Evaluation of Intra-Arterial Nimodipine Therapy in Post-Subarachnoid Hemorrhage Refractory Cerebral Vasospasm
Article Information
Shrey Jain1*, Ajit K Sinha1, Sumit Goyal2
1Department of Neurosurgery, Sir Ganga Ram Hospital, Delhi, India
2Department of Neurosciences, Pushpawati Singhania Research Institute Hospital, Delhi, India
*Corresponding author: Shrey Jain, Department of Neurosurgery, Sir Ganga Ram Hospital, Delhi, India.
Received: 11 July 2022; Accepted: 20 July 2022; Published: 26 July 2022
Citation: Shrey Jain, Ajit K Sinha, Sumit Goyal. Evaluation of Intra-Arterial Nimodipine Therapy in Post-Subarachnoid Hemorrhage Refractory Cerebral Vasospasm. Cardiology and Cardiovascular Medicine 6 (2022): 383-389.
View / Download Pdf Share at FacebookAbstract
Background: Cerebral vasospasm is a major cause of morbidity and mortality in patients with subarachnoid hemorrhage. Vasospasm is managed with triple H and vasodilators but sometimes, patients do not respond. Intra-arterial vasodilator infusion has been shown to improve outcome in such patients. In this study, we try to evaluate the efficacy of intra-arterial nimodipine therapy in 43 patients of post-aneurysmal subarachnoid hemorrhage refractory cerebral vasospasm.
Methods: It is a prospective observational study of a group of 43 patients presenting with refractory cerebral vasospasm as per the inclusion criteria. Pre-procedure neurological assessment and Transcranial Doppler (TCD) monitoring were done. Endovascular spasmolysis was conducted and post-operative morbidity and outcomes were noted. Follow up of the patients was done at the time of discharge and at 6 months according to the Modified Rankin Scale and NCCT head.
Results: Most of the patients developing refractory cerebral vasospasm belonged to Hunt and Hess Grade 2 and 3 and Fisher grade 3 and 4. 87.5% of the patients showed clinical recovery following endovascular spasmolysis and 58% of the patients showed complete angiographic recovery. Outcome after 6 months was good in 76%, moderate in 12% and poor in 12% patients. NCCT head showed no infarct in 58%, minor infarct in 28% and major vascular territorial infarct in 14% patients.
Conclusion: Intra-arterial nimodipine infusion is a safe and effective therapy with minimum risk of complications if adhered to standard endovascular practice. By timely intervention, major ischemic insult to the brain can be averted, thereby significantly improving the prognosis.
Keywords
Aneurysm; Vasospasm; Subarachnoid hemorrhage; Intraarterial nimodipine; Transcranial Doppler
Article Details
1. Introduction
Cerebral vasospasm is a major cause of morbidity and mortality in patients of Subarachnoid Hemorrhage (SAH), occurring in 30-50% of the patients. It is an important factor to determine the prognosis and eventual outcome of the patient. It results in a decrease in cerebral blood flow, disturbance in autoregulation and delayed cerebral ischaemia.
Pathogenesis of vasospasm after SAH has been related to hemolysis of the subarachnoid blood [1]. Contraction of vessels also occurs in response to vasoactive substances released from erythrocyte hemosylate, the key components being haemoglobin and other vasoactive factors including serotonin, norepinephrine, bradykinins, angiotensin, prostaglandin, thromboxanes and free radicals [2,3].
Vasospasm can be diagnosed by clinical examination, Doppler ultrasound or by angiography. Transcranial Doppler uses low frequency ultrasound (1-2 MHz) to assess the blood flow velocity in the cerebral vessels. It is a safe, inexpensive and readily available method, that promptly identifies vessels in spasm. Angiography is the main modality of investigation and offers combined advantage of a diagnostic as well as therapeutic tool, in the form of mechanical (balloon angioplasty) and pharmacological angioplasty.
Management of vasospasm requires double or triple H therapy as hypervolemia is not recommended nowadays [4]. In addition, calcium channel blockers are particularly useful in vasospasm management. Nimodipine is most commonly used agent in view of its cerebro-selective nature. Jan et al. [5] reported a 36% reduction in bad outcome in patients treated with nimodipine. Other calcium channel blockers including nicardipine and verapamil, free oxygen radical scavengers, statins, endothelin receptor antagonists and nitric oxide donor drugs have also been used in the vasospasm management.
In patients with cerebral vasospasm, not responding to medical management, cerebrospinal fluid drainage and endovascular intervention remain viable options. Balloon angioplasty has shown promising results in dilatation of proximal major cerebral arteries but has no effect in distal cerebral artery vasospasm. In cases of distal vessel spasm, pharmacological dilatation of the vessels intra-arterially is useful. Authors attempt to study the efficacy and complications of intra-arterial instillation of nimodipine in patients with refractory cerebral vasospasm.
2. Materials and Methods
It is a single institutional observational prospective study wherein, 43 patients were analyzed for efficacy and complications of intra-arterial nimodipine from December 2013 to June 2017. Patients considered were those who developed cerebral vasospasm in post-aneurysmal rupture subarachnoid hemorrhage, not responding to medical management. Patients excluded from the study were those with traumatic SAH, children, pregnant or lactating women or patients with well-developed infarct.
A detailed history and thorough physical examination was done on patients, along with their clinical grading according to Hunt and Hess scale Non Contrast Computed Tomography (NCCT) head was done for confirmation of SAH along with grading according to Fisher grade. Angiography was done for confirmation of aneurysm and Transcranial Doppler (TCD) was performed for baseline mean velocity of intracranial vessels. Definitive treatment of the aneurysm (clipping/coiling) was done according to variable aneurysm and patient factors.
Post-procedure, patient was monitored and managed under strict guidelines on triple H therapy. Intravenous or oral nimodipine was initiated post-procedure to prevent vasospasm. Intravenous fluids were given to the patients to maintain the central venous pressure. Regular monitoring of these patients was done and daily detailed neurological examination as well as Transcranial Doppler monitoring was conducted.
In patients who developed delayed neurological deficits clinically, alternative causes of neurological deterioration like hydrocephalus, re-bleeding, hyponatraemia or seizure were investigated for and managed accordingly. After excluding such causes, mean blood pressure was raised upto 110 mm Hg. With persistence of neurological deficit or showing TCD mean velocity of greater than 150 cm/sec or an increase of 40 cm/s or more from the established baseline, patients were considered in refractory vasospasm and were taken up for Angiography. Vasospasm was classified as mild (<25% decrease in vessel lumen), moderate (25-50% decrease in vessel lumen) and severe (>50% decrease in vessel lumen).
After taking the informed consent, patient underwent check cerebral DSA and intra-arterial nimodipine infusion using microcatheter. Upto 3 mg of Nimodipine per vascular territory was given slowly at the rate of 0.15 to 0.3 mg per hour. In case of hypotension during nimodipine infusion, inotropic support was added. However, in case of inability to maintain blood pressure, nimodipine infusion was discontinued. The details of the procedure were noted.
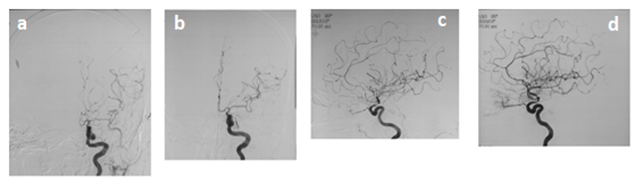
The outcome of the patient, post-procedure, was noted angiographically, clinically and radiologically (Figure 1).
Figure 1: a: Antero-posterior projection of digital subtraction angiography of brain showing spasm in anterior and middle cerebral arteries b: Antero-posterior projection of digital subtraction angiography of brain showing post-spasmolysis dilatation in anterior and middle cerebral arteries c: Lateral projection of digital subtraction angiography of brain showing spasm in anterior and middle cerebral arteries d: Lateral projection of digital subtraction angiography of brain showing post-spasmolysis dilatation in anterior and middle cerebral arteries.
Angiographic response was classified into mild, moderate and complete depending on the circulation time and dilation of the vessel previously in spasm, i.e.,
- Mild if only the circulation time is improved with no change in spastic vessel caliber.
- Moderate if partial dilation of the vessels previously in spasm.
- Complete if complete dilation of the vessels previously in spasm.
Clinical outcome was noted in terms of improvement in focal deficit, improvement in clinical status and post-procedure TCD mean velocity in the affected vessels. Any requirement of repeat procedure was noted depending on the outcome of the patient or in case of continued deterioration of the above parameters.
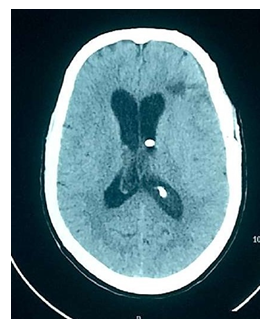
Radiological outcome was noted on the presence or absence of any infarct (major arterial territory or minor) post-procedure done at the time of discharge and after 6 months of follow up (Figure 2). Follow up of the patient was done at the time of discharge and 6 months clinically, in terms of Modified Rankin Scale. Outcome was considered as:
- Good with mRS score of 0 to 2
- Moderate with mRS score of 3 to 4
- Poor with mRS score 5 to 6
3. Statistical Analysis
Data entry was done in Microsoft excel and Data analysis was done using stata [6]. Demographic and clinical data were described as percentages. Comparison of pre-procedural and post-procedural neurological status was analyzed using paired t test. A p-value of less than 0.05 was considered significant.
4. Results
43 patients were evaluated in the study, whose demographic and clinical profiles are illustrated in Table 1. Male to female ratio was 20:23, with average age or presentation being 53 years and range from 20 to 73 years. 58% of the patients presented with Glasgow Coma Scale (GCS) above 14 with 51% of the patients presenting with focal neurological deficit. Majority of the patients belonged to Hunt and Hess grade II and III (67%); and Fisher grade III and IV (47% and 32%, respectively). 56% of the patients underwent coiling and 44% underwent clipping as definitive treatment of the aneurysm.
|
Total number of Patients |
43 |
|
Sex (Male : Female) |
20:23 |
|
Age in years (Range, Mean) |
20-73; 53 |
|
Glasgow coma scale (GCS), no. (%) |
|
|
Greater than equal to 14 |
25(58) |
|
Nov-13 |
13(30) |
|
0-10 |
5(12) |
|
Focal Deficits at admission, no. (%) |
21 (49) |
|
Hunt & Hess grade, no. (%) |
|
|
I |
9 (21) |
|
II |
16 (37) |
|
III |
13 (30) |
|
IV |
5 (12) |
|
V |
0 (0) |
|
Fisher grade, no. (%) |
|
|
I |
0 (0) |
|
II |
9 (21) |
|
III |
20 (47) |
|
IV |
14 (32) |
|
Location of Aneurysm, no. (%) |
|
|
Anterior communicating artery |
13 (30) |
|
Middle cerebral artery |
9 (21) |
|
Posterior communicating artery |
5 (12) |
|
Vertebro-basilar artery |
7 (16) |
|
Internal carotid artery |
4 (9) |
|
Anterior choroidal artery |
3 (7) |
|
Distal anterior cerebral artery |
2 (5) |
|
Treatment of anueryms, no. (%) |
|
|
Clipping |
19 (44) |
|
Coiling |
24 (56) |
Table 1: 43 patients were evaluated in the study, whose demographic and clinical profiles are illustrated in the table.
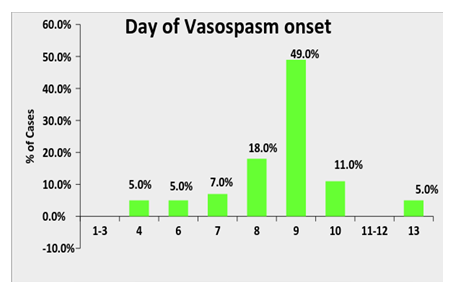
49% of the patients developed vasospasm on post-ictal day 9 with earliest ranging from day 4 to latest on day 13 as depicted in Graph 1. Details about the cerebral vasospasm development and its management are illustrated in Table 2. 56% of the patients presented with clinical deterioration while 88% of the patients were found to be developing vasospasm on the basis of elevated flow velocity on TCD. 54% of the patients were found to have vasospasm in unilateral vessels while severity of vasospasm was found to be moderate and severe in 37% and 37% patients, respectively.
|
Patients with delayed neurological deficit, no. (%) |
24 (56) |
|
Site of Vasospasm, no. (%) |
|
|
Middle cerebral artery |
21 (49) |
|
Anterior cerebral artery |
16 (47) |
|
Internal carotid artery |
21 (49) |
|
Posterior cerebral artery |
3 (7) |
|
Bilateral vessels |
20 (46) |
|
Degree of vasospasm, no. (%) |
|
|
Mild |
11 (26) |
|
Moderate |
16 (37) |
|
Severe |
16 (37) |
|
Dosage of nimodipine (mg) instilled, no. (%) |
|
|
3 |
10 (23) |
|
6 |
24 (56) |
|
7 |
1 (2) |
|
8 |
2 (5) |
|
9 |
6 (14) |
|
Angiographic recovery, no. (5%) |
|
|
Mild |
9 (21) |
|
Moderate |
9 (21) |
|
Complete |
25 (58) |
|
Clinical recovery, no. (%) |
21 (87.5) |
|
Repeat spasmolysis, no. (%) |
5 (12) |
Table 2: Details about the cerebral vasospasm development and its management are illustrated in the table.
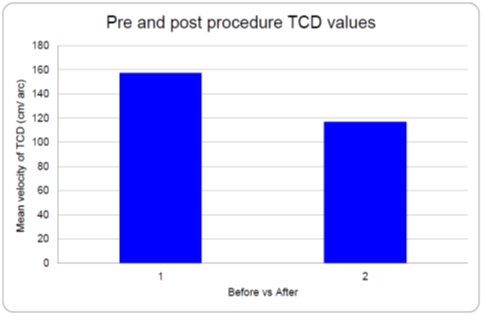
As per the protocol, all patients were locally administered intra-arterial nimodipine infusion up to a maximum dosage of 3 mg per vascular territory. 58% of the patients showed complete angiographic recovery while 87.5% of the patients showed clinical recovery after the intervention. Post-procedural TCD velocity was found to decrease in accordance with angiographic recovery in the patients with the mean difference of 40.46 cm/sec (Graph 2). Out of all the patients, 5 patients (12%) needed a repeat procedure at a later date in view of inadequate recovery.
Final Outcome has been illustrated in Table 3. At the time of discharge, 72% of patients had good outcome, 16% had moderate with 12% had poor outcome. On NCCT head, 58% had no infarcts while 28% had minor infarcts. On follow-up after 6 months, 76% patients had good clinical outcome while 12% patient died before the follow up period. NCCT head findings remained the same as at the time of discharge.
|
Clinical outcome on discharge, no. (%) |
|
|
Good |
31 (72) |
|
Moderate |
7 (16) |
|
Poor |
5 (12) |
|
Vasospasm related infarction on discharge, no. (%) |
|
|
No infarct |
25 (58) |
|
Minor infarct |
12 (28) |
|
Major Discharge |
6 (14) |
|
Mortality, no. (%) |
5 (12) |
|
Clinical outcome at 6 moths follow up, no. (%) |
|
|
Good |
33 (76) |
|
Moderate |
5 (12) |
|
Poor |
0 (0) |
|
Vasospasm related infarction at 6 months follow up, no. (%) |
|
|
No infarct |
25 (58) |
|
Minor infarct |
12 (28) |
|
Major infarct |
1 (3) |
Table 3: Final outcome has been illustrated in the table.
5. Discussion
Cerebral vasospasm is one of the most common treatable complication with aneurysmal subarachnoid hemorrhage, causing death and disability in 12-17% of SAH patients [7-10]. Clinical features of vasospasm depends on the vascular territory being affected. Although, other causes of neurological deterioration must be ruled out including electrolyte disturbance (hyponatremia), infection (meningitis and sepsis), hydrocephalus and seizures. TCD provides a cheap, reliable, repeatable and portable test for early diagnosis of vasospasm. Sensitivity and specificity of TCD in detecting vasospasm is high and is reported to be 84-85% and 89-98% respectively [11]. Angiography remains the gold standard for the diagnosis of vasospasm. CT head can be used for prognostication but is not a useful technique for early diagnosis of vasospasm.
Management of vasospasm requires good intensive care including measurement of central venous pressure, cardiac output monitoring along with good neuro-nursing care. Triple H therapy is targeted at increasing Cerebral Blood Flow (CBF) and decreasing hematocrit, improving the rheological properties of blood. Solomon et al. [12] suggested that immediate aneurysm surgery and aggressive post-operative prophylactic volume expansion in all patients can substantially reduce re-bleeding and delayed cerebral ischaemia, potential cause of morbidity after aneurysmal SAH. According to latest international guidelines, hypervolaemia is not recommended anymore and HH therapy is preferred nowadays [4].
Among pharmacological prevention of vasospasm, calcium channel antagonist are drugs of choice. Nimodipine has selective Central Nervous System (CNS) action and blocks dihydropyridine sensitive (L-type) calcium channels. It blocks calcium influx in smooth muscle cell and leads to reduced vascular smooth muscle constriction and consequent improved cerebral perfusion. Other possible mechanisms having direct neuroprotective effects include decrease in the release of vasoactive substances from endothelium and platelets, blockage of free-radical attack on the intra neuronal mitochondria, and improvement of CO2 reactivity and cerebral oxygen metabolism, or a reduction of tissue damage caused by calcium overload at reperfusion. Its short half-life requires frequent dosing in order to maintain sustained concentration. Recently, several trials have been conducted to assess efficacy of statins, endothelin-1 antagonists and magnesium sulfate [13].
Patients who fail to respond adequately, endovascular therapy including balloon angioplasty and chemical vasodilatation, have been shown to play crucial role. Mechanical angioplasty has demonstrated permanent reversal of vasospasm in treated vessels, but it can be applied to only proximal vessel segments [14,15]. Numaguchi et al. [16] demonstrated successfully that pharmacologic dilation by means of intra-arterial papaverine has the advantage of also acting on smaller distal branches and diffuse vasospasm. However, its beneficial effects are transient, and repeat treatment sessions are often necessary. Use of papaverine for intra-arterial infusion has largely been abandoned in view of limited success, frequent dosing and serious side effects including exacerbation of vasospasm [17], thrombocytopenia [18] and increase in intracranial pressure [19]. Milrinone was shown to have recurrence rate of cerebral vasospasm more than 20% following intra-arterial bolus injection [20,21] Other drugs used for intra-arterial infusion include nicardipine, verapamil, colforsin daropate, but no studies have shown significant advantage of these drugs over nimodipine.
There have been several studies performed to assess efficacy of intra-arterial nimodipine infusion in management of refractory cerebral vasospasm [6,22-29] It was observed that majority of the patients developing cerebral vasospasm belonged to Hunt and Hess Grade II and III along with Fisher grade III and IV. The number of patients in refractory cerebral vasospasm were found to be more in the coiling group (56%) in the present study however, this does not suggest that patients who undergo coiling have more chances of going into cerebral vasospasm as it was evaluated in the study. Out of 43 patients, 24 patients (56%) developed delayed neurological deficits at the onset of vasospasm while 40 patients (93%) were detected on the basis of increased flow velocity on TCD. TCD velocity corroborated with clinical deterioration in 21 off 24 patients (88%). This stresses over the importance of regular TCD monitoring and clinical examination as effective means of early detection and management of vasospasm.
In our study, local administration of intra-arterial nimodipine was done at a dosage of 3 mg/territory. Total nimodipine dose used in 43 patients ranged from a minimum of 3 mg to a maximum of 9 mg. Inotropic support was started intra-procedure to maintain hemodynamic status of the patient. In 5 patients (12%), we were not able to give nimodipine sufficiently in view of inability to maintain hemodynamic status. In a study conducted by Biondi et al. [23], 1-3 mg of nimodipine was given per vessel. The total dose of nimodipine injected intra-arterially for a given patient was maintained within 5 mg. Cho et al. [24] kept the dose of nimodipine from 3 to 6 mg at a rate of 6 mg/h per vessel. When transient hypotension occurred during procedure, infusion was stopped temporarily until blood pressure became normalized.
In our study, 25 patients off 43 (58%) showed complete angiographic recovery and 9 patients (21%) showed moderate angiographic recovery. 21 off 24 patients (87.5%) who had developed neurological deficits, clinically improved after spasmolysis while 3 patients (12.5%) did not show any improvement. When compared with Bondi et al. [23], only 7% showed excellent angiographic response, 37% showed good and 57% showed poor response. 76% patients showed substantial and stable clinical improvement in their clinical condition. On the other hand, in study conducted by Cho et al. [24], 83% cases showed good angiographic response post-intervention while neurological deficits improved in 69% of the patients after spasmolysis. A correlation can be established with the dosage of nimodipine infused intra-arterially with better response being seen in studies where high dosage was used.
On discharge and follow up, it was observed in our study that by timely intervention, mortality of refractory cerebral vasospasm was reduced to 12% as compared to 14-26% without endovascular intervention as reported by Kassell et al. [30] Patient with good clinical outcome was comparable to other studies assessing efficacy of intra-arterial nimodipine therapy. Most of the patients tolerated intra-arterial nimodipine infusion well with only 12% of the patients developing systemic hypotension. No other systemic side effect was noticed in any of the patient following intra-arterial nimodipine therapy. There was no procedural complication found in any of the patients.
Authors are of the opinion that the study conducted was of small sample size and a larger study is required to really assess the effect of alteration in dosage of nimodipine during intra-arterial instillation for a better outcome. Also, with intra-arterial nimodipine infusion, 58% of the patients showed complete angiographic recovery while 87.5% showed clinical recovery. This warrants additional investigation in combination of nimodipine with alternative pharmaceutical agents like milrinone and fasudil for better outcome in patients.
6. Conclusion
Cerebral vasospasm remains the major cause of morbidity and mortality in post-subarachnoid period and one should be watchful for timely detection and intervention of vasospasm for good outcome. With regular detailed neurological examination and TCD monitoring, it is possible to timely diagnose and manage cerebral vasospasm. Intra-arterial nimodipine infusion is safe and effective therapy with minimum risk of complications if adhered to standard endovascular practice. By timely intervention, major ischaemic insult to the brain can be averted as seen by low number of large infarcts in follow up CT scan and thereby significantly improving the prognosis.
Funding
No funding was received for this project.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Authors’ contributions
Shrey Jain, Ajit K Sinha and Sumit Goyal conceived and designed research. Ajit K Sinha and Sumit Goyal conducted interventions. Shrey Jain and Ajit K Sinha analyzed data. Shrey Jain wrote the manuscript. All authors read and approved the manuscript.
References
- Sonobe M, Suzuki J. Vasospasmogenic substance produced following subarachnoid hemorrhage and its fate. Acta Neurochir (Wien) 44 (1978): 97-106.
- Asano T, Takakura K, Sano K, et al. Effects of hydroxyl radical scavenger on delayed ischaemic neurological deficits following aneurysmal subarachnoid hemorrhage: results of a multicentre placebo-controlled double-blind trial. J Neurosurg 84 (1996): 792-803.
- Asano T, Tanishima T, Sasaki T, et al. Possible participation of the free radical reaction initiated by clot lysis in the pathogenesis of vasospasm following subarachnoid hemorrhage. In Wilkins RH (Ed). Cerebral Arterial Spasm. Williams and Wilkins, Baltimore (1980): 190-201.
- Connolly SE, Carhuapoma JR, Higashida RT, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke 43 (2012): 1711-1737.
- Jan M, Buchheit F, Tremoulet M. Therapeutic trial of intravenous nimodipine in patients with established cerebral vasospasm after rupture of intracranial aneurysms. Neurosurgery 23 (1988): 154-157.
- Hanggi D, Turowski B, Beseoglu K, et al. Intra-arterial Nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid hemorrhage: influence on clinical course and cerebral perfusion. AJNR Am J Neuroradiol. 29 (2008): 1053-1060.
- Cooke D, Seiler D, Hallam D, et al. Does treatment modality affect vasospasm distribution in aneurysmal subarachnoid hemorrhage: differential use of intra-arterial interventions for cerebral vasospasm in surgical clipping and endovascular coiling populations. J NeuroIntervent Surg 2 (2010): 139-144.
- Dorsch NWC, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Part I. Incidence and effects. Journal of Clinical Neuroscience 1 (1994): 19-26.
- Komotar R, Zacharia B, Otten ML, et al. Controversies in the endovascular management of cerebral vasospasm after intracranial aneurysm rupture and future directions for therapeutic approaches. Neurosurgery 62 (2008): 897-905.
- Komotar RJ, Zacharia BE, Valhora R, et al. Advances in vasospasm treatment and prevention. J Neurol Sci 261 (2007): 134-142.
- Jindal A, Mahapatra A. Transcranial Doppler in neurological disorders. Neurol India 47 (1999): 166-167.
- Solomon RA, Fink ME, Lennihan L. Prophylactic volume expansion therapy for the prevention of delayed cerebral ischaemia after early aneurysm surgery. Arch Neurol 45 (1988): 325-332.
- Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 9 (2010): 504-519.
- Livingston K, Guterman LR, Hopkins LN. Intraarterial papaverine as an adjunct to transluminal angioplasty for vasospasm induced by subarachnoid hemorrhage. AJNR Am J Neuroradiol 14 (1993): 346-347.
- Rosenwasser RH, Armonda RA, Thomas JE, et al. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery 44 (1999): 975-979.
- Numaguchi Y, Zoarski GH, Clouston JE, et al. Repeat intra-arterial papaverine for recurrent cerebral vasospasm after subarachnoid hemorrhage. Neuroradiology 39 (1997): 751-759.
- Clyde BL, Firlik AD, Kaufmann AM, et al. Paradoxical aggravation of vasospasm with papaverine infusion following aneurysmal subarachnoid hemorrhage. Case report J Neurosurg 84 (1996): 690-695.
- Miller JA, Cross DT, Moran CJ, et al. Severe thrombocytopenia following intraarterial papaverine administration for treatment of vasospasm. J Neurosurg 83 (1995): 435-437.
- McAuliffe W, Townsend M, Eskridge JM, et al. Intracranial pressure changes induced during papaverine infusion for treatment of vasospasm. J Neurosurg 83 (1995): 430-434.
- Fraticelli AT, Cholley BP, Losser MR, et al. Milrinone for the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 39 (2008): 893-898.
- Shankar JJS, dos Santos MP, Deus-Silva L, Lum C. Angiographic evaluation of the effect of intra-arterial milrinone therapy in patients with vasospasm from aneurysmal subarachnoid hemorrhage. Neuroradiology 53 (2011): 123-128.
- Bele S, Proescholdt MA, Hochreiter A, et al. Continuous intra-arterial nimodipine infusion in patients with severe refractory cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a feasibility study and outcome results. Acta Neurochir (Wien) 157 (2015): 2041-2050.
- Biondi A, Ricciardi GK, Puybasset L, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol 25 (2004): 1067-1076.
- Cho WS, Kang HS, Kim JE, et al. Intra-arterial Nimodipine infusion for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Interv Neuroradiol 17 (2011): 169-178.
- Goel R, Aggarwal A, Salunke P, et al. Is intra-arterial nimodipine really beneficial in vasospasm following aneurysmal subarachnoid hemorrhage? Br J Neurosurg 30 (2016): 407-410.
- Hui C, Lau KP. Efficacy of intra-arterial nimodipine in the treatment of cerebral vasospasm complicating subarachnoid hemorrhage. Clin Radiol 60 (2005): 1030-1036.
- Musahl C, Henkes H, Vajda Z, et al. Continuous local intra-arterial nimodipine administration in severe symptomatic vasospasm after subarachnoid hemorrhage. Neurosurgery 68 (2011): 1541-1547.
- Ott S, Jedlicka S, Wolf S, et al. Continuous selective intra-arterial application of nimodipine in refractory cerebral vasospasm due to aneurysmal subarachnoid hemorrhage. Biomed Res Int (2014):
- Wolf S, Martin H, Landscheidt JF, et al. Continuous selective intra-arterial infusion of nimodipine for therapy of refractory cerebral vasospasm. Neurocrit Care 12 (2010): 346-351.
- Kassell NF, Torner JC, Haley EC, et al. The international cooperative study on the timing of aneurysm surgery. J Neurosurg 79 (1990): 18-47.