Extramedullary Plasmacytoma Over the scar from Long-lasting Past Surgery as Presenting Feature of Multiple Myeloma
Article Information
David F. Moreno1, Xavier Setoain2,3, Joan Bladé1, Laura Rosiñol1*
1Amyloidosis and Myeloma Unit, Department of Hematology, Hospital Clínic, IDIBAPS, University of Barcelona, Spain
2Nuclear Medicine Department, Hospital Clínic, University of Barcelona, Spain
3Biomedical Research Networking Center in Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Barcelona, Spain
*Corresponding Author: Laura Rosiñol, MD, Amyloidosis and Myeloma Unit, Department of Hematology, Hospital Clínic, IDIBAPS, University of Barcelona, Barcelona, Spain, 08036.
Received: 26 November 2020; Accepted: 03 December 2020; Published: 09 December 2020
Citation: David F. Moreno, Xavier Setoain, Joan Bladé, Laura Rosiñol. Extramedullary Plasmacytoma Over the scar from Long-lasting Past Surgery as Presenting Feature of Multiple Myeloma. Archives of Clinical and Biomedical Research 4 (2020): 766-774.
View / Download Pdf Share at FacebookAbstract
Plasmacytomas can have different clinical presentations. They can be observed as a single tumor mass without bone marrow involvement (solitary plasmacytoma) or in the context over multiple myeloma. In rare instances plasmacytomas are triggered by invasive surgical procedures. These different types of plasmacytomas can be found in the same patient which suggests a special biology of the malignant plasma cell that facilitates the spread outside the bone marrow. Here, we report the unique case of a 37-year-old man with solitary bone plasmacytoma with soft-tissue involvement who progressed to multiple myeloma with a huge mass at the scar of inguinal hernia surgery performed 10 years before. Throughout the course of the disease, he developed extensive multiple paraskeletal and extramedullary plasmacytomas at different locations.
Keywords
Plasmacytoma; Surgery; Scar; Myeloma
Plasmacytoma articles; Surgery articles; Scar articles; Myeloma articles
Article Details
Introduction
Multiple myeloma (MM) is a heterogeneous disease representing 1% of all human cancers [1]. It consists of aberrant plasma cell proliferation in the bone marrow resulting in bone disease, anemia, hypercalcemia or renal failure [2]. Furthermore, malignant plasma cells can escape the bone marrow microenvironment in form of plasmacytomas that can be classified into two categories according their origin: extramedullary (involving soft tissue or viscera with no contact with bone) and bone related (paraskeletal) [3,4]. Rarely, plasmacytomas can arise from sites that previously had suffered traumatic injury or any other invasive procedure [5].
There is a clinical entity defined as solitary plasmacytoma, which consists of the presence of single bone (solitary bone plasmacytoma) or viscera involvement (solitary extramedullary plasmacytoma) without detectable disease in the bone marrow (or minimal). Treatment and prognosis is quite different from multiple myeloma with extramedullary involvement [6]. Solitary plasmacytoma has an incidence of 0.5/100.000 [7] and most of them (70%) are bone plasmacytomas that localizes in femurs, vertebrae or pelvis. However, solitary extramedullary plasmacytomas are more prevalent in head and neck soft tissues [8,9].
Here, we report a case with SBP that progressed to MM as extramedullary plasmacytoma arising along the scar from inguinal surgery performed 10 years before the diagnosis of MM.
Case report
A 37-year-old male went to the medical consult because of progressive back pain. He had a past medical history of bilateral inguinal hernia repaired by open surgery 10 years before, with no other clinical conditions. His primary care center performed an MRI that showed T6 fracture with posterior wall displacement and a soft tissue mass surrounding the spine cord. There was no evidence of myelopathy or spinal cord compression. The PET/CT showed high metabolic activity in the T6 mass (standardized uptake value – SUV of 3.7) without other relevant findings. In this scenario, a CT guided biopsy showed an infiltration of plasma cells that were positive for kappa light chain in the immunohistochemistry (IHC) study. Given the suspicion of MM, he was referred to his local hematologist and laboratory tests revealed a serum M-protein of 6 g/L. The immunofixation was positive for IgA kappa and serum free light chain (FLC) kappa was 23.8 mg/L (normal range 3.3 – 19.4). Urine immunofixation was positive for kappa light chain. However, there was no proteinuria, and the rest of laboratory parameters were in the normal range. Bone marrow aspirate showed 7% of plasma cells with normal phenotype. With this data, the patient was diagnosed with solitary plasmacytoma of bone. He was treated with radiation therapy (36 Gy fractionated in 12 sessions) and then he was referred to our Myeloma and Amyloidosis Unit for follow-up. Four months later, the PET/CT showed complete metabolic response with persistence of serum M-protein of 8 g/L.
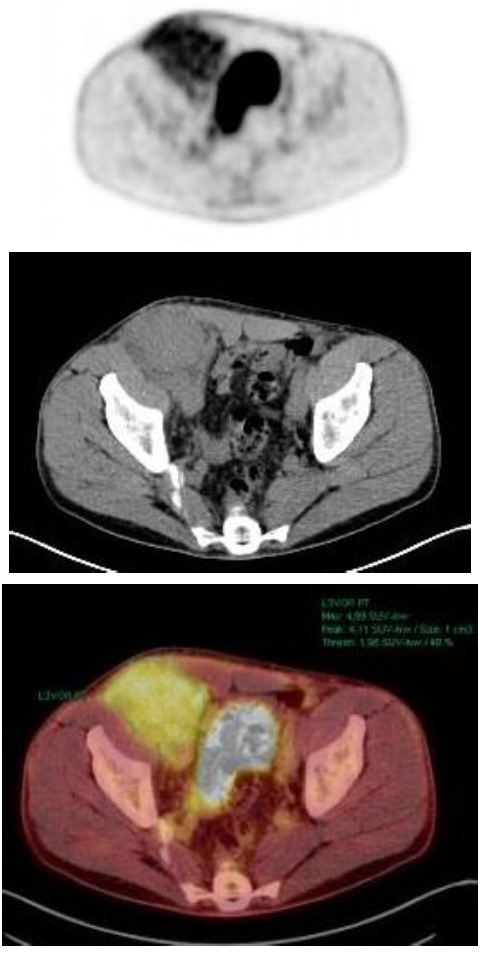
Eight months later, the patient complained of right inguinal pain and a palpable tumor. With the suspicion of recurrence of his past inguinal hernia, a CT scan showed a solid inguinal mass of 10x6 cm. The tumor biopsy showed a plasma cell infiltration with plasmablastic differentiation. IHC revealed CD56+, CD19-, CD117- with kappa restriction and 60% Ki-67 expression. PET/CT showed a mass of 1 cm3 with no contact with bone and high metabolic activity in the right inguinal hernia (SUV 4.8), as seen in Figure 1. The bone marrow aspirate had 10% of plasma cells and 38% of them had abnormal phenotype (CD38+, CD56+, CD19-, CD45-, CD27-, CD81+, CD117-). Cytogenetics by FISH analysis showed no abnormalities. At this time, new laboratory results showed serological progression: M-protein size was 27.9 g/L and serum kappa FLC had increased to 94.1 mg/L. Serum albumin and β2-microglobulin were 41 g/L and 2.1 mg/L, respectively. LDH and the rest of laboratory values were in the normal range. In this context, the patient was diagnosed with stage ISS 1 IgA kappa MM with extramedullary involvement.
The patient was included in the GEM12 trial. It consisted of 6 cycles of VRD (bortezomib, lenalidomide and dexamethasone) induction treatment followed by autologous stem cell transplant (SCT) and consolidation with 2 additional cycles of VRD. He achieved a very good partial response and started on maintenance treatment under GEM14 trial (lenalidomide, dexamethasone and ixazomib) achieving a complete response.
After 15 cycles of maintenance therapy, the disease relapsed with spinal cord compression that required urgent radiation therapy. Serum immunofixation was positive for IgA kappa and the electrophoresis showed an M-protein of 3.4 g/L. Bone marrow plasmacytosis was 11%, 91% of them with abnormal phenotype. Cytogenetic analysis did not show any high-risk abnormality. Given the poor prognosis and disease aggressiveness, he was treated with 4 cycles of D-PACE chemotherapy (dexamethasone, cisplatin, doxorubicin, cyclophosphamide and etoposide). He achieved complete metabolic response regarding extramedullary disease and serological partial response. Then, he underwent an allogeneic SCT from an unrelated donor with non-myeloablative conditioning.
Three months later, the patient had a persistent IgA kappa by immunofixation and 6% bone marrow plasma cells. However, the patient complaint of back pain and PET/CT showed high metabolic activity in multiple paraskeletal tumors localized at vertebrae, pelvis, ribs and scapulae. Additionally, a single plasmacytoma in the liver was observed.
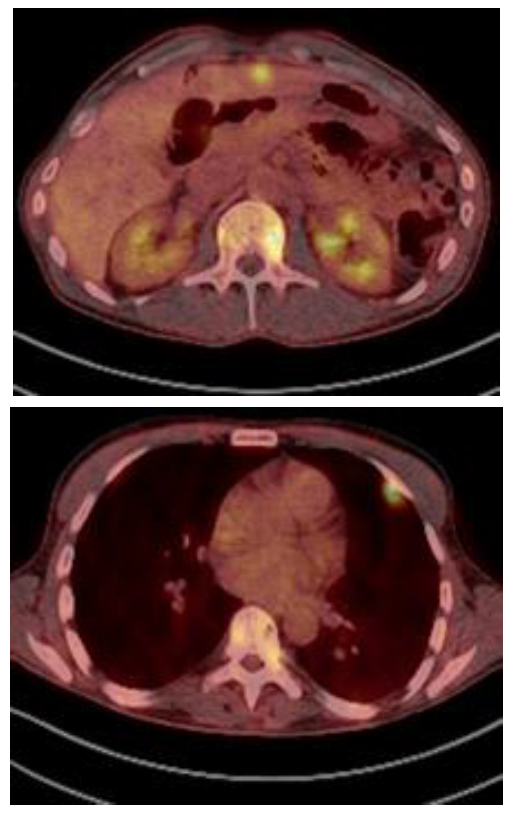
In this situation, he was treated with 3 cycles of Kd (carfilzomib and dexamethasone). The re-evaluation showed that the M-protein size was 4.3 g/L and the bone marrow aspirate showed 3% of plasmacytosis. The PET/CT showed partial response in some of the previous paraskeletal plasmacytomas but development of multiple new paraskeletal plasmacytomas (vertebrae, ribs, pelvis, iliac) and multiple tumor masses in liver and pleura (Figure 2). Daratumumab monotherapy was considered as salvage therapy. After 3 cycles, he achieved a serological very good partial response. Moreover, the PET/CT showed partial response in both paraskeletal and extramedullary plasmacytomas. After 9 cycles of daratumumab, the PET/CT showed normalization of the metabolic uptake in all of them except in iliac plasmacytoma, which significantly increased with invasion of the gluteal muscles with high metabolic activity (SUV 13) Local radiation therapy followed by daratumumab were given for three additional cycles.
Afterwards, the patient complained of dyspnea and generalized pain. A new PET scan showed an extramedullary spread with multiple plasmacytomas in skin, pericardium, mediastinum, and retroperitoneal structures. PoCyDex (pomalidomide, cyclophosphamide and dexamethasone) was indicated but performance status deteriorated, and the patient died few weeks later.
Discussion
A patient that had SBP at T6 vertebrae with adjacent soft-tissue mass, resolved with radiation therapy, progressing to symptomatic MM with an extensive extramedullary plasmacytoma all along the scar from an inguinal hernia surgery performed 10 years before is described. Throughout the course of the disease, the patient presented successive relapses with paraskeletal and extramedullary involvement and finally died because of disease progression with extensive extramedullary spread.
The risk of progression of SBP to MM described for our patient varies around 65 – 84% at 10 years [6]. This could have been higher in presence of clonal bone marrow infiltration [10], additional lesions in the PET/CT [11] and an abnormal FLC ratio [12]. The patient did not have any of these adverse prognostic factors. However, and despite the good response of T6 plasmacytoma, he was still at high risk because of persistent M-protein of 8 g/L after treatment, as it is recognized that the presence of serum M-protein more than 5 g/L after treatment is a predictor of progression to MM [13,14].
MM is characterized by a proliferation of malignant plasma cells with a strong dependence of the bone marrow microenvironment. However, plasma cells escape the microenvironment influence resulting in extramedullary plasmacytomas. The most frequent are paraskeletal plasmacytomas resulting from tumor masses arising from focal bone lesions that disrupt the cortical bone while the remaining tumors result from hematogenous metastatic spread [4].
There is no agreement in the medical community in the definition of extramedullary disease. Thus, while some authors consider both, paraskeletal and extramedullary (those without contact with bone) plasmacytomas [15–17], other authors only consider the extramedullary plasmacytomas as extramedullary disease [18–20]. Our patient is a paradigm of both, paraskeletal and extramedullary. The patient developed paraskeletal involvement at the time of SBP (resolved with radiation therapy) and progressed to MM with extensive extramedullary disease (resolved with chemotherapy). At first relapse he had paraskeletal involvement while from third relapse he developed simultaneously both extensive paraskeletal and extramedullary involvement. The evolution of our patient supports the idea that the inherent characteristics of the plasma cells and/or the microenvironment of the patient facilitates the spread of malignant plasma cells outside the bone marrow [3,4,21–23].
Sometimes plasmacytomas can be triggered by surgical invasive procedures performed during the course of the disease, such as laparotomy scars, catheter insertions or bone surgery [24-31]. The mechanisms of this type of relapse remain unclear but it has been suggested that the inflammatory process associated with tissue injury can facilitate the migration of myeloma cells into the skin or muscles and constitute a reservoir of dormant viable myeloma cells able to proliferate [32]. Supporting this hypothesis is a pre-clinical model of cells extracted from extramedullary disease that were injected to fetal bone graft and later developed soft tissue masses [33]. Of interest, the reported cases of extramedullary plasmacytomas in scars are always cases developing over the scars or procedures performed in the context of active MM. Our case is unique because the plasmacytoma grew from an old scar performed 10 years before the diagnosis of SBP. We can hypothesize that resistant plasma cells to radiation therapy migrate to the scar and proliferated resulting in an aggressive behavior. Of interest, this patient had no biology findings such as high-risk cytogenetics or CD56 negative plasma cells associated with extramedullary involvement [34,35].
In conclusion, we present a paradigmatic case of MM with extramedullary disease starting with an atypical plasmacytoma that grew on a scar. Clinicians need to be aware of this unusual disease presentation and more prospective studies are needed to clarify better the tumor biology and optimize treatment options.
Author contributions:
DFM, XS, JB and LR wrote and reviewed the manuscript. All authors approved the final version of the manuscript.
Competing interests:
There are no conflicts of interests to declare.
References
- Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA: a Cancer Journal for Clinicians 66 (2016): 443-459.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet Oncology 15 (2014): e538-e548.
- Bhutani M, Foureau DM, Atrash S, et al. Extramedullary multiple myeloma. Leukemia 34 (2020): 1-20.
- Bladé J, Fernandez de Larrea C, Rosinol L, et al. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. Journal of Clinical Oncology 29 (2011): 3805-3812.
- Bladé J, de Larrea CF, Rosiñol L. Extramedullary involvement in multiple myeloma. Haematologica 97 (2012): 1618-1619.
- Caers J, Paiva B, Zamagni E, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. Journal of Hematology & Oncology 11 (2018): 10.
- Dimopoulos MA, Moulopoulos LA, Maniatis A, et al. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood, The Journal of the American Society of Hematology 96 (2000): 2037-2044.
- Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. International Journal of Radiation Oncology* Biology* Physics 64 (2006): 210-217.
- Liebross RH, Ha CS, Cox JD, et al. Clinical course of solitary extramedullary plasmacytoma. Radiotherapy and Oncology 52 (1999): 245-249.
- Warsame R, Gertz MA, Lacy MQ, et al. Trends and outcomes of modern staging of solitary plasmacytoma of bone. American Journal of Hematology 87 (2012): 647-651.
- Fouquet G, Guidez S, Herbaux C, et al. Impact of initial FDG-PET/CT and serum-free light chain on transformation of conventionally defined solitary plasmacytoma to multiple myeloma. Clinical Cancer Research 20 (2014): 3254-3260.
- Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 23 (2009): 215-224.
- Wilder RB, Ha CS, Cox JD, et al. Persistence of myeloma protein for more than one year after radiotherapy is an adverse prognostic factor in solitary plasmacytoma of bone. Cancer 94 (2002): 1532-1537.
- Dingli D, Kyle RA, Rajkumar SV, et al. Immunoglobulin free light chains and solitary plasmacytoma of bone. Blood 108 (2006): 1979-1983.
- Varettoni M, Corso A, Pica G, et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Annals of Oncology 21 (2010): 325-330.
- Bladé J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. British Journal of Haematology 93 (1996): 345-351.
- Varga C, Xie W, Laubach J, et al. Development of extramedullary myeloma in the era of novel agents: no evidence of increased risk with lenalidomide–bortezomib combinations. British Journal of Haematology 169 (2015): 843-850.
- Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 97 (2012): 1761-1767.
- Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood, The Journal of the American Society of Hematology 127 (2016): 971-976.
- Short KD, Rajkumar SV, Larson D, et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 25 (2011): 906-908.
- Sheth N, Yeung J, Chang H. p53 nuclear accumulation is associated with extramedullary progression of multiple myeloma. Leukemia Research 33 (2009): 1357-1360.
- De Larrea CF, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 27 (2013): 780-791.
- Mulligan G, Lichter DI, Di Bacco A, et al. Mutation of NRAS but not KRAS significantly reduces myeloma sensitivity to single-agent bortezomib therapy. Blood, The Journal of the American Society of Hematology 123 (2014): 632-639.
- De Larrea CF, Rosiñol L, Cibeira MT, et al. Extensive soft-tissue involvement by plasmablastic myeloma arising from displaced humeral fractures. European Journal of Haematology 85 (2010): 448-451.
- Yoo J, Jo M, Kim MS, et al. Cutaneous Plasmacytoma: Metastasis of Multiple Myeloma at the Fracture Site. Annals of Dermatology 29 (2017): 483-486.
- Muchtar E, Raanani P, Yeshurun M, et al. Myeloma in scar tissue-an underreported phenomenon or an emerging entity in the novel agents' era? A single center series. Acta Haematologica 132 (20140: 39-44.
- Carmel B, Delost GR, Stern J, et al. Cutaneous plasmacytoma: Metastasis of multiple myeloma and invasion of sternotomy scar. JAAD Case Reports 5 (2019): 94-97.
- Trullemans F, Schots R, Storme G, et al. Late and localized extramedullary relapse of a light chain kappa myeloma after syngeneic bone marrow transplantation. Bone Marrow Transplantation 25 (2000): 115-117.
- Arango M, Echeverri C. Catheter insertion site plasmacytoma. Hematology/Oncology and Stem Cell Therapy 9 (2016): 34-35.
- Gaba RC, Kenny JP, Gundavaram P, et al. Subcutaneous plasmacytoma metastasis precipitated by tunneled central venous catheter insertion. Case Reports in Oncology 4 (2011): 315-322.
- Rosenblum MD, Bredeson CN, Chang CC, et al. Subcutaneous plasmacytomas with tropism to sites of previous trauma in a multiple myeloma patient treated with an autologous bone marrow transplant. American Journal of Hematology 72 (2003): 274-277.
- Rosiñol L, de Larrea CF, Bladé J. Extramedullary myeloma spread triggered by surgical procedures: an emerging entity?. Acta Haematologica 132 (2014): 36-38.
- Huang SY, Tien HF, Su FH, et al. Nonirradiated NOD/SCID-human chimeric animal model for primary human multiple myeloma: a potential in vivo culture system. The American Journal of Pathology 164 (2004): 747-756.
- Chang H, Bartlett ES, Patterson B, et al. The absence of CD56 on malignant plasma cells in the cerebrospinal fluid is the hallmark of multiple myeloma involving central nervous system. British Journal of Haematology 129 (2005): 539-541.
- Dahl IM, Rasmussen T, Kauric G, et al. Differential expression of CD56 and CD44 in the evolution of extramedullary myeloma. British Journal of Haematology 116 (2002): 273-277.