Extremely Low Frequency Magnetic Fields Increase the Expression of HIF-1α in Prostate Cancer Cells
Article Information
Sharon Amir1, Snir Dekalo1,2, Joseph Friedman3, Nicola J Mabjeesh1,4*
1Prostate Cancer Research Laboratory, Tel Aviv Medical Center, Tel Aviv, Israel
2Department of Urology, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
3Department of Biological Regulation, The Weizmann Institute of Science, Rehovot, Israel
4Department of Urology, Soroka University Medical Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel
*Corresponding Authors: Dr. Nicola J Mabjeesh, Department of Urology, Soroka University Medical Center, Beer-Sheva, POB 151, Israel
Received: 01 December 2020; Accepted: 30 December 2020; Published: 19 January 2021
Citation:
Sharon Amir, Snir Dekalo, Joseph Friedman, Nicola J Mabjeesh. Extremely Low Frequency Magnetic Fields Increase the Expression Of HIF-1 in Prostate Cancer Cells. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 094-105.
View / Download Pdf Share at FacebookAbstract
Background: Increased levels of hypoxia-inducible factor 1α (HIF-1α) are related to poor prognosis and treatment failure in prostate cancer. The effect of extremely low frequency magnetic fields (ELF-MF), generally emitted from electronic devices on HIF-1α in cancer cells and, specifically, in prostate cancer is not well described. This study aimed to investigate the effects of ELF-MF on HIF- 1α protein expression and HIF-1 transcriptional activity in prostate cancer cells.
Methods: Prostate cancer cells were irradiated with ELF-MF by means of a frequency generator at 875 MHz with an intensity of 0.07 mW/cm2 at various time periods and with an exposure system housed in a tissue culture incubator. The expression of HIF-1α protein and HIF-1 transcriptional activity at the different conditions were evaluated.
Results: HIF-1α expression in different prostate cancer cells was higher after one hour of exposure to ELF-MF. All exposed cells also demonstrated a significant 50% increase in HIF-1 transcriptional activity as measured by an HRE-dependent reporter gene assay after ELF-MF irradiation. Cells exposed to ELF-MF exhibited 3-fold longer HIF-1α half-life compared to non-exposed control cells, while its mRNA levels were not affected. Immunofluorescence staining confirmed nuclear HIF-1α accumulation following exposure to ELF-MF. Cell proliferation was increased following ELF-MF exposure and significantly reduced when HIF-1α was silenced even after exposure to ELF-MF.
Conclusions: Exposure of prostate cancer cells to ELF-MF increases HIF-1α protein stability and upregulates HIF-1 transcriptional activity. These findings provide new insights into the effects of ELF-MF on the hypoxic pathway which are important for guiding future implications.<
Keywords
Prostate cancer; Cancer; HIF-1; Extremely low frequency magnetic fields
Prostate cancer articles; Cancer articles; HIF-1 articles; Extremely low frequency magnetic fields articles
Prostate cancer articles Prostate cancer Research articles Prostate cancer review articles Prostate cancer PubMed articles Prostate cancer PubMed Central articles Prostate cancer 2023 articles Prostate cancer 2024 articles Prostate cancer Scopus articles Prostate cancer impact factor journals Prostate cancer Scopus journals Prostate cancer PubMed journals Prostate cancer medical journals Prostate cancer free journals Prostate cancer best journals Prostate cancer top journals Prostate cancer free medical journals Prostate cancer famous journals Prostate cancer Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals HIF-1 articles HIF-1 Research articles HIF-1 review articles HIF-1 PubMed articles HIF-1 PubMed Central articles HIF-1 2023 articles HIF-1 2024 articles HIF-1 Scopus articles HIF-1 impact factor journals HIF-1 Scopus journals HIF-1 PubMed journals HIF-1 medical journals HIF-1 free journals HIF-1 best journals HIF-1 top journals HIF-1 free medical journals HIF-1 famous journals HIF-1 Google Scholar indexed journals Extremely low frequency magnetic fields articles Extremely low frequency magnetic fields Research articles Extremely low frequency magnetic fields review articles Extremely low frequency magnetic fields PubMed articles Extremely low frequency magnetic fields PubMed Central articles Extremely low frequency magnetic fields 2023 articles Extremely low frequency magnetic fields 2024 articles Extremely low frequency magnetic fields Scopus articles Extremely low frequency magnetic fields impact factor journals Extremely low frequency magnetic fields Scopus journals Extremely low frequency magnetic fields PubMed journals Extremely low frequency magnetic fields medical journals Extremely low frequency magnetic fields free journals Extremely low frequency magnetic fields best journals Extremely low frequency magnetic fields top journals Extremely low frequency magnetic fields free medical journals Extremely low frequency magnetic fields famous journals Extremely low frequency magnetic fields Google Scholar indexed journals solid malignancy articles solid malignancy Research articles solid malignancy review articles solid malignancy PubMed articles solid malignancy PubMed Central articles solid malignancy 2023 articles solid malignancy 2024 articles solid malignancy Scopus articles solid malignancy impact factor journals solid malignancy Scopus journals solid malignancy PubMed journals solid malignancy medical journals solid malignancy free journals solid malignancy best journals solid malignancy top journals solid malignancy free medical journals solid malignancy famous journals solid malignancy Google Scholar indexed journals hypoxia-inducible articles hypoxia-inducible Research articles hypoxia-inducible review articles hypoxia-inducible PubMed articles hypoxia-inducible PubMed Central articles hypoxia-inducible 2023 articles hypoxia-inducible 2024 articles hypoxia-inducible Scopus articles hypoxia-inducible impact factor journals hypoxia-inducible Scopus journals hypoxia-inducible PubMed journals hypoxia-inducible medical journals hypoxia-inducible free journals hypoxia-inducible best journals hypoxia-inducible top journals hypoxia-inducible free medical journals hypoxia-inducible famous journals hypoxia-inducible Google Scholar indexed journals Immunofluorescence articles Immunofluorescence Research articles Immunofluorescence review articles Immunofluorescence PubMed articles Immunofluorescence PubMed Central articles Immunofluorescence 2023 articles Immunofluorescence 2024 articles Immunofluorescence Scopus articles Immunofluorescence impact factor journals Immunofluorescence Scopus journals Immunofluorescence PubMed journals Immunofluorescence medical journals Immunofluorescence free journals Immunofluorescence best journals Immunofluorescence top journals Immunofluorescence free medical journals Immunofluorescence famous journals Immunofluorescence Google Scholar indexed journals heterodimeric articles heterodimeric Research articles heterodimeric review articles heterodimeric PubMed articles heterodimeric PubMed Central articles heterodimeric 2023 articles heterodimeric 2024 articles heterodimeric Scopus articles heterodimeric impact factor journals heterodimeric Scopus journals heterodimeric PubMed journals heterodimeric medical journals heterodimeric free journals heterodimeric best journals heterodimeric top journals heterodimeric free medical journals heterodimeric famous journals heterodimeric Google Scholar indexed journals carcinogenic articles carcinogenic Research articles carcinogenic review articles carcinogenic PubMed articles carcinogenic PubMed Central articles carcinogenic 2023 articles carcinogenic 2024 articles carcinogenic Scopus articles carcinogenic impact factor journals carcinogenic Scopus journals carcinogenic PubMed journals carcinogenic medical journals carcinogenic free journals carcinogenic best journals carcinogenic top journals carcinogenic free medical journals carcinogenic famous journals carcinogenic Google Scholar indexed journals epithelial-mesenchymal articles epithelial-mesenchymal Research articles epithelial-mesenchymal review articles epithelial-mesenchymal PubMed articles epithelial-mesenchymal PubMed Central articles epithelial-mesenchymal 2023 articles epithelial-mesenchymal 2024 articles epithelial-mesenchymal Scopus articles epithelial-mesenchymal impact factor journals epithelial-mesenchymal Scopus journals epithelial-mesenchymal PubMed journals epithelial-mesenchymal medical journals epithelial-mesenchymal free journals epithelial-mesenchymal best journals epithelial-mesenchymal top journals epithelial-mesenchymal free medical journals epithelial-mesenchymal famous journals epithelial-mesenchymal Google Scholar indexed journals
Article Details
1. Introduction
Prostate cancer is the most common solid malignancy in men [1]. Advanced disease is characterized by high-grade cancer and distant metastases. Hypoxic and angiogenetic pathways are well known to be major contributors to the development of metastases and poor prognosis [2, 3]. Hypoxia is common among advanced human tumors in need of uncontrolled angiogenesis, and it is often associated with metastatic dissemination. Hypoxia-inducible factor (HIF)-1 and -2 are responsible for the primary mechanism that mediates adaptive responses to hypoxia. Cancer-specific HIF activity, especially in regions of intratumoral hypoxia, has been shown to mediate angiogenesis, epithelial-mesenchymal transition, stem cell maintenance, metastasis, and resistance to radiation therapy and chemotherapy [4, 5]. HIF-1 is a heterodimeric transcription factor composed of a constitutively expressed HIF-1β subunit and an oxygen-regulated HIF-1a subunit. Under normal oxygen conditions, HIF-1a is ubiquitinated by the tumor-suppressor protein, Von Hippel-Lindau, and is targeted for proteasomal degradation. Under hypoxic conditions, HIF-1a rapidly accumulates in the cytoplasm and translocates into the nucleus where it dimerizes with HIF-1β to recruit co-activators and drive transcription of many genes critical for key aspects of cancer progression. Increased levels of HIF-1a have been demonstrated in the majority of primary human cancers and their metastases, and were shown to be related to poor prognosis and treatment failure [6].
Extremely low frequency magnetic fields (ELF-MF) are usually defined as radio waves with frequencies ranging from 3 Hz to 3 kHz, which are emitted from electronic devices. They are classified as being possibly carcinogenic, although the effect is not well described or understood. It is known that ELF-MF cannot traverse membranes or cause DNA damage. Many other mechanisms of actions have been studied among various diseases [7], however and some of the hypotheses that were put forth to explain them involved correlations between ELF-MF exposure and regulation of cell proliferation [8]. To the best of our knowledge, a correlation between ELF-MF and HIF-1a in general and, specifically, a correlation between ELF-MF and prostate cancer have not been published to date. This study aimed to determine whether exposure to ELF-MF changes the expression of HIF-1a and its transcriptional activity in prostate cancer cells.
2. Materials and Methods
2.1 Culture
Human prostate cancer PC-3, LNCaP, and DU145 cells were maintained in RPMI 1640. Human embryonic kidney HEK293 cells were maintained in DMEM. The media were supplemented with 10% fetal calf serum and antibiotics. All cells were cultured at 37°C in a humidified atmosphere and 5% CO2 in air. For hypoxic exposure, the cells were placed in a sealed modular incubator chamber (Billups-Rothenberg, Del Mar, CA) flushed with 1% O2, 5% CO2, and 94% N2 and then cultured at 37°C.
2.2 ELF-MF irradiation
The cells were irradiated inside a humidified incubator. Irradiation was by a frequency generator (TGR1040 signal generator; Thurlby Thandar Instruments, UK) at 875 MHz with an intensity of 0.07 mW/cm2. The generator was set to the desired power and connected to the power amplifier, which was connected to a panel antenna (MA-CL 67-12) that was fixed in the incubator. Amplification was by an ERA-3SM device (Minicircuits, USA). The emitting antenna was placed in the center of a shelf in the incubator, and the walls of the incubator were covered to avoid reflection of the sound waves.
2.3 Protein extraction and Western blot
Whole cellular extracts were prepared and analyzed as previously described.23 Protein concentration was determined with a bicinchoninic acid protein assay kit (Pierce Biotechnology, USA). Protein extracts were analyzed by SDS-PAGE and immunoblotted with the indicated antibodies.
2.4 Antibodies, reagents and siRNA
The primary antibodies were mouse monoclonal anti-HIF-1a (BD Biosciences, USA) and mouse monoclonal anti-tubulin. The secondary antibodies were conjugated with horseradish peroxidase (Jackson ImmunoResearch, USA) used for Western blotting. Immunofluorescence was by Alexa Flour 594 donkey anti-mouse (Invitrogene, USA) Cycloheximide (CHX), an inhibitor of protein synthesis, was purchased from Sigma-Aldrich. On-TARGET plus human HIF-1a siRNA-SMART pool and non-target SMART pool were obtained from Dharmacon.
2.5 Transient transfection
Subconfluent cells were transfected with 1 µg DNA by means of the TransIT-X2 transfection reagent (Mirus Bio, USA) according to the manufacturer's protocol. The cells were transfected with siRNA by the Dharmafect transfection reagent (Dharmacon).
2.6 Luciferase luminescence assay
HIF-HRE-dependent luciferase activity was performed with the pBI-GL construct (pBI-GL V6L) containing six tandem copies of the VEGF hypoxia response element. The cells were grown in six-well plates and then transiently transfected in triplicate with 1 µg DNA reporter plasmid. Forty eight hours after transfection, the cells were either exposed or unexposed to irradiation for one hour, and lysed and analyzed for luciferase luminescence assay six hours later.
2.7 RNA purification and quantitative real-time PCR
Total RNA was extracted from the cells with NucleoSpin RNA II kit (Macherey-Nagel, Germany) following the manufacturer’s instructions. One µg of total RNA was reverse transcribed into cDNA by means of a VersoTM cDNA kit (ABgene, Epsom, UK) with anchored oligo (dT) as the first-strand primer. Quantitative real-time PCR (qRT-PCR) analyses were performed to determine the expression of HIF-1a mRNA. qRT-PCR reaction was performed in triplicates with a LightCycler FastStart DNA Master SYBR Green I (Roche Applied Science, Germany).
2.8 In vitro cell proliferation assay
The cells were transfected with 40 μM non-target siRNA or siRNA to HIF-1a. Forty-eight hours after transfection, they were re-seeded on 96-well plates, 1000 cells/well, and exposed or unexposed to irradiation every day for one hour for the following 7 days. After the indicated times, cell proliferation was measured using a 3-bis-(2-methoxy-4-nitro-5 sulfanyl)-(2H)-tetrazolium-5-carboxanilide (XTT) kit (Biological Industries Ltd, Israel) following the manufacturer’s instructions.
2.9 Immunofluorescence staining and microscope analysis
The cells were plated into six-well plates with 13-mm diameter cover glasses. After incubation and irradiation, the cells were fixed and permeabilized with cold Phemo buffer (0.068M PIPES, 0.025M HEPES, 0.015M EGTANa2, 0.003M MgCl26H2O, 10% DMSO, pH 6.8), 3.7% formaldehyde, 0.05% glutaraldehyde, and 0.5% Triton X-100 for 10 minutes. Briefly, blocking was done in 1% BSA/10% normal donkey serum/PBS and subsequently incubated with primary HIF-1a antibody diluted 1:50 in primary antibody dilution buffer (Biomeda, USA) followed by incubation with Alexa Flour 594 donkey anti-mouse diluted 1:200. The cells were then mounted with a fluorescence mounting medium (GBI labs, Israel), and fluorescence digital images were captured by an Olympus ix81-ZDC microscope. Image analysis was performed with ImageJ software. The cytosolic region of interest (ROI) was calculated by subtracting the nuclear ROI from whole-cell HIF-1a staining of the ROI for each cell individually.
2.10 Statistical analysis
The experiments presented in the figures are representative of three or more independent repetitions. The data are expressed as means ± SD. Student's t test was applied to compare differences between particular conditions. Group differences were assessed with a two-way ANOVA test. All tests were two-tailed, and statistical significance was defined as a P<0.05.
3. Results
3.1 ELF-MF increased HIF-1a expression and its transcriptional activity
We tested HIF-1a expression and its transcriptional activity following radiation in order to study whether HIF-1 is regulated by ELF-MF. ELF-MF exposure increased HIF-1a protein levels in PC-3 (Figure 1A) and LNCaP (Figure 1B) cells by two- to three-fold. The maximum effect of this induction was seen immediately after the end of the radiation, and interestingly, this effect diminished over time. Similar results were also obtained from DU145 prostate cancer cells (not shown). HIF-1a levels increased dramatically under hypoxic conditions, serving as a positive control. In addition, ELF-MF significantly increased HIF-1 transcriptional activity in these cells by 50-60%, as measured with a reporter plasmid expressing luciferase under the control of hypoxia-response elements (HRE) (Figure 2A and 2B). A similar increase in HRE-dependent luciferase activity was also seen in the non-cancerous HEK293 cells (Figure 2C).
3.2 ELF-MF regulates HIF-1α at the posttranscriptional level
We determined HIF-1a protein and mRNA following exposure to ELF-MF in order to characterize at which level it affects HIF-1a expression. For this purpose, we measured the half-life of HIF-1a after exposure to ELF-MF using the inhibitor of protein synthesis CHX. The HIF-1a half-life in irradiated cells was prolonged by three-fold compared to the control cells (6 min vs. 2 min) (Figure 3A and 3B). These changes were not evident at the mRNA level as determined by real-time PCR (Figure 3C). These results indicated that ELF-MF increased HIF-1a protein stability.
3.3 ELF-MF promoted HIF-1a accumulation into the nucleus
PC-3 cells were exposed to ELF-MF and processed for immunofluorescence labeling with HIF-1a antibody in order to test whether the increase in HIF-1 transcriptional activity following radiation was due to accumulation in the nucleus. Figure 4 demonstrates that the accumulation of nuclear HIF-1a was significantly higher in the irradiated cells compared with the control cells.
3.4 ELF-MF influenced cell proliferation in a HIF-1a-dependent manner
We applied an XTT proliferation assay in order to test whether ELF-MF could affect cell proliferation. ELF-MF caused a significant increase in cell proliferation in PC-3 and LNCaP during a period of 6 days in the irradiated cells compared to the control cells (PC-3 P<0.001, LNCaP and P<0.05, two-way ANOVA, Figure 5A and 5B). We applied siRNA to HIF-1a in PC-3 cells in order to further explore the role of HIF-1a in irradiation-induced changes in proliferation (Figure 5C). As shown in Figure 5D, ELF-MF failed to increase proliferation when HIF-1a was silenced. In contrast, the proliferation was reduced, even after exposure to ELF-MF (P<0.05, two-way ANOVA). In parallel, the irradiated cells expressing the non target siRNA demonstrated significantly higher proliferation compared to the control cells (data not shown). This phenomenon is most probably explained by the fact that the induction in cell proliferation by ELF-MF depends, at least partly, upon HIF-1a.
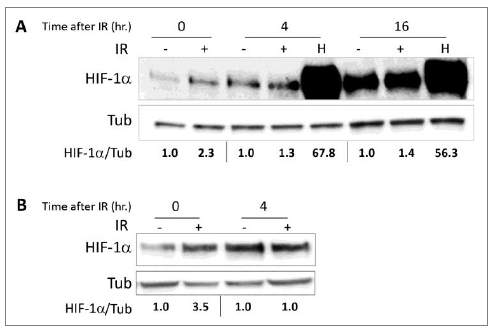
Figure 1: The ELF-MF irradiation increases HIF-1a expression. (A) PC-3 and (B) LNCaP cells were irradiated (IR) for 1 hour. After irradiation, the cells were lysed immediately (0) or after the indicated times (4 and 16 hours). The cells that had been subjected to no treatment (-) or to hypoxia (H) for the same duration served as controls. Whole cell extracts were analyzed by SDS-PAGE and immunoblotted with antibodies to HIF-1a and tubulin. Densitometric quantification of HIF-1a levels that normalized to tubulin is denoted under the lanes.
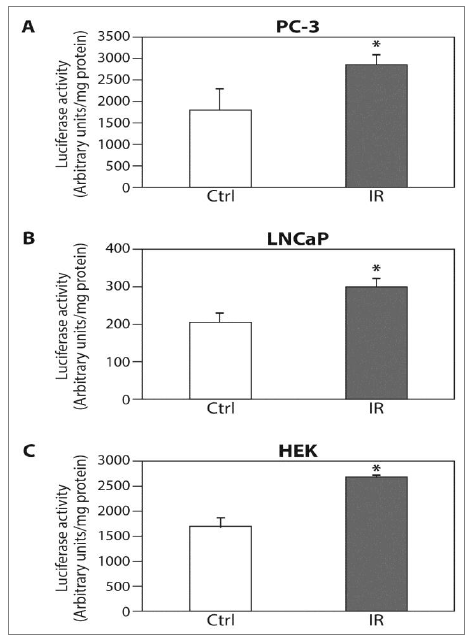
Figure 2: The ELF-MF irradiation increases HIF-1a transcriptional activity. (A) PC-3; (B) LNCaP and (C) HEK cells were transiently transfected with expression vector encoding pBI-GL V6L expressing luciferase under the control of HRE. After 48 hours of transfection, the cells were exposed (IR) or unexposed (Ctrl) to irradiation for 6 hours, and then lysed and analyzed for luciferase luminescence assay. Relative luciferase activity, units/mg protein at each assay point. Columns show means (n = 3); bars show SD; *P<0.05 compared to control.
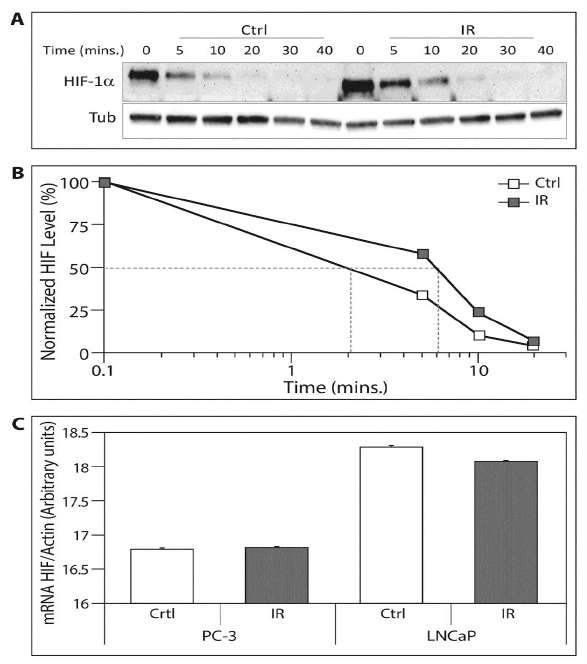
Figure 3: The ELF-MF irradiation upregulates HIF-1a protein on the pottranscriptional level. (A) 10 μM CHX was added to the PC-3 cell medium. Immediately afterwards, the cells were exposed (IR) or unexposed (Ctrl) to irradiation for 1 hour and lysed at the indicated times. Whole cell extracts were analyzed by SDS-PAGE and immunoblotted with antibodies to HIF-1a and tubulin; (B) Chart displaying densitometric quantification of HIF-1a levels in normalized to tubulin. The 50% decrease of HIF-1a levels is delineated in grey lines. This is a representative experiment of three independent repetitions; (C) PC-3 and LNCaP cells were exposed (IR) or unexposed (Ctrl) to irradiation for1 hour. RNA was isolated and reverse transcribed for HIF-1a and actin mRNA real-time PCR.
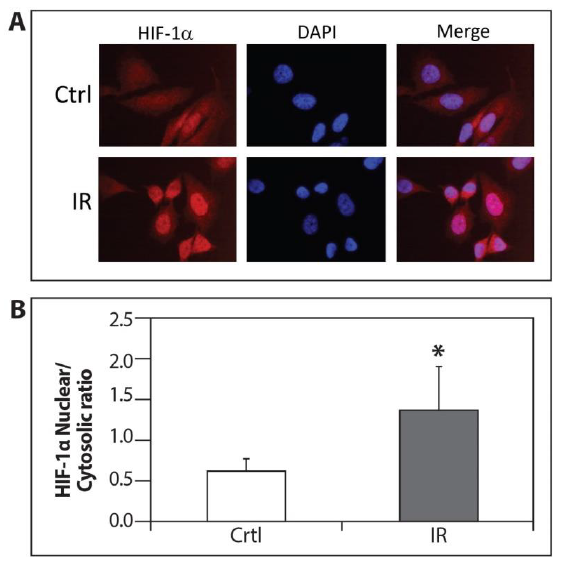
Figure 4: Accumulation of nuclear HIF-1a by ELF-MF irradiation. (A) PC-3 cells were seeded on cover glasses. After 24 hours, the cells were exposed (IR) or unexposed (Ctrl) to irradiation for 1 hour and processed for immunofluorescence labeling with HIF-1a antibody. Staining was analyzed by fluorescence microscopy (magnification x64); (B) The densitometric quantification of HIF-1a fluorescence signal was measured by Image J software. This is a representative experiment of three independent repetitions. A signal of all cells from five different fields was measured from each experiment. *P<0.05 compared with control.
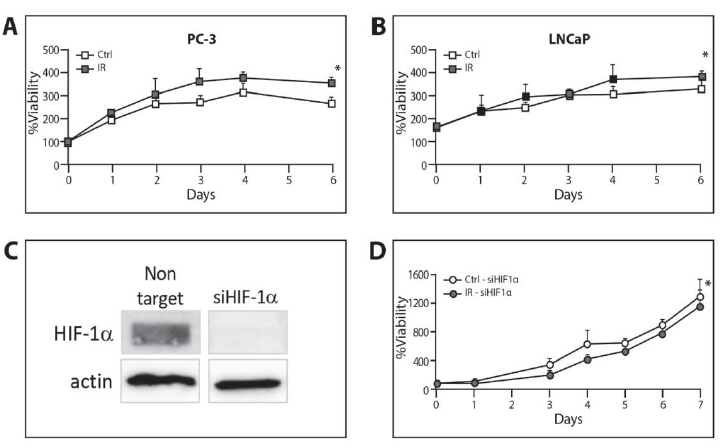
Figure 5: Increase of cell proliferation by ELF-MF irradiation in a HIF-1a-dependent manner. (A) PC-3 and (B) LNCaP cells were seeded on 96-well plates, 2000 cells/well for PC-3 and 5000 cells/well for LNCaP. The cells were exposed or unexposed to irradiation every day for 1 hour for the following 6 days. The proliferation rate was measured with XTT assay and expressed as an increase in percentage of initial absorbance that was measured before irradiation (100%).*P<0.05 compared with control; (C) PC-3 cells transfected with 40 μM non-target siRNA or siRNA against HIF-1a. The cells were lysed 72 hours after transfection and whole cell extracts were analyzed by SDS-PAGE and immunoblotted with antibodies to HIF-1a and tubulin; (D) PC-3 cells transfected with 40 μM siRNA against HIF-1a. They were re-seeded on 96-well plates, 1000 cells/well, 48 hours after transfection and exposed or unexposed to irradiation every day for 1 hour for the following 7 days. The proliferation rate was measured with an XTT assay and expressed as an increase in percentage of initial absorbance that was measured before irradiation (100%).
4. Discussion
The relation between ELF-MF and cancer has been the object of scientific and public discussions for decades. The World Health Organization published recommendations and concluded by stating that there is no convincing scientific evidence that weak radiofrequency signals cause adverse health effects [9], although many studies raise further concerns regarding their effect on cancer development [10-12]. Although prostate cancer is a very common type of malignancy, evidence of its relationship with ELF-MF is lacking [13]. This study, therefore, investigated the effects of ELF-MF on HIF-1a protein expression and HIF-1 transcriptional activity in prostate cancer cells.
, therefore,gnancyrated xplain them HIF-1a is a key element in the hypoxic pathway, which is one of the most well-described and understood pathways important for cell survival and cancer progression [14]. Interestingly, there is a paucity of data on the correlation between HIF-1a and ELF-MF. Only a single study evaluated the effect of ELF-MF on neuron-like cells, and it demonstrated that ELF-MF exposure significantly counteracted hypoxia damage by reducing cell death and apoptosis and inhibited the activation of the HIF-1a pathway [15].
Our results are in agreement with reports in the literature that different ELF-MF exposures can elicit various effects on various cells [16]. Prostate cancer cells that were exposed to ELF-MF in the current study showed immediate higher expression of HIF-1a, although the effect was transient (Figure 1). The effects that were exhibited in our in vitro cannot be applied to the in vivo setting in which exposure is long-standing.
Another interesting finding is that exposure to ELF-MF prolonged the half-life of HIF-1a (Figure 3A & B), leading to an increase in HIF-1 transcriptional activity (Figure 2) without affecting HIF-1a mRNA (Figure 3C). Many prior studies showed that HIF-1a mRNA levels were not affected by hypoxia or other interventions in both in vitro and in vivo models, whereas HIF-1a protein levels were drastically increased. It is possible that HIF-1a is regulated at the post-mRNA level [17-19]. We also demonstrated that prostate cancer cells exposed to ELF-MF showed greater levels of proliferation compared to cells control counterpart cells, but these cells did not show any increase in proliferation when HIF-1a was silenced (Figure 5). The results may indicate on such undiscovered mechanisms where HIF-1a in involved in the response to ELF-MF irradiation.
HIF-1a phosphorylation is another level of regulation which promotes nuclear accumulation of HIF-1a and leads to an increase of HIF-1 transcriptional activation [20-22]. Our findings showed that exposure to ELF-MF enhanced the accumulation of nuclear HIF-1a compared to the non-exposed cells (Figures 3 and 4). Further studies are warranted to explore the exact mechanism of how ELF-MF stabilizes HIF-1a protein.
It must be borne in mind that exposure to ELF-MF at all strengths is too low to induce any physiological or pathological effects by themselves, and that our results cannot be regarded as evidence of the involvement of ELF-MF in human cancer. Conclusions from in vitro studies performed on cells cannot be applied to interpret epidemiological findings in human populations. Our findings that the exposure of prostate cancer cells to ELF-MF increases HIF-1a activation suggest that ELF-MF might facilitate and promote dormant insignificant prostate tumors, and they call for further investigation of these issues in the clinical setting.
Acknowledgment
This project was funded by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (AMRF). In addition, we would like to thank Ms. Esther Eshkol for editorial assistance.
Conflicts of Interest
The authors have no financial interest or benefit that has arisen from the direct applications of this research.
References
- Prashanth Rawla. Epidemiology of Prostate Cancer. World J Oncol 10 (2019): 63-89.
- Ghinea N, Robin B, Pichon C, et al. Vasa nervorum angiogenesis in prostate cancer with perineural invasion. Prostate 79 (2019): 640-646.
- Russo G, Mischi M, Scheepens W, et al. Angiogenesis in prostate cancer: Onset, progression and imaging. BJU Int 110 (2012).
- Zapatero A, Morente M, De Vidales CM, et al. HIF1A expression in localized prostate cancer treated with dose escalation radiation therapy. Cancer Biomarkers 15 (2015): 41-46.
- Fraga A, Ribeiro R, Coelho A, et al. Genetic polymorphisms in key hypoxia-regulated downstream molecules and phenotypic correlation in prostate cancer. BMC Urol 17 (2017): 1-12.
- Gladek I, Ferdin J, Horvat S, et al. HIF1α gene polymorphisms and human diseases: Graphical review of 97 association studies. Genes Chromosom Cancer 56 (2017): 439-452.
- Kapri-Pardes E, Hanoch T, Maik-Rachline G, et al. Activation of Signaling Cascades by Weak Extremely Low Frequency Electromagnetic Fields. Cell Physiol Biochem 43 (2017): 1533-1546.
- Mezei G, Vergara XP. Adult cancer and extremely low-frequency magnetic fields. In: Epidemiology of Electromagnetic Fields (2014): 161-184.
- World Health Organization. Electromagnetic fields: What are electromagnetic fields? World Heal Organ (2014): 1-6.
- Schüz J. Exposure to extremely low-frequency magnetic fields and the risk of childhood cancer: Update of the epidemiological evidence. Prog Biophys Mol Biol 107 (2011): 339-342.
- Koeman T, Piet A van den Brandt, Pauline Slottje, et al. Occupational extremely low frequency magnetic field exposure and cancer incidence in a large prospective cohort study. Occup Environ Med 70 (2013): 82-83.
- Koeman T, Van Den Brandt PA, Slottje P, et al. Occupational extremely low-frequency magnetic field exposure and selected cancer outcomes in a prospective Dutch cohort. Cancer Causes Control 25 (2014): 203-214.
- Zhu K, Weiss NS, Stanford JL, et al. Prostate cancer in relation to the use of electric blanket or heated water bed. Epidemiology 10 (1999): 83-85.
- Masoud GN, Li W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm Sin 5 (2015): 378-389.
- Vincenzi F, Ravani A, Pasquini S, et al. Pulsed Electromagnetic Field Exposure Reduces Hypoxia and Inflammation Damage in Neuron-Like and Microglial Cells. J Cell Physiol 232 (2017): 1200-1208.
- Grellier J, Ravazzani P, Cardis E. Potential health impacts of residential exposures to extremely low frequency magnetic fields in Europe. Environ Int 62 (2014): 55-63.
- Wenger RH, Kvietikova I, Rolfs A, et al. Hypoxia-inducible factor-1α is regulated at the post-mRNA level. Kidney International 51 (1997): 560-563.
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor. J Biol Chem 270 (1995): 1230-1237.
- Jiang BH, Semenza GL, Bauer C, et al. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O 2 tension. Am J Physiol - Cell Physiol 271 (1996): 440-444.
- Burroughs SK, Kaluz S, Wang D, et al. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem 5 (2013): 553-572.
- Richard DE, Berra E, Gothié E, et al. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia- reducible factor (HIF-1α) and enhance the transcriptional activity of HIF-1. J Biol Chem 274 (1999): 32631-32637.
- Mylonis I, Chachami G, Samiotaki M, et al. Identification of MAPK phosphorylation sites and their role in the localization and activity of hypoxia-inducible factor-1α. J Biol Chem 281 (2006): 33095-33106.
- Manisterski M, Golan M, Amir S, et al. Hypoxia induces PTHrP gene transcription in human cancer cells through the HIF-2α. Cell Cycle 9 (2010): 3747-3753.
