Factors Associated with Liver Injury After Intravenous Gamma Globulin Treatment in Children with Kawasaki Disease
Article Information
Zhipeng Xu1, Huan Yu2, Biyao Hang2, Yuhan Xia2, Jie Li2, Jianjie Wang2, Qihao Zhang2, Xiaoshan Zhao2, Yue Ren2* and Maoping Chu2#
1Hangzhou Fuyang Women and Children hospital, hangzhou,311400, Zhejiang, China and Children’s Heart Center, The Second Affiliated Hospital and Yuying Children’s Hospital, Institute of Cardiovascular Development and Translational Medicine, and Wenzhou Medical University, Wenzhou, 325000 Zhejiang, China
2Children’s Heart Center, The Second Affiliated Hospital and Yuying Children’s Hospital, Institute of Cardiovascular Development and Translational Medicine, and Wenzhou Medical University, Wenzhou, 325000 Zhejiang, China
*Corresponding Author: Yue Ren, Children’s Heart Center, The Second Affiliated Hospital and Yuying Children’s Hospital, Institute of Cardiovascular Development and Translational Medicine, and Wenzhou Medical University, Wenzhou, 325000 Zhejiang, China
#Co-corresponding author: Maoping Chu, Children’s Heart Center, The Second Affiliated Hospital and Yuying Children’s Hospital, Institute of Cardiovascular Development and Translational Medicine, and Wenzhou Medical University, Wenzhou, 325000 Zhejiang, China
Received: 26 October 2022; Accepted: 02 November 2022; Published: 08 December 2022
Citation: Zhipeng Xu, Huan Yu, Biyao Hang, Yuhan Xia, Jie Li, Jianjie Wang, Qihao Zhang, Xiaoshan Zhao, Yue Ren and Maoping Chu. Factors Associated with Liver Injury After Intravenous Gamma Globulin Treatment in Children with Kawasaki Disease. Archives of Internal Medicine Research 5 (2022): 504-507
View / Download Pdf Share at FacebookAbstract
Background: The etiology of liver injury in children with Kawasaki disease (KD) is not yet clear. It is common for children who are responded to intravenous gamma globulin (IVIG) therapy to develop liver injury after IVIG treatment. This research is to explore related factors of liver injury after IVIG treatment in children with KD who responded retrospectively to IVIG.
Methods: A total of 806 children with KD were included in this analysis. The clinical characteristics, laboratory findings, and drug use before IVIG were collected. Difference analysis, ROC curve analysis and logistic regression analysis were performed to obtain possible risk factors for liver injury after IVIG treatment.
Results: Among the clinical symptoms of the two groups of children, children with lymphadenopathy had a lower risk of developing liver injury after IVIG treatment (p=0.040), while there were no significant differences in other symptoms. Among laboratory indicators, the liver injury group had higher levels of platelet (PLT), eosinophil (EO) and brain natriuretic peptide (BNP) levels and lower hemoglobin (HB),erythrocyte sedimentation rate(ESR) and prothrombin time (PT) levels before IVIG treatment (p<0.05). There were no significant difference in c-reactive protein (CRP) and Procalcitonin (PCT) (p>0.05). The use of antibiotics, dipyridamole and aspirin doses between two groups had statistically significant differences (p>0.05). Further ROC curve analysis of aspirin dose found the optimal cut-off point of aspirin was 34.7 mg/(k*d) (the 95% CI: 0.504-0.601, p=0.026). The logistic regression analysis showed highdose aspirin (≥34.7mg/(kg*d)) was a risk factor for liver damage after IVIG treatment in KD children. Further multivariate regression analysis prompted that the use of antibiotics and higher doses of aspirin (≥34
Keywords
Kawasaki disease, liver injury, aspirin, antibiotics
Kawasaki disease articles, liver injury articles, aspirin articles, antibiotics articles
Article Details
Background
Kawasaki disease (KD), also known as mucocutameous lymph node syndrome (MCLS), is an acute febrile rash disease that mainly occurs in infants under 5 years of age. Since it was first reported by Mr. Tomisaku Kawasaki in Japan in 1967, KD has been studied all over the world. And as the incidence rate is increasing year by year, KD has become the most common cause of acquired heart disease in developed countries and even in China.
With immune-mediated systemic small-sized and medium-sized vasculitis as the main pathological change, KD can lead to multiple organ dysfunctions, among which coronary complications are the most common. However, in recent years, non-cardiovascular complications have gradually been paid attention to, among which liver damage is the most prominent. In 2011, Mohammed et al [1] summarized the case characteristics of 240 children with KD who were admitted to the Children's Hospital of Denver, Colorado, USA from 2004 to 2009. Their research showed that 109 patients (45.4%) had abnormitality in at least one liver function index. Among them, 81.7% of children had elevated alanine aminotransferase (ALT), only 37.6% had abnormal bilirubin. Most cases showed mild elevations of liver function indexs, but 10% had ALT elevations greater than 5-fold upper limit of normal (ULN).The laboratory features of hepatic impairment in KD were described for the first time in this study, but the hepatic outcome was not further analyzed statistically. With the gradual deepening understanding of liver function damage in children with KD, more and more researchers have begun to pay attention to this aspect. As far as we know, elevated liver enzymes are the main manifestations of liver injury in children with KD.
Among them, elevated ALT, aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GT) are the most common. Most of them can return to normal quickly, [2] only a small number of children may develop serious complications related to liver damage, including hydrocele, acute cholecystitis, acute cholangitis, enterocolitis, hepatitis, and even acute liver failure. [3-8]
At present, most clinical studies only collect cases of liver damage in the acute phase for research, and tend to use liver function indicators as Independent predictors for the construction of predictive models for immune globulin resistance or coronary artery damage. [9, 10]
However, in clinical work, we found that it is not uncommon for liver damage to appear in the subacute stage or even recovery period of KD. In a retrospective analysis of 210 children with KD, [10] up to 191 cases (90.95%) had liver damage, and 12 cases (5.71%) of them developed liver damage after IVIG treatment.
In 2021, Italian scholars also reported a case of a 4-month-old boy who received IVIG combined with high-dose aspirin (80 mg/(kg*d)) in the acute phase after the diagnosis of KD; The aspirin dose was reduced to 3mg/(kg*d) 48 hours after the fever subsided, and taken orally for 8 weeks; At that time, the clinical outcome was good, and there was no coronary dilatation; However, at the 9th week of follow-up, the child had an increase in ALT, with a peak of 240IU/L, and it returned to normal after 7 months.[11]
In order to further seek out the features of these patients, this study collected 806 children with KD who admitted to our hospital from January 1, 2015 to December 31, 2019. We retrospectively analyzed their clinical case data and laboratory test results, used the most common indicator of liver damage, ALT, as the breakthrough point, and perfomed difference analysis, univariate regression analysis and multivariate logistic regression analysis on each index.
Methodology
Patients
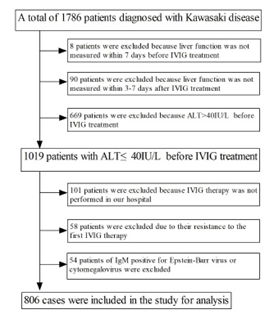
We collected 806 children admitted to the Second Affiliated Hospital of Wenzhou Medical University who were diagnosed with KD from January 1, 2015 to December 31, 2019. Due to missing resulting of liver function test within 7 days before IVIG treatment and 3-7 days after IVIG treatment, 98 patients were excluded. And 101 patients who did not use IVIG treatment in our hospital were excluded. For children who did not respond to initial IVIG treatment, considering more influencing factors would affect the liver function results after the second IVIG treatment, they were also excluded in this study. In addition, considering the influence of hepatotropic virus and non-hepatotropic virus on liver function, 54 cases of IgM positive for Epstein-Barr virus or cytomegalovirus were excluded (Figure 1).
Patients were divided into two groups according to whether ALT was greater than 40 IU/L after IVIG treatment: the liver injury group as the case group (ALT>40 IU/L after IVIG treatment) and the no liver injury group as the control group (ALT≤40IU/L after IVIG treatment).
Data collection
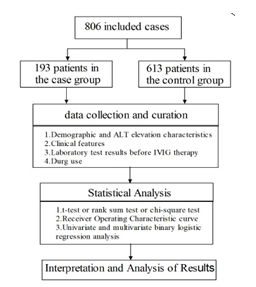
We collected the demographic and clinical characteristics, results of laboratory examinations and drug use of the selected children (Figure 2).
- Demographic and clinical characteristics: including age of onset, gender, weight, classic KD, recurrence of KD, and related clinical symptoms (fever, rash, conjunctivitis, oral changes, cervical lymphadenopathy, swelling and peeling of fingers and toes, scarring and swelling), PPD results.
- Laboratory examinations: The liver function results of the children within 7 days before IVIG treatment and 3-7 days after IVIG treatment were collected. And the laboratory test results of the two groups of children within 3 days before IVIG treatment were collected, including WBC, Hb, PLT, Anc, Eo, CRP, ESR, PT, activated partial thromboplastin time (APTT), fibrinogen (Fib), thrombin time (TT), D-dimer (D2), BNP, PCT.
- Drug use: The drug use of all children before IVIG treatment were collected, including hormones, antibiotics, clopidogrel, dipyridamole, high-dose application of aspirin, and days from fever to IVIG therapy.
Relative diagnostic criteria
We mainly adopted the 2017 American Heart Association (AHA) Guidelines [12], including: Diagnostic criteria for classic KD, Diagnostic criteria for incomplete KD and Criteria for IVIG resistance. And the Criteria for liver function damage after IVIG treatment is as follows: liver function test should be carried out within 3-7 days after IVIG treatment; When ALT>40 IU/L, it is considered there is liver function damage.
Statistical methods
We mainly use SPSS version 26 for statistical analysis. Continuous variables are expressed as mean ± standard deviation or interquartile range, while categorical variables are expressed as numbers and percentage. For Continuous variables, t-test or rank-sum test are used to compare count data according to whether the data conformed to a normal distribution; for categorical variables, chi-square test, corrected chi-square or Fisher test were used. The correlation between quantitative data (aspirin dose (mg/(kg*d)) and liver function damage after IVIG treatment was further explored by ROC curve. Binary logistic regression analysis was used to evaluate the effects of the use of antibiotics, aspirin and dipyridamole on liver damage after IVIG treatment before and after adjustment for age, weight, and gender. Results were considered statistically significant when p value < 0.05.
Results
Demographic and ALT elevation characteristics
In the 806 children with KD in this study, there were 193 children in the liver injury group, with an age of 11.1 months (6.15 months-22.57 months), and a weight of 9.5 kg (8 kg -12 kg). And there were 613 children in the no liver injury group with an age of 24.1 months (13.00 months-39.80 months), and a weight of 12 kg (9.5 kg-14.5 kg). And there were significant differences in age and weight between the two groups (p<0.001). (Table 1)
In the liver injury group, 109 patients (56.5%) had significantly elevated ALT (>60 IU/L), most of which fluctuated within 200 IU/L,9 children had ALT rising to 500 IU/L or more, 3 cases were even as high as 1000 IU/L or more, and one 22-month-old boy even had ALT as high as 3419 IU/L. In addition, 123 patients had elevated AST (>40 IU/L), which was basically synchronous with ALT.
Considering the possibility of Reye Syndrome in children treated with aspirin, we discontinued aspirin therapy for children with ALT greater than 500 U/L, and changed to clopidogrel for antiplatelet aggregation. ALT returned to normal within 7 days after liver-protective symptomatic treatment in 95% of the children, but 7 cases of ALT elevation continued for 1 month, and one child returned to normal after 3 months. Fortunately, most of the above cases did not experience other complications related to liver damage, including hyperbilirubinemia, acute cholangitis, or acute liver failure.
|
Group |
Case Group |
Control Group |
X2-Value/Z-value |
p-Value |
|
N |
193 |
613 |
||
|
Sex |
1.057 |
0.304 |
||
|
Male |
121(62.7%) |
409(66.7%) |
||
|
Female |
72(37.3%) |
204(33.3%) |
||
|
Age (month) |
11.10 |
24.10 |
-7.64 |
<0.001 |
|
(6.15, 22.57) |
(13.00, 39.80) |
|||
|
Weight (Kg) |
9.50 |
12.00 |
-7.149 |
<0.001 |
|
(8.00, 12.00) |
(9.50, 14.50) |
|||
|
KD initial /relapse % |
||||
|
Initial |
188 (97.4%) |
593 (96.7%) |
- |
|
|
Relapse |
5 (2.6 %) |
20(3.3%) |
- |
|
|
Classic/Incomplete KD % 2.265 0.132 |
||||
|
Classic KD |
119(61.7%) |
414(67.5%) |
- |
|
|
Incomplete KD |
74(38.3%) |
199(32.5%) |
- |
|
|
Fever (%) |
193(100%) |
612(99.8%) |
0.000 |
1.000 |
|
Rash (%) |
141(73.1%) |
439(71.6%) |
0.151 |
0.697 |
|
Conjunctivitis (%) |
174(90.2%) |
542(88.4%) |
0.447 |
0.504 |
|
Oral change (%) |
172(89.1%) |
557(90.9%) |
0.518 |
0.472 |
|
Extremity Changes (%) |
119(61.7%) |
402(65.6%) |
0.987 |
0.320 |
|
Cervical |
64(33.2%) |
254(41.4%) |
4.208 |
0.040 |
|
Lymphadenopthy (%) |
||||
|
Erythema and Induration at BCG inoculation site (%) 0.704 0.703 |
||||
|
Positive |
8(4.1%) |
18(2.9%) |
- |
|
|
Negative |
161(83.4%) |
520(84.8) |
- |
|
|
Missing |
24(12.4%) |
75(12.2%) |
- |
|
|
Positive tuberculin test (%) |
41(21.2%) |
119(19.4) |
0.309 |
0.5878 |
|
Quantitative data (age, weight) are not normally distributed, they are displayed as median (interquartile range), and compared by rank sum test; qualitative data are displayed by number (percentage), according to the sample size and theoretical value, select the card square, corrected chi-square, or Fisher's exact test. |
||||
Table 1: General Conditions of children with Kawasaki disease before intravenous gamma globulin (IVIG) treatment in the two groups.
Clinical features
The difference of clinical features between the two groups had academic significance (p<0.05) was only in whether the lymph nodes were enlarged before treatment. The incidence of lymphadenopathy in the liver injury group were lower than that in the other group. In terms of whether it was complete KD or KD recurrence, whether there was fever, rash, conjunctival hyperemia, change of lips, swelling and peeling of fingers and toes, redness or swelling of stuck scars, or whether the result of PPD was positive, there were no significant differences between the two group (all p>0.05). (Table 1).
Laboratory test results before IVIG therapy
The liver injury group had higher levels of PLT, EO, and BNP, while the levels of HB, ESR, and PT before IVIG treatment were lower than those in the no liver injury group (p<0.05).There were no significant difference between the two groups in other laboratory indicators such as WBC, ANC, CRP, Na+, APTT, FIB, TT, D-dimer and PCT (all p>0.05). (Table 2).
|
Group |
Case Group (X±s)/[M(P25, P75] |
Control Group (X±s)/[M(P25, P75] |
t-Value/Z-value |
p-Value |
|
Wbc (10^9/l) |
15.93±0.37 |
15.62±0.23 |
-0.685 |
0.493 |
|
Hb (g/dl) |
110.46±0.84 |
112.51±0.42 |
2.314 |
0.021 |
|
Plt (10^9/l) |
413.36±10.73 |
387.54±5.35 |
-2.28 |
0.022 |
|
Anc (10^9/l) |
8.98 (6.69, 12.04) |
9.60 (7.25, 12.70) |
-1.733 |
0.083 |
|
Eo (10^9/l) |
0.35 (0.11, 0.70) |
0.26 (0.11, 0.52) |
-2.205 |
0.027 |
|
CRP (mg/dl) |
69.92 (4.41, 107.75) |
67.07 (36.45, 104.05) |
-0.709 |
0.478 |
|
ESR (mm/h) |
34.99±1.08 |
37.74±0.67 |
2.042 |
0.042 |
|
Na+ (mmol/l) |
135.89±0.17 |
135.93±0.10 |
0.218 |
0.828 |
|
PT (s) |
13.50 (13.00, 14.10) |
13.75 (13.20, 14.40) |
-2.902 |
0.004 |
|
APTT (s) |
44.18±0.50 |
44.52±0.27 |
0.606 |
0.545 |
|
FIB (g/l) |
6.04(5.36, 5.84) |
6.20(5.41, 7.05) |
-0.794 |
0.427 |
|
TT(s) |
14.70(14.20, 15.30) |
14.70 (14.30, 15.30) |
-2.073 |
0.785 |
|
D2 (ug/ml) |
1.25 (0.77, 1.80) |
1.25(0.80, 1.90) |
-0.604 |
0.546 |
|
BNP(pg/ml) |
556.00 (242.00,1560.00) |
441.00 (197.00, 1090.00) |
-2.356 |
0.018 |
|
PCT (ng/ml) |
0.23 (0.13, 0.47) |
0.27 (0.15, 0.51) |
-0.904 |
0.366 |
|
Quantitative data are presented as mean (standard deviation) or median (interquartile range) and compared by t-test or rank-sum test. |
||||
Table 2: Comparison of Laboratory Test results before IVIG treatment in patients with Kawasaki disease in two groups.
Drug use
- There were statistically significant differences between the two groups in whether use dipyridamole, antibiotics and high-dose aspirin (p<0.05).No significant difference esisted in whether use hormones or clopidogrel. And days from fever to IVIG treatment, days of high-dose aspirin use, and days × dose of high-dose aspirin use also did not show significant differences (p>0.05) (Table 3).
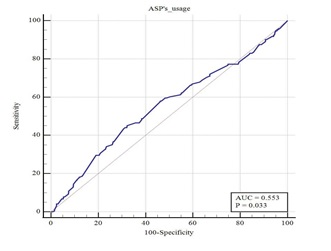
- Further ROC curve analysis was performed on the aspirin dose (mg/(kg*d)) (Figure 3), the area under the ROC curve was 0.553 (95% CI: 0.504-0.601, p=0.026) and the optimal cut-off point was 34.7 mg/(kg*d). We further divided two groups of aspirin ≥34.7mg/(kg*d)and aspirin<34.7 mg/(kg*d). And the univariate regression analysis showed that there was a statistically significant association between aspirin≥34.7 mg/(kg*d) and liver damage after IVIG treatment (unadjusted: OR=1.720, 95% CI: 1.236-2.393, p=0.001) (Table 4).
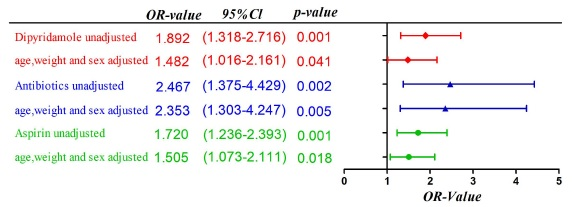
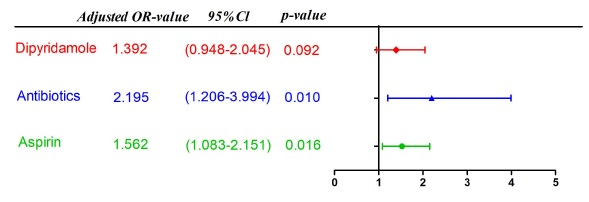
- The regression analysis results of further adjustment of age, weight and gender found that the adjustment factors have the greatest impact on the p-value of whether use dipyridamole; Logistic multivariate regression analysis showed that the use of antibiotics and the use of high-dose aspirin (≥34.7 mg/(kg*d)) were independent risk factors for liver damage after IVIG treatment (use of antibiotics: OR=2.195, 95% CI:1.206-3.994, p=0.01; aspirin≥34.7 mg/(kg*d):OR=1.526, 95% CI:1.083-2.151, p=0.016). Whether use dipyridamole was not an independent risk factor (OR=1.392, 95% CI:0.948-2.045, p=0.092). (Figure 4 and Figure 5).
|
Group |
Case Group |
Control Group |
X2-Value/Z-value |
p-Value |
|
Hormone (%) |
16 (8.3 %) |
54 (8.8 %) |
2.812 |
0.245 |
|
Clopidogrel (%) |
33 (17.1%) |
87 (14.2 %) |
0.978 |
0.323 |
|
Dipyridamole (%) |
62 (32.3%) |
123 (20.1 %) |
12.185 |
<0.001 |
|
Antibiotic (%) |
179 (92.7%) |
513 (83.8 %) |
9.681 |
0.002 |
|
IVIG for a new days after a fever |
6 (6,8) |
6 (5,7) |
-0.28 |
0.978 |
|
High dose aspirin use |
||||
|
Days of use |
4.00 (3.00, 4.00) |
4.00 (3.00, 5.00) |
-0.466 |
0.641 |
|
Dosage mg/ (kg d) |
33.33 (30.00, 37.50) |
32.26 (29.75, 36.00) |
-2.221 |
0.026 |
|
Use days*dose (mg/kg) |
138.16 (100.00, 169.84) |
131.25 (97.98, 163.33) |
-0.823 |
0.411 |
Table 3: Comparison of drug use before IVIG treatment in Kawasaki disease patients between the two groups.
|
Group |
Case Group |
Control Group |
p-Value |
OR-Value |
95 % Cl for OR value |
||
|
Lower limit |
Upper limit |
||||||
|
< 34.7 mg/kg/d* |
106 (%) |
415 (%) |
0.001 |
1.720 |
1.236 |
2.393 |
|
|
≥ 34.7 mg/kg/d |
87 (%) |
198 (%) |
|||||
Table 4: Comparison of the results of high dose aspirin use before IVIG in patients with Kawakasi disease between the two groups.
Discussion
As the incidence of KD increases year by year, scholars pay more and more attention to KD and its related complications. We studied much on its cardiovascular complications, especially coronary artery damage which is most common in KD, but studies on non-cardiovascular complications are relatively few. In 2011,American scholar Mohammed[1] conducted a retrospective analysis on KD complicated with liver damage for the first time; this study only described the clinical characteristics and briefly analyzed the relationship between liver damage and related inflammatory indicators. The duration or outcome of hepatic impairment was not described. In 2019, Japanese scholar Tomita analyzed the data of 381 children with KD admitted to Kobe Medical Center General Hospital from 1983 to 2001, and found that 199/381 cases (52.2%) had liver damage, among which ALT elevation was most notable. And most ALT value reached the peak 1-3 days after the disease onset, returned to normal within 15-17 days.[13]At present, most studies believe that KD complicated with liver damage is only a transient injury, and abnormal liver enzyme markers can be rapidly improved with the control of acute inflammation.[14]
However, with the deepening of people's understanding and follow-up of KD, we found that it is not rare that liver damage occurs in the subacute stage and even the recovery stage of the disease. According to a report at the 2014 China Conference, [15] 6.7% (13/195) of children had this condition. In our study, the incidence of liver damage after IVIG treatment of KD patients with effective IVIG treatment was 10.8% (193/1786). In some cases, the ALT value could even be as high as 3419 IU/L or more, and the liver damage in some cases could last for several months. The incidence of subacute liver damage in different centers varies greatly, and we consider that it may be related to the heterogeneity of genetic background or the different reference intervals of ALT among different centers. However, although the differences are large, the conclusions are similar. The incidence of liver damage in the subacute phase or convalescent phase of KD is not uncommon. So it is necessary enough for clinicians to pay more attention to those patients, and it may be a signal to adjust aspirin or other treatments in time to avoid the possibility of Reye syndrome or even acute liver failure.
So, what is the cause of liver damage in children with KD in the subacute stage? This study retrospectively analyzed a total of 1,786 children with KD who were admitted to our center from January 2015 to December 2019. After multiple screening, a total of 806 cases were finally included in the study. The differences in clinical characteristics and laboratory data between the two groups were analyzed and compared, and the correlations between these characteristics and liver damage after IVIG treatment were analyzed.
The difference analysis results showed that the liver injury group was younger in age and weight, less lymphadenopathy in the acute phase, and no significant difference in other clinical features. And the liver injury group had a younger age and weight. The first reason we consider is that when ALT>40 IU alone is used as the standard for liver injury, the differences in the basic values of different ages and genders influenced the result. According to the Reference interval of the first edition of children's clinical routine test index in my country in 2021 and related studies, [16] we can know that although the difference of median ALT value in children of different ages is not large, the younger the age, the larger the fluctuation range. This makes the liver injury group more likely to have ALT>40 IU/L during the course of the disease, but it is also more likely that the ALT value is still within the normal range.
Comparing the laboratory test indicators before IVIG treatment, we found the HB in the live injury group was lower than that in the other group (110.46±0.84 g/dL vs. 112.51±0.42 g/dL, p=0.021), suggesting the liver injury group was more prone to anemia. According to Ying-Hsien Huang and Ho-Chang Kuo et al., [17,18] KD children with anemia have a longer duration of active inflammation, which may be related to inflammation-related transient iron deficiency anemia, and hepcidin may play an important role in it. So the liver damage after IVIG treatment may be a show of a longer duration of active inflammation. And the difference of ESR in the two groups was not obvious (34.99±1.08 mm/h vs. 37.74±0.67 mm/h, p=0.042). As one of the inflammatory indicators, ESR was not like CRP, or PCT in this study, but it also suggested that the case group did not have a higher inflammatory response in the acute phase, which may be related to the detection time, and need to be further explored by further studying the ESR levels at different times in the course of KD.
At the same time, the platelet counts in the liver injury group before IVIG treatment was significantly higher than that in the control group (413.36±10.73×10^9/L vs. 387.54±5.35×10^9/L, p=0.022), indicating platelet activation early in KD are more prone to appear liver damage after IVIG treatment. According to the current researches, platelets, as a kind of multifunctional cells with inflammatory and coagulation functions, are characterized by the increase at 2-3 weeks in the natural course of KD. This is related to the activation of inflammatory cells mediated by abnormal immune complexes in KD lead to platelet recruitment.[19] Whether the higher platelet count in acute phase of the liver injury group in this study means this group of children had a stronger inflammatory response in the acute phase is not yet fully supported. At the same time, the analysis of this study found that the eosinophil counts in the case group were also significantly higher than that in the control group (0.35×10^9/L vs.0.26×10^9/L, p=0.027). According to current study, the mechanism of the increase of eosinophils in children with KD is not completely clear, and the accumulation of eosinophils in blood or tissues may reflect the host's immune response to KD-related antigens.[20] BNP is a peptide synthesized and secreted by myocardial cells after myocardial stimulation, such as pressure load, ischemia, and inflammatory stimulation. It can be used as one of the important markers to reflect myocardial injury. It is recommended as a predictor of incomplete KD and coronary artery damage, and indirectly reflects the inflammatory response of KD. [21, 22] In this study, we found that the BNP level in the acute phase of the liver injury group (556 pg/ml) was significantly higher than that of the control group (441 pg/ml), suggesting that the liver injury group might have a stronger inflammatory response in the acute phase of the disease.
But interestingly, we compared the two groups of CRP and PCT before IVIG treatment, which representing the body's inflammatory response, and found that there was no significant difference between the two groups (p>0.05). Most of the comparative analysis of acute liver function damage found that ALT elevation was positively correlated with CRP and PCT, and it was speculated that acute liver damage may be related to inflammatory mediators and infectious factors. In 1979, Amano et al. [23] studied the distribution of vascular lesions in 37 autopsy cases of KD and found that hepatic vasculitis developed in 6 of them. Some scholars speculate that cytokines in the acute phase of KD activate natural killer cells, which accumulate in the vascular endothelium and hepatic sinusoids and may be involved in hepatocyte injury and vascular endothelial cell injury.[24] Therefore, the liver damage in the acute phase of KD can reflect the severity of systemic inflammation to a certain extent. However, Japanese scholar Tomita put forward different opinions.[13] He compared the changes of liver function indicators in different stages of KD and found that for children with liver damage in the acute stage, the recovery of liver damage occurred at the initial stage of the disease. That is, the liver damage can be improved during the exacerbation of CRP, suggesting that the recovery of liver damage does not fully reflect the level of systemic inflammation. This is consistent with our findings, and also suggests that the liver function damage that occurs after IVIG treatment of children with KD may be not entirely a secondary complication of the systemic inflammatory state.
In terms of drug use, the rank sum test showed that the dose of aspirin used in the acute phase was associated with the occurrence of liver damage after IVIG treatment (p= 0.026).Further ROC curve analysis and univariate regression analysis prompted that Aspirin dose ≥34.7mg/(kg*d) was a risk factor for liver damage after IVIG treatment of KD (OR value:0.720, 95% CI: 1.236-2.393, p=0.001). According to the study of Zimmerman HJ et al.,[25] aspirin-use is dose-dependent with the occurrence of liver damage, and short-term use of low-dose aspirin causes liver damage is rare. Related reports suggest that high-dose aspirin (80-100 mg/(kg*d) in children with KD can lead to severe liver damage and even Reye syndrome. [26] In addition, there was no statistically significant difference between the two groups in the "high-dose-use dose × time" in this study, which may be because the liver injury group stopped aspirin treatment in time after the liver damage was found.
In addition, both univariate regression analysis and multivariate regression analysis showed the use of antibiotics in the acute phase of children with KD was a risk factor for liver damage after IVIG treatment of KD (Before adjustment: OR value: 2.467, 95% confidence interval: 1.375-4.492, p=0.002; After adjusted: OR: 2.353, 95% confidence interval: 1.303-4.247, p=0.005). It is worth noting that in this study, the rate of antibiotic use in the acute phase of children with KD in our hospital was as high as 85.8% (692/806). According to Seung Beom Han's report, [27, 28] in a university hospital in Seoul from 2015 to 2016, the antibiotic use rate in the acute phase of children with KD was 54.3%. For children with KD, the difficulty of early diagnosis of the disease, the markedly elevated blood counts, and persistent fever, as well as the significant increase in the incidence of incomplete KD in recent years, have forced clinicians to use antibiotics more. But antibiotics are not considered to be helpful in the treatment of KD, and even the ineffectiveness of antibiotic treatment is an important clue for clinicians to be alert to KD. Its use not only increases the risk of acute liver damage in children with KD, but also increases the incidence of liver damage after IVIG treatment after discontinuation of antibiotics. According to the report of Mitsuharu Fukazawa Jr. et al.,[29] this may be related to the disturbance of intestinal flora associated with antibiotic use and the increased liver burden caused by the metabolism of antibiotics in the liver.
In the rank sum test and univariate regression analysis of whether dipyridamole was used, the differences between the two groups were statistically significant; But after adjusting for age, weight, and gender, the OR value was significantly reduced and the p value significantly increased (Before adjustment: OR value: 1.892, 95% confidence interval: 1.318-2.716, p=0.001; After adjusted: OR: 1.482, 95% confidence interval: 1.016-2.161,p=0.041). After multivariate logistic regression analysis with antibiotics and aspirin, its p>0.05, suggesting that the use of dipyridamole is not an independent risk factor for liver damage after IVIG treatment in children with KD. According to current studies, dipyridamole, as a vasodilator and platelet aggregation inhibitor, may cause mild elevation of liver enzymes during use, but it is not associated with significant acute liver damage. [30] Moreover, according to the research of G Sansoè, T Ueda et al., the use of dipyridamole has a protective effect on the liver.[31]
This study is not without limitations. First, as a single-center retrospective study, this study has shorting comings such as selection bias and missing data, but our study also reflects the real clinical situation to a certain extent. In addition, we selectively defined liver damage as ALT > 40 IU/L, making the study likely to mask or exaggerate the role of some relevant factors. However, this choice is based on our summary of previous studies: according to most current research reports, when children with KD complicated with liver damage, ALT elevation is the main manifestation. In addition, the liver function results of 193 children in the liver function damage group summarized in this study showed that the proportion of liver damage with ALT combined with AST elevation as the main manifestation was as high as 90%, and the two elevations were basically synchronized, which was consistent with most studies.
In addition, our study did not include infection factors, including the detection of hepatotropic virus and non-hepatotropic virus. Considering most of related clinical tests were only performed in the case of severe liver damage, there are many data missing. And other site infections such as respiratory tract infection, urinary tract infection, etc. have not been studied and discussed in this study. Considering that the use of antibiotics in the early stage of the disease could not completely rule out co-infection, especially in children with relevant clinical symptoms; and our research subjects for KD children with effective IVIG treatment, these children had no fever after IVIG treatment, and the possibility of liver damage after IVIG treatment of KD caused by persistent infection is very small. Finally, we focused on the differences between the two groups of children before IVIG treatment, selectively ignoring the effect of gamma globulin itself on liver function in children with KD, laboratory indicators after IVIG treatment in the two groups of children, which to some extent limited the analysis of the study results.
Conclusion
For children with normal liver function in the acute phase, the younger the age of onset, the smaller the body weight, and the absence of lymphadenopathy in the acute phase, with elevated PLT, EO, BNP and reduced HB, ESR, PT in laboratory examinations in acute stage were more likely to develop subacute liver damage in KD children. There was no significant correlation between systemic inflammation (CRP and PCT levels) in children with KD in acute phase and liver damage after IVIG treatment; but a longer duration of active inflammation may be a very important cause of liver injury delayed. Applications of antibiotics and high-dose aspirin (≥34.7 mg/(kg*d)) in the acute phase of children with KD may be the risk factors for liver damage after IVIG treatment of children with KD.
Abbreviations
KD: Kawasaki disease; IVIG: Intravenous immunoglobulin; AHA: American heart association; ROC: Receiver Operating Characteristic; PLT: platelet; EO: eosinophiland; BNP: brain natriuretic peptide; HB: lower hemoglobin; ESR: erythrocyte sedimentation rate; PT: prothrombin time; CRP: c-reactive protein; PCT: Procalcitonin; PPD: Tuberculin purified protein derivative; APTT: activated partial thromboplastin time; Fib: fibrinogen; TT: thrombin time; D2: D-dimer; CI: Confidence interval; OR: Odds ratio.
Declaration
Ethics approval and consent to participate
The study was approved by the local ethics committee of The Second Affiliated Hospital and Yuying Children’s Hospital, China.
Written informed consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated during and analysed during the current study are not publicly available due to data protection but are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
The work was supported by the Natural Science Foundation of China (No.81970435), Zhejiang Provincial Science and Technology Project of Traditional Chinese Medicine (No. 2018ZZ019), and the Special Project for Significant New Drug Research and Development in the Major National Science and Technology Projects of China (No. 2020ZX09201002).
Authors’ contributions
All authors have made substantial contributions to (1) the conception and design of the study, acquisition of data, or analysis and interpretation of data; (2) drafting the article or critically revising it for important intellectual content; and (3) final approval of the submitted version of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
References
- Eladawy Mohammed, SR Dominguez, MS Anderson et al., Abnormal liver panel in acute kawasaki disease. Pediatr Infect Dis J, 30 (2011): 141-144.
- Tremoulet AH, S Jain, D Chandrasekar et al., Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J, 30 (2011): 1022-1026.
- Gear JH, KE Meyers and M Steele. Kawasaki disease manifesting with acute cholangitis. A case report. S Afr Med J, 81 (1992): 31-33.
- Lipe DN, LC Bridges. Kawasaki Disease Presenting as Acute Acalculous Cholecystitis. Clinical Practice and Cases in Emergency Medicine, 3 (2019): 383-386.
- Munro AR, SW Beasley, PK Pattemore et al., Fatal late onset necrotising enterocolitis in a term infant: Atypical Kawasaki disease or polyarteritis nodosa of infancy? J Paediatr Child Health, 39 (2003): 555-557.
- Ohnishi T, S Sato, K Kinoshita et al., A Case of Intravenous Immunoglobulin-Resistant Kawasaki Disease With Yersinia enterocolitica Enterocolitis Successfully Treated With Cefotaxime Following Infliximab and Cyclosporine. J Pediatric Infect Dis Soc, 10 (2021): 10: 225-226.
- Anjani G, R Deglurkar, RK Pilania et al., Fulminant acute liver failure as an unusual presentation of Kawasaki disease. Scand J Rheumatol, 50 (2021): 327-329.
- Mammadov G, HH Liu, WX Chen et al., Hepatic dysfunction secondary to Kawasaki disease: characteristics, etiology and predictive role in coronary artery abnormalities. Clin Exp Med, 20 (2020): 21-30.
- Liu L, W Yin, R Wang et al., The prognostic role of abnormal liver function in IVIG unresponsiveness in Kawasaki disease: a meta-analysis. Inflamm Res 65 (2016): 161-168.
- Hepatic dysfunction secondary to Kawasaki disease: characteristics, etiology and predictive role in coronary artery abnormalities. Clinical and Experimental Medicine, 20 (2020): 21-30.
- Paglia P, L Nazzaro AGE, De Anseris et al., Atypically Protracted Course of Liver Involvement in Kawasaki Disease. Case Report and Literature Review. Pediatr Rep, 13 (2021): 357-362.
- McCrindle BW, AH Rowley, JW Newburger et al., Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation, 135 (2017): e927-e999.
- Tomita Y, T Fukaya, Y Yamaura et al., Implications of hepatic dysfunction in Kawasaki disease: Time-related changes in aspartate aminotransferase, alanine aminotransferase, total bilirubin, and C-reactive protein levels. Pediatr Investig, 3 (2019): 19-26.
- Jang M, MS Oh, SC Oh et al., Distribution of Diseases Causing Liver Function Test Abnormality in Children and Natural Recovery Time of the Abnormal Liver Function. J Korean Med Sci, 31 (2016): 1784-1789.
- Yue Ren, Rongzhou Wu, Songyue Zhang. Analysis of Clinical Characteristics of Kawasaki Disease Children with Liver Function Impairment in Convalescent Period. The 2014 China Conference.
- Stirnadel-Farrant HA, N Galwey, C Bains et al., Children's liver chemistries vary with age and gender and require customized pediatric reference ranges. Regul Toxicol Pharmacol 73 (2015): 349-355.
- Huang YH, HC Kuo, FC Huang et al., Hepcidin-Induced Iron Deficiency Is Related to Transient Anemia and Hypoferremia in Kawasaki Disease Patients. Int J Mol Sci 17(2016).
- Huang YH, HC Kuo. Anemia in Kawasaki Disease: Hepcidin as a Potential Biomarker. Int J Mol Sci 18 (2017).
- Menikou S, PR Langford, M Levin. Kawasaki Disease: The Role of Immune ComplexesRevisited. Front Immunol, 10 (2019): 1156.
- Kuo HC, KD Yang, CD Liang et al., The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol, 18 (2007): 354-359.
- Zheng X, Y Zhang, L Liu et al., N-terminal pro-brain natriuretic peptide as a biomarker for predicting coronary artery lesion of Kawasaki disease. Sci Rep 10 (2020): 5130.
- Wu L, Y Chen, S Zhong et al., Blood N-terminal Pro-brain Natriuretic Peptide and Interleukin-17 for Distinguishing Incomplete Kawasaki Disease from Infectious Diseases. Indian Pediatr, 52 (2015): 477-480.
- Amano S, F Hazama, Y Hamashima. Pathology of Kawasaki disease: II. Distribution and incidence of the vascular lesions. Jpn Circ J, 43 (1979): 741-748.
- Seki S, Y Habu, T Kawamura et al., The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev, 174 (2000): 35-46.
- Zimmerman HJ. Effects of aspirin and acetaminophen on the liver. Arch Intern Med, 141 (1981): 333-342.
- Wei CM, HL Chen, PI Lee et al., Reye's syndrome developing in an infant on treatment of Kawasaki syndrome. J Paediatr Child Health, 41 (2005): 303-304.
- Lee JH, HY Hung, FY Huang. Kawasaki disease with Reye syndrome: report of one case. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 33 (1992): 67-71.
- Han SB, SY Lee. Antibiotic use in children with Kawasaki disease.

- Fukazawa M, M Fukazawa, E Nanishi et al., Previous antibiotic use and the development of Kawasaki disease: a matched pair case-control study. Pediatrics International, 62(2020).
- Kerndt CC, S Nagalli. Dipyridamole, in StatPearls. 2022: Treasure Island (FL).
- Sansoè G, A Ferrari, P D'Alimonte et al., Beneficial hemodynamic effects of dipyridamole on portal circulation in cirrhosis. Am J Gastroenterol, 93(1998): 429-433.