False Positive Tuberculosis Cases (Xpert MTB/RIF Assay) Among People Living With HIV Attending Bahati Comprehensive Care Centre Nairobi, Kenya
Article Information
Lucy Obonyo Nyang’au1*, Evans Amukoye2, Stanley Kangethe3, Jackson Onyuka4
1Mount Kenya University, Thika, Nairobi, Kenya
2Kenya Medical Research Institute, Nairobi, Kenya
3Mount Kenya University, Thika, Nairobi, Kenya
4Mount Kenya University, Thika, Nairobi, Kenya
*Corresponding Author: Lucy Obonyo Nyang’au, Department of Medical Laboratory Sciences, Mount Kenya University, P.O Box 342-01000, Thika, Kenya
Received: 06 April 2020; Accepted: 16 April 2020; Published: 24 April 2020
Citation: Lucy Obonyo Nyang’au, Evans Amukoye, Stanley Kangethe, Jackson Onyuka. False Positive Tuberculosis Cases (Xpert MTB/RIF Assay) Among People Living With HIV Attending Bahati Comprehensive Care Centre Nairobi, Kenya. Archives of Microbiology & Immunology 4 (2020): 57-65.
View / Download Pdf Share at FacebookAbstract
Abstract
The introduction of GeneXpert MTB/RIF assay has impacted positively in tuberculosis diagnosis, providing a rapid way of identifying tuberculosis patients in high burden, low income countries. However Mycobacterium tuberculosis (MTB) detection in previously treated patients, which may be due to old deoxyribonucleic acid or active disease, still remains a diagnostic dilemma for diagnosis of tuberculosis. A retrospective cohort study was conducted among consenting patients with signs and symptoms of tuberculosis attending Bahati comprehensive care centers. A total of three hundred and forty six patients were sampled and their sputa collected, subsequently laboratory analysis was carried out for detection and culture of Mycobacterium tuberculosis. Seventy seven (22%) sputa had Mycobacterium tuberculosis detected on Xpert MTB/RIF assay sputa from these patients with bacteriologically confirmed pulmonary tuberculosis were subjected to culture on Mycobacterium Growth Indicator Tube (MGIT) media. Detection of Mycobacterium tuberculosis on Xpert MTB/RIF assay with no isolation of growth on culture indicated a false positive tuberculosis diagnosis. Out of 77 isolates subjected for culture a total of 0(0%) and 5(7.5%); P=0.484, isoniazid preventive therapy and non- isoniazid preventive therapy patients had false positive tuberculosis cases, while 0(0%) and 5(25%); P=0.001 new and retreatment patient’s had false positive tuberculosis. Our study concluded that there was no significant association between isoniazid preventive therapy and tuberculosis false positivity but there was significant association between patient treatment status and tuberculosis false positivity. Previously treated tuberculosis patients were significantly associated with false positivity, this call for clinicians to exercise caution when interpreting results from previously treated tuberculosis patients.
Keywords
False positive tuberculosis; GeneXpert MTB/RIF assay; Mycobacterium tuberculosis
False positive tuberculosis articles, GeneXpert MTB/RIF assay articles, Mycobacterium tuberculosis articles
Article Details
1. Introduction
Tuberculosis is one of the top ten causes of death globally and the leading cause of death from a single infectious agent (ranking above HIV/AIDS) [1]. Despite declining global incidence and mortality tuberculosis (TB) remains a major challenge worldwide, an estimated 10.0 million people fell ill with TB in 2018, with estimated 1.2 million TB deaths among HIV negative people and an additional 2,51,000 deaths among HIV positive people were recorded respectively [1]. TB affects people of all age groups and gender, the highest burden was in Men aged >15 years in 2018, they accounted for 57% of all TB cases, while Women accounted for 32% and children aged < 15 years accounted for 11% TB cases globally [1].
People living with HIV (PLHIV) accounted for 8.6% TB cases in 2018 [1]. HIV co-infection is associated with unusual presentations of TB such as smear negative and abnormal chest radiographs this causes a diagnostic challenge, poor treatment outcome and subsequent increased mortality [2]. Previous studies have shown that pauci-bacillary forms of TB are more commonly identified in patients who are HIV positive and these patients happen to be sputum smear negative, but because microscopy is less sensitive in these populations these groups are the ones most likely to benefit from Xpert MTB/RIF assay [2].
The introduction of GeneXpert MTB/RIF assay has contributed a huge positive impact in tuberculosis (TB) diagnosis, by providing a rapid way of identifying TB patients in high burden, low income countries [3]. The Xpert MTB/RIF assay is an automated cartridge based nucleic acid amplification test (NAAT) capable of simultaneously detecting Mycobacterium tuberculosis complex (MTBC) and Rifampicin (RIF) resistance within 2 hours; this assay was endorsed by the WHO in 2010, and approved by FDA in 2013, and it is regarded as a breakthrough in TB diagnostics [3]. The assay is performed on the Cepheid GeneXpert multi-disease instrument system which integrates sample purification, nucleic acid amplification, and detection of target sequences [3]. It uses hemi-nested real time PCR (polymerase chain reaction) for the detection of MTBC specific sequence of the rpoB gene and five molecular probes to detect mutations within the genes rifampicin resistance determining region (RRDR). The assay can be performed directly on raw sputum or concentrated sediments. Nevertheless post implementation studies have identified several challenges [4], emphasizing the need for deeper understanding of clinical and operational factors affecting performance.
2 Materials and Methods
Following approval by the ethics review committee of Mount Kenya University (Ref. No.MKU/ERC/1305) and research clearance by National Commission for Science Technology and Innovation (NACOSTI/P/19/13045/31000), 346 respondents were recruited for the study using cluster random sampling.
2.1 Study DesignRetrospective cohort study design was used where eligible HIV positive participants with or without use of isoniazid preventive therapy (IPT) were recruited through cluster random sampling, only those who gave consent were enrolled in the study.
2.2 Study SiteThe study was conducted in Makadara sub-county, Nairobi the capital city of Kenya. The sub-county covers an area of 13Km2 and comprises of five wards; Maringo, Hamza, Viwandani, Harambee, and Makongeni [5]. Viwandani ward is an informal settlement which is characterized by increased population. Tuberculosis being airborne, congestion especially in the houses facilitates increases in tuberculosis transmission.
2.3 Sample Size DeterminationData from National tuberculosis, leprosy and Lung disease program (NTLD-P) annual report (2017) for Nairobi county, a TB prevalence of 0.1% (147per 100,000) in HIV positive people was established. Using Habib et al., (2014) [6] formula and 0.1% as the working prevalence rate (P) for tuberculosis and assuming a standard error (Z) from the mean of 1.96 and in absolute precision (d) of 5%, and design effect (D) being taken as (2) sample size (n) was calculated as follows.
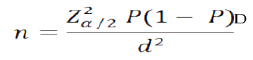
The estimated sample size was 276; it was adjusted to allow for attrition /refusals which was estimated for 20% [7] thus n= 276/ (1-0.2) =346 Clients.
2.4 Inclusion and Exclusion CriteriaPatients who were HIV positive, on care, above 15 years, with signs and symptoms of tuberculosis, one year post isoniazid preventive therapy (IPT), were included in the study upon consent. While patients with isoniazid preventive therapy and age records not clear, less than one year post isoniazid preventive therapy, unable to consent, with other samples other than sputum were excluded from the study.
2.5 Laboratory Methods 2.5.1 Identification of Mycobacterium tuberculosisUsing the geneXpert MTB/RIF assay sputa and the reagent buffer were mixed according to the standard operating procedure and loaded into the Xpert MTB/RIF assay cartridge and test started on Xpert MTB/RIF assay machine platform [8].
2.6 Culture of Mycobacterium tuberculosisMycobacterium tuberculosis (MTB) culture was performed using non-radiometric method Mycobacterium Growth Indicator Tube (MGIT) BACTEC 960. Sputa decontamination was performed using sodium hydroxide solution (40% w/v) combined with 2.9% sodium citrate solution and N-acetyl-L-cystein (NALC) powder [9]. Sterile phosphate buffer was added and the organisms concentrated by centrifugation at 3,000 rpm for 15 minutes. The supernatant was decanted and the sediment suspended with phosphate buffer and inoculated in liquid MGIT media and incubated along with negative control (un-inoculated MGIT media) and positive control (H37Rv ATCC 27294) [9]. The MGIT tubes were incubated in the BACTEC MGIT 960 machine at 37oC until the instrument flagged them positive. After a maximum of six weeks the instrument flagged the tubes negative, if there was no growth at 37oC [10-13]. Confirmative identification of MTB was done using BD MGIT TBc, on all positive cultures. Positive culture for MTB confirmed diagnosis of active disease.
3. Data management and Statistical Analysis
Data was tabulated in a computer database designed using MS-Access, and then transferred to statistical package for the social sciences (SPSS) version 20.0 for analysis. Pearson’s Chi-square test was applied to determine the differences in proportion for both groups in isoniazid preventive therapy (IPT) status, type of patient and gender against the detection of Mycobacterium tuberculosis (MTB). Pearson’s Chi-square test was applied to determine the differences in proportion for both groups in isoniazid preventive therapy (IPT) status, type of patients and demographics against TB and TB false positives. While Fisher’s exact test was applied to determine the difference in proportions among the MTB detection levels in GeneXpert MTB/RIF assay and among the age groups. These results were presented by appropriate tabulations based on the determined variables, odds ratio (OR) with 95% confidence interval (CI) and the corresponding p values. The threshold for statistical significance was set at P≤ 0.05.
4. Results
Prevalence of Tuberculosis false positives in relation to Isoniazid Preventive Therapy and Treatment status
Of the 77 Mycobacterium Tuberculosis (MTB) positive Xpert MTB/RIF assay samples subjected to culture 5(7.5%) and 0(0%) were false positive for tuberculosis (TB) among the non-isoniazid preventive therapy and isoniazid preventive therapy arms respectively, P=0.484 while on the other hand 0(0%) and 5(25%) were false positive for TB among new and retreatment patients P= 0.001 (Table 1). This indicates that there was no significant association between isoniazid preventive therapy and TB false positivity, while on the other hand there was significant association between TB false positivity and retreatment patients. Further study findings indicated that 10(100%) and 62(92.5%) were true Xpert MTB/RIF assay TB results in isoniazid preventive therapy and Non- isoniazid preventive therapy patients respectively (Table 1). While 57(100%) and 15(75%) were true Xpert MTB/RIF assay TB results among new and retreatment TB patients respectively (Table 1). The true results were concordant in Xpert MTB/RIF assay and BACTEC MGIT 960 culture results while the false positive cases had discordant results in the two methods. Culture was taken as the reference standard.
Table 1: False positive tuberculosis cases among study patients|
Variables |
Total (N) |
True Xpert results n (%) |
False positives n (%) |
OR (95% CI) |
P Value |
|
MTB+ve/Growth |
MTB+ve /No Growth |
||||
|
IPT status |
|||||
|
IPT patients |
10 |
10 (100) |
0 (0) |
UD |
0.484 |
|
Non-IPT patients |
67 |
62 (92.5) |
5 (7.5) |
||
|
Type of patients |
|||||
|
New patients |
57 |
57 (100) |
0 (0) |
UD |
0.001 |
|
RT patients |
20 |
15 (75) |
5 (25) |
||
|
MTB detection levels |
|||||
|
MTB detected high |
26 |
25 (96.2) |
1 (3.8) |
||
|
MTB detected medium |
45 |
41 (91.1) |
4 (8.9) |
0.690 (0.205-1.226) |
0.999 |
|
MTB detected low |
4 |
4 (100) |
0 (0) |
UD |
0.895 |
|
MTB detected very low |
2 |
2(100) |
0 (0) |
UD |
0.999 |
|
Gender |
|
|
|
|
|
|
Female |
30 |
28 (93.3) |
2 (6.7) |
0.92 (0.144-5.867) |
0.652 |
|
Male |
47 |
44 (93.6) |
3 (6.4) |
||
|
Age (years) |
|
|
|
|
|
|
< 20 |
4 |
4 (100) |
0 (0) |
||
|
20 – 39 |
47 |
42 (89.4) |
5 (10.6) |
0.111 (0.09-1.271) |
0.388 |
|
40 – 59 |
23 |
23 (100) |
0 (0) |
UD |
0.999 |
|
60 + |
3 |
3 (100) |
0 (0) |
UD |
0.999 |
Key: MTB: Mycobacterium Tuberculosis; +Ve: Positive; IPT: Isoniazid Preventive Therapy; RT: Retreatment; OR: Odds Ratio; C.I: Confidence Interval
Further the findings revealed that there was no significant difference between TB false positivity and the Mycobacterium tuberculosis detection levels (high, medium, low and very low) gender and age of the patients (Table 1). In all age categories of the isoniazid preventive therapy arm there were 0(0%) false positive TB cases in males and females respectively, the study further revealed that there were 3(4.5%) and 2(3%) false positive TB cases in male and female patients from the non-isoniazid preventive therapy arm respectively, in the age category (20-39) (Table 2). This indicates the age category 20-39 was more prone to false positive TB cases, and males had higher rate of false positivity than females having 4.5% and 3% respectively.
Table 2: Distribution of false positives tuberculosis Cases among study patients
|
True Xpert MTB/RIF assay Positive results; |
False Xpert MTB/RIF assay positive results; |
Total |
||||
|
IPT status |
Gender |
MTB+ve /Growth |
MTB+ve /No Growth |
|||
|
IPT patients |
Male |
Age (Years) |
60+ |
1(10%) |
0(0%) |
1 |
|
40 – 59 |
2(20%) |
0(0%) |
2 |
|||
|
20 – 39 |
3(30%) |
0(0%) |
3 |
|||
|
Total |
6(60%) |
0(0%) |
6 |
|||
|
Female |
Age (Years) |
60+ |
1(10%) |
0(0%) |
1 |
|
|
40 – 59 |
1(10%) |
0(0%) |
1 |
|||
|
20 – 39 |
1(10%) |
0(0%) |
1 |
|||
|
< 20 |
1(10%) |
0(0%) |
1 |
|||
|
Total |
4(40%) |
0(0%) |
4 |
|||
|
Total |
Age (Years) |
60+ |
2(20%) |
0(0%) |
2 |
|
|
40 – 59 |
3(30%) |
0(0%) |
3 |
|||
|
20 – 39 |
4(40%) |
0(0%) |
4 |
|||
|
< 20 |
1(10%) |
0(0%) |
1 |
|||
|
Total |
10(100%) |
0(0%) |
10 |
|||
|
Non IPT patients |
Male |
Age (Years) |
60+ |
1(1.5%) |
0(0%) |
1 |
|
40 – 59 |
12(17.9%) |
0(0%) |
12 |
|||
|
20 – 39 |
24(35.8%) |
3(4.5%) |
27 |
|||
|
< 20 |
1(1.5%) |
0(0%) |
1 |
|||
|
Total |
38(56.7%) |
3(4.5%) |
41 |
|||
|
Female |
Age (Years) |
40 – 59 |
8(11.9%) |
0(0%) |
8 |
|
|
20 – 39 |
14(20.9%) |
2(3%) |
16 |
|||
|
< 20 |
2(3%) |
0(0%) |
2 |
|||
|
Total |
24(35.8%) |
2(3%) |
26 |
|||
|
Total |
Age (Years) |
60+ |
1(1.5%) |
0(0%) |
1 |
|
|
40 – 59 |
20(29.9%) |
0(0%) |
20 |
|||
|
20 – 39 |
38(56.7%) |
5(7.5%) |
43 |
|||
|
< 20 |
3(4.5%) |
0(0%) |
3 |
|||
|
Total |
62(92.5%) |
5(7.5%) |
67 |
|||
|
Total |
72 |
5 |
77 |
|||
Key: IPT: Isoniazid Preventive Therapy; +Ve: Positive; MTB: Mycobacterium Tuberculosis
Discussion
Xpert MTB/RIF assay is a molecular technique widely used currently all over the World for diagnosis of active tuberculosis (TB) [1]. This assay’s positivity for Mycobacterium tuberculosis deoxyribonucleic acid (DNA) can remain detected for years after treatment in the absence of viable organisms for culture [4]. Understanding these Xpert false positive results is of paramount importance given the large global burden of symptomatic patients who present in health institutions for investigation of active TB but have previously been treated for active TB disease [4]. The current study revealed that 25% (P=0.001) false positive TB cases (MTB detection on Xpert MTB/RIF assay and negative result on BACTEC MGIT 960 culture) among previously treated TB patients, while there were 0(0%) false positive TB cases among the new patients. These study findings revealed significant association between TB false positivity and retreatment TB patients, but there was no significant association between TB false positivity and the IPT status of the patients, MTB detection levels, age and gender of the patients. These findings concur with previous studies where the rate of TB false positivity among retreatment patients was South Africa 14% [14] 7 % [4], Egypt 2% [15], India 5.3% [16] and China 0.8% [17].
The current study findings confirm with other previous studies that false positive TB cases are significantly associated with previously treated TB patients who had active TB disease. These findings can be attributed to the fact that Xpert MTB/RIF assay being a molecular technique cannot differentiate between viable and non-viable mycobacterial DNA, hence therefore the assay can detect DNA from dead bacilli of previously treated patients who had active TB disease [4]. Xpert MTB/RIF assay results of this nature can lead to baseless treatment of the patients, increase the health care costs and delay in reaching the correct diagnosis for the patients which can even lead to death [4]. In this regard patients started on medication based on wrong diagnosis due to false positive TB results, may end up dying because of wrong diagnosis, since the really problem remains unknown and untreated. This calls for clinicians to exercise caution when interpreting results from previously treated TB patients [4].
Further the current study findings revealed that there were 3(4.5%) false positive TB cases in male cases, in the age category (20-39). These findings concur with previous finding in Brazil where there was 73.6% false TB positivity among male patients [18]. These findings would be attributed to the fact that males in this age category (20-39), exhibit some characteristics which expose them to recurrent episodes of TB disease, making them vulnerable to false positivity because of harboring deoxyribonucleic acid from the previous episodes [18]. This characteristics include overcrowding since most of these people are found in institutions of learning, which are often crowded increasing transmission of this air borne disease, peer pressure which is common during this period leads them to engage in irresponsible behavior like drug and alcohol abuse coupled with the already depressed immunity due to HIV makes the body vulnerable to TB disease [18].
Conclusions
Xpert MTB/RIF assay positivity for Mycobacterial DNA can remain detected for years after treatment in the absence of viable organisms for culture [4]. Understanding such assay results is of paramount importance given the huge global burden of symptomatic patients who present for investigation of active TB and have history of previous active TB disease treatment. There was significant association between previously treated TB patients and TB false positivity. But TB false positivity was not significantly associated with the MTB detection levels, IPT status, gender or age of the patients. Clinicians should wait confirmatory testing in Xpert positive TB results for retreatment patients before commencing them on treatment, they should also take detailed history especially accurate classification of the patient and this will lead to proper patient management. GeneXpert should not be used to follow up patients who are on treatment, this is because the mycobacterial deoxyribonucleic acid will still be detected and the results will be positive for MTB detection, therefore smear microscopy still remains a major test in TB treatment follow up. The findings of this study have important policy implications which include the gaps in TB management guidelines and the need for revision and standardization to avoid exposing patients to unwarranted treatment.
Recommendation
Studies should be conducted to monitor Xpert MTB/RIF assay tuberculosis positive patients after treatment completion to ascertain duration of mycobacterial DNA survival, also clinicians should be very careful when dealing with retreatment patients to avoid baseless treatment which is not only expensive but toxic.
Acknowledgement
The authors owe special gratitude to the staffs of Central Reference Laboratory, Bahati Comprehensive Care Centre staffs and all those who directly or indirectly contributed to the success of this study.
Competing interests
The authors declare that they have no competing interests.
References
- World Health Organization. Global tuberculosis report 2019. Geneva (Switzerland): World Health Organization (2019).
- Rakha EB, Abdel Hakeem MA. GeneXpert MTB/RIF Assay: A Revolutionizing Method for Rapid Molecular Detection of Mycobacterium Tuberculosis in Comparison to Other Conventional Methods. Int. J. Curr. Microbiol. App. Sci 6 (2017): 2573-2580.
- Ocheretina O, Byrt E, Mabou MM, et al. False-positive rifampin resistant results with Xpert MTB/RIF version 4 assay in clinical samples with a low bacterial load. Diagnostic Microbiology and Infectious Disease 85 (2016): 53-55.
- Theron G, Venter R, Smith L, et al. False-positive Xpert MTB/RIF results in retested patients with previous tuberculosis: frequency, profile, and prospective clinical outcomes. Journal of Clinical Microbiology 56 (2018): e01696-17.
- Nairobi County. Nairobi County integrated Development Plan (2014).
- Habib A, Johargy A, Mahmood K, et al. Design and determination of the sample size in medical research. IOSR J Dent Med Sci (IOSR-JDMS) 13 (2014): 21-31.
- Sakpal T. Sample size estimation in clinical trial. Perspectives in Clinical Research 1 (2010): 67.
- Lee JJ, Suo J, Lin CB, et al. Comparative evaluation of the BACTEC MGIT 960 system with solid medium for isolation of mycobacteria. The International Journal of Tuberculosis and Lung Disease 7 (2003): 569-574.
- Yan JJ, Huang AH, Tsai SH, et al. Comparison of the MB/BacT and BACTEC MGIT 960 system for recovery of mycobacteria from clinical specimens. Diagnostic Microbiology and Infectious Disease 37 (2000): 25-30.
- Ng'ang'a ZW, Nyang’au LO, Amukoye E. First Line Anti-Tuberculosis Drug Resistance Among Human Immunodeficiency Virus Infected Patients Attending Maryland Comprehensive Care Centre Mathare 4a Nairobi Kenya.
- Aono A, Hirano K, Hamasaki S, et al. Evaluation of BACTEC MGIT 960 PZA medium for susceptibility testing of Mycobacterium tuberculosis to pyrazinamide (PZA): compared with the results of pyrazinamidase assay and Kyokuto PZA test. Diagnostic Microbiology and Infectious Disease 44 (2002): 347-352.
- Banaiee N, Bobadilla-del-Valle M, Riska PF, et al. Rapid identification and susceptibility testing of Mycobacterium tuberculosis from MGIT cultures with luciferase reporter mycobacteriophages. Journal of Medical Microbiology 52 (2003): 557-561.
- Pfyffer GE, Palicova F, Rusch-Gerdes S. Testing susceptibility of mycobacterium tuberculosis to Pyrazinamide with non-radiometric BACTEC MGIT 960 system. J Clin Microbiol 40 (2002): 1670-1674.
- Theron G, Venter R, Calligaro G, et al. Xpert MTB/RIF results in patients with previous tuberculosis: can we distinguish true from false positive results?. Clinical Infectious Diseases 62 (2016): 995-1001.
- Omar A, Elfadl AE, Ahmed Y, et al. Valuing the use of GeneXpert test as an unconventional approach to diagnose pulmonary tuberculosis. Egyptian Journal of Bronchology 13 (2019): 403.
- Agrawal M, Bajaj A, Bhatia V, et al. Comparative study of GeneXpert with ZN stain and culture in samples of suspected pulmonary tuberculosis. Journal of Clinical and Diagnostic Research (JCDR) 10 (2016): DC09.
- Tang T, Liu F, Lu X, et al. Evaluation of GeneXpert MTB/RIF for detecting Mycobacterium tuberculosis in a hospital in China. Journal of International Medical Research 45 (2017): 816-822.
- Fernandes P, Ma Y, Gaeddert M, et al. Sex and age differences in Mycobacterium tuberculosis infection in Brazil. Epidemiology & Infection 146 (2018): 1503-1510.
