design. Additionally, not all patients underwent comprehensive genetic analysis, such as MLPA or next-generation sequencing, which may have missed some mutations. Further studies involving larger cohorts are needed to better characterise genotype-phenotype correlations and long-term outcomes.
7. Conclusions
Familial adenomatous polyposis (FAP) in children presents a unique clinical challenge due to its silent onset, variable phenotype, and inevitable progression to colorectal cancer if left untreated. Our case series highlights the importance of early identification through family screening, even in asymptomatic children, and supports the value of genetic testing for precise risk stratification.
The correlation observed between specific APC mutations and clinical severity—particularly the more aggressive presentation associated with the p.Ser1213X variant—emphasizes the role of genotype-phenotype analysis in guiding management. The presence of high-grade dysplasia and even early carcinoma in adolescence further underscores the need for early and regular endoscopic surveillance and consideration of timely prophylactic surgery. While gastric involvement was minimal in our cohort, it reinforces that upper GI screening should not be delayed in select high-risk patients. Ultimately, a multidisciplinary approach, including gastroenterology, genetics, surgery, and oncology, is essential for optimal long-term outcomes in pediatric FAP patients.
Larger prospective studies are needed to validate these findings and refine screening and management guidelines tailored to pediatric populations.
Acknowledgments:
We thank doctors: Prof. Antigoni Siamopoulou – Mavridou+ MD, PhD, and Prof Epameinondas Tsianos MD, PhD for their valuable contribution in the management of these cases.
Authors contribution:
Dr Rogalidou is the first author and wrote the paper and interpreted the data. Dr Rogalidou, is the Pediatric Gastroenterologist responsible for the patients. Dr Batistatou is the pathologist who interpreted the biopsies. Prof Tzoufi, Prof. Katsanos, Prof. Christodoulou and Prof Siomou assisted in editing the draft. All authors have participated in the concept and design, analysis and interpretation of data, drafting or revising of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding Source:
No external funding for this manuscript
Financial Disclosure:
All authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest:
All authors have indicated they have no potential conflicts of interest to disclose.
References
- Hyer W, Cohen S, Attard T, et al. Management of Familial Adenomatous Polyposis in Children and Adolescents: Position Paper from the ESPGHAN Polyposis Working Group. JPGN 68 (2019): 428-441
- Attard TM, Tajouri T, Peterson KD, et al. Familial Adenomatous Polyposis in Children Younger than Age Ten Years: A Multidisciplinary Clinic Experience. Dis Colon Rectum 51 (2008): 207-212.
- Alkhouri N, Franciosi JP, Mamula P. Familial Adenomatous Polyposis in Children and Adolescents. JPGN 51 (2010): 727-732
- Munck A, Gargouri L, Alberti C, et al. Evaluation of Guidelines for Management of Familial Adenomatous Polyposis in a Multicenter Pediatric Cohort. JPGN 53 (2011): 296-302
- Attard TM, Cuffari C, Tajouri T, et al. Multicenter Experience with Upper Gastrointestinal Polyps in Pediatric Patients with Familial Adenomatous Polyposis. Am J Gastroenterol 99 (2004): 681-686.
- Campos FG, Habr-Gama A, Kiss D, et al. Extracolonic Manifestations of Familial Adenomatous Polyposis: Incidence and Impact on the Disease Outcome. Arq Gastroenterol 40 (2003): 92-98.
- Kennedy R, Potter D, Moir C, et al. The Natural History of Familial Adenomatous Polyposis Syndrome: A 24-Year Review of a Single Center Experience in Screening, Diagnosis, and Outcomes. J Pediatr Surg 49 (2014): 82-86.
- Galiatsatos P, Foulkes WD. Familial Adenomatous Polyposis. Am J Gastroenterol 101 (2006): 385-398.
- Cazorla A, Viennet G, Uro-Coste E, et al. Mucoepidermoid Carcinoma: A Yet Unreported Cancer Associated with Familial Adenomatous Polyposis. J Craniomaxillofac Surg 42 (2014): 262-264.
- Barnard J. Screening and Surveillance Recommendations for Pediatric Gastrointestinal Polyposis Syndromes. JPGN 48 (2009): S75-S78.
- Stone J, El-Matary W. Early-Onset Gastric Polyps in Children with Familial Adenomatous Polyposis: A Case Series. J Can Assoc Gastroenterol 3 (2020): 102-103.