Evaluation of Analgesic, Anti- Inflammatory Activities, Acute and Sub-Acute Toxicity Profile of Methanolic Extract of Lettuce Cornuta Leaves
Article Information
Maibouge Tanko Mahamane Salissou*,1,2, Sibongile Chituku2, Idi Claire Musimwa1, Brenda Makonyere1, Vigillance Zibako1, Blessing Chikore1, Brooklyn Nhema1
1Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences Africa University Mutare Zimbabwe
2Department of public health and nursing, College of Health Agriculture and Natural Sciences Africa University Mutare Zimbabwe
*Corresponding author: Maibouge Tanko Mahamane Salissou. Department of Biomedical and Laboratory Sciences, College of Health Agriculture and Natural Sciences Africa University Mutare Zimbabwe.
Received: 17 November 2025; Accepted: 26 November 2025; Published: 19 December 2025
Citation: Maibouge Tanko Mahamane Salissou, Sibongile Chituku, Idi Claire Musimwa, Brenda Makonyere, Vigillance Zibako, Blessing Chikore, Brooklyn Nhema. Evaluation of Analgesic, Anti- Inflammatory Activities, Acute and Sub- Acute Toxicity Profile of Methanolic Extract of Lettuce Cornuta Leaves. Journal of Pharmacy and Pharmacology Research. 9 (2025): 163-170.
View / Download Pdf Share at FacebookAbstract
Background: Despite being beneficial, conventional Non-steroidal anti- inflammatory, anti-nociceptive drugs triggers serious adverse side effects associated with their usage as synthetic compounds which include heart attack, gastric ulcers, and liver and kidney diseases. Hence the necessity to continue investigating in natural product as alternatives. However the possible toxicity of natural products must be tested before being used in the market. Although Launaea cornuta ( LC ) has been used traditionally to pain and inflammation and other oxidative-stress-related syndromes; however,Its analgesic, anti-inflammatory efficacy including its toxicity has not been extensively investigated and validated, prompting this study.
Methods: The methanolic extract of Lettuce cornuta leaves (LC ) was generated through extraction with methanol using Soxhlet extractor apparatus. The phytochemical screening of the extract was conducted according to methods describe by Trease α Evans. Acute oral toxicity and sub-acute toxicity of the extract was examined in rats. In the sub-acute toxicity study lasting (28 days), the animals were divided into three groups (5 rats): control, low-dose group (food supplemented with 250 mg/kg of the LC extract), and high-dose group (500 mg/kg of LC extract). While the formalin test was used to test the analgesic anti-inflammatory activity of LC extract.
Results: The phytochemical screening of the extract showed presence of phenols, alkaloids, flavonoids, glycosides. In acute toxicity study the extract was safe and did not cause any clinical signs of acute oral toxicity in rat all doses and no mortality was observed (LD50 > 5000 mg/kg BW) however in sub-acute toxicity study despite no mortality was observed the histo-pathological examination of the kidney as vital organs showed mild lesion in the higher dose group. Moreover, the extract significantly showed analgesic anti-inflammatory potential in a dose-dependent manner, in formalin test compared with respective controls group (p<0.05).
Conclusion: The extract dose of 500 mg/kgbw had higher potency than the standard drug tramadol. Some of these phytochemicals like flavonoids are antioxidant- and anti-inflammatory-associated phytochemicals and were likely responsible for the reported pharmacologic efficacy further empirical studies are needed to determine and characterize the extract’s specific analgesic anti-inflammatory compounds, specific mechanisms of action, and complete toxicity profiles.
Keywords
lettuce cornuta, methanolic extract, analgesic, toxicity
lettuce cornuta articles, methanolic extract articles, analgesic articles, toxicity articles
Article Details
Background
In Africa and the Western World, the use of traditional medicine is on the rise The World Health Organization (WHO) has reported that between 70% and 95% of individuals in developing countries use traditional medicine also known as “complementary”, “alternative”, or “nonconventional” medicines for disease management [1].Traditionally several plants including Launaea cornuta have been used to cure diseases such as diseases associated with pain and inflammation enough evidence on their toxicity and pharmacological properties are still lacking . Launaea cornuta Hochst (Ex Oliv. and Hiern.) is a small, erect herb of the Asteraceae (Compositae) commonly known as the ‘bitter lettuce’ and grows up to 1.5 Metres above the ground. The plant is indigenous to many African countries including Zimbabwe. It is referred to as “Mchunga” in Swahili, Nyamagogo in Shona. It is used to treat gonorrhoea, typhoid, and inflammatory diseases including enlarged testicles, earaches, stomach-aches, chronic joint problems, diabetes, hypertension, and memory loss. Past studies have shown that this plant is highly effective in reducing inflammation and free radicals, and it also contains antioxidant phyto-compounds that may improve cognitive function [2]. This research aim to offer an alternative to conventional analgesic anti-inflammatory agents, and evaluate the toxicity profile of Launaea cornuta highlighting the potential of natural herbs in promoting health Hence this study evaluated the Phytochemical constituent of the LC extract, its acute and subacute toxicity profile of the extract as well as its therapeutic dosage in achieving its analgesic and anti- inflammatory activities.
Methodology
Collection of the plant materials
The plants were haversteed in the Karoi forest in Zimbabwe .Karoi is a town in Zimbabwe. Karoi is located in Karoi District, Mashonaland West Province, in central northern Zimbabwe. It is located approximately 85 kilometres, by road, northwest of Chinhoyi. The collection took place around april 2025 and thier identification and autnetification were done at Harare national herbarium Zimbabwe, below is pictorial images of Letuce cornuata plant collected.
Preparation of the extract
The extraction was carried out at the Department of biomedical and laboratory sciences Africa University. The leaves of the plant were dried under shade for five days, then pounded and grounded into a fine powder using mortar and pestle. Powdered sample (250 g) of Lettuce. cornuta was dissolved in 1000 ml of methanol and macerated for 48 hours decanted, filtered (Whatman No. 1 filter paper; vacuum pump), separately which was evaporated to dryness in an oven at 40°C and kept in a sealed container at 4°C in a refrigerator until use. The percentage (%) yield was calculated using the formula (Eq. 1) stipulated by Truong et al.: An air-tight sealed universal glass bottle containing the extract was stored in a refrigerator (4°C) awaiting bioassays.

Wt = Weight in grams
Acute Toxicity Study
Acute toxicity study was conducted by Lorke's method this method has two phases which are phases 1 and 2 respectively to get LD50 of the methanolic extract of lettuce cornuta (LC) in rats.
Phase 1
This phase requires nine animals. The nine animals are divided into three groups of three animals each. Each group of animals are administered different doses (10, 100 and 1000 mg/kg) of the methanolic extract of lettuce cornuta (LC). The animals are placed under observation for 24 hours to monitor their behavior as well as if mortality occur.
Phase 2
This phase involves the use of three animals, which are distributed into three groups of one animal each. The animals were administered higher doses (1600, 2900 and 5000 mg/kg) of the methanolic extract of lettuce cornuta (LC) and then observed for 24 hours for behavior as well as mortality.[3]
The LD50 is calculated as the square root of the product of the lowest lethal dose and the highest non-lethal dose, i.e. the geometric mean of the consecutive doses in which 0 and 100% survival rates were recorded.
Then the LD50 is calculated by the formula:

D0 = Highest dose that gave no mortality,
D100 = Lowest dose that produced mortality.
Sub-acute toxicity study
The experiment was performed following OECD guideline number 407 12 rats were randomly divided into three groups of 4. The control group received the usual food for 28 days. The second group received the food supplemented with the methanolic extract of lettuce cornuta (LC) at a dose of 250 mg/kg/day.
The third group received the food supplemented with 500 mg/kg/day of the product for 28 days. During the experiment, animals were monitored for behavioral signs of toxicity as mentioned above. At the end of the study, the animals were fasted and euthanized after deep anesthesia, using chlorehydrate at a dose of 400mg/kg body weight intra peritoneal (IP) and the kidney was removed and fixed in formalin and subjected to routine processing (dehydration, embedding in paraffin, preparing 5 μm sections) for staining with Haematoxylin and eosin and histopathological examination
Phytochemical screening
The methanolic extract of lettuce cornuta ( LC) leaves was subjected to preliminary phytochemical screening tests for the presence of carbohydrate, flavonoids, alkaloids, saponins, tannins, and glycosides according to the method described by Trease and Evans (1983).
- Test for Alkaloids
- a) Dragendroff’s Test: About 0.2 g of the extracts was warned with 2% H2S04 for two minutes. It was filtered and few drops of Dragendroff’s reagent were added. Orange red precipitate indicates the presence of alkaloids[4]
- b) Mayer’s test: To a few ml of filtrate, a few drops of Mayer’s reagent were added by the side of the tube. A creamy white precipitate indicates the presence of alkaloids [5].
- Test for Flavonoid
- a) Alkaline reagent test: Extract was treated with 10 % NaOH solution; formation of intense yellow color indicates presence of Flavonoid.
- b) NH4OH test: 3 ml of extract was 10 % NH4OH solution development of yellow fluorescence indicates a positive test.
- c) Mg turning test: Extract was treated with Mg turning and add conc. HCl to this solution add 5ml of 95 % ethanol, formation of crimson red colour indicates Flavonoid.
- d) Zn test: 2 ml extract was treated with Zn dust and conc. HCl development of red colour indicates presence of Flavonoid [6]
- Test for Phenolic compounds
The extract (500 mg) was dissolved in 5 ml of distilled water. To this, few drops of neutral 5% ferric chloride solution were added. A dark green color indicated the presence of phenolic compounds [7].
- Test for glycoside
Glycosides are compounds which upon hydrolysis give rise to one or more sugars (glycones) and a compound which is not a sugar (aglycone or genuine). To the solution of the extract in glacial acetic acid, few drops of ferric chloride and concentrated sulphuric acid are added, and observed for a reddish brown coloration at the junction of two layers and the bluish green color in the upper layer. [8].
- Test for tannins
To 0.5 ml of extract solution 1ml of water and 1- 2 drops of ferric chloride solution were added. Blue color was observed for Gallic tannins and green black for catecholic tannins [9].
Evaluation of analgesic and anti-inflammatory activity of the extract
Formalin test was be used to evaluate analgesic and anti-inflammatory activities, this has been proven effective in evaluating both anti-inflammatory and analgesic activity in various studies,In the first phase of formalin test (early, acute phase) which begins immediately (0–5 min) after administration of formalin and coincide with neurogenic pain, and the second phase (late, tonic phase) usually beginning 15 min after formalin administration and it indicates inflammatory pain, LC . Formalin nociception is associated with tissue injury and is therefore believed to more closely resemble clinical pain when compared to other tests that use mechanical or thermal stimuli[10] [Tanko Mahamane Salissou, Maibouge, et al [11].In this test, the nociceptive response was in two distinct phases involving different mechanisms. The first phase (neurogenic pain) which began immediately after formalin injection resulted from the direct chemical stimulation of myelinated and un-myelinated nociceptive afferent fibers, mainly C fibers [12]. The second phase noted 20 - 30 min after formalin injection resulted from the release of inflammatory mediators in the peripheral tissues as well as facilitation of synaptic transmission at the spinal level[13] . Hence we conducted formalin test in rats to evaluate the analgesic of LC according to the method described by Hunskaar and Hole. A total of 20 Rats, fasted for 12hours, were divided in to 4 groups of five (5). - Group 1 (control group) received normal saline at 10 mL/kg body weight ;Groups 2, (test groups) plant extract of LC at doses of 250mg/kg body weight; group 3 received extract at a dose of 500 mg/kg Groups 4 (reference drug groups) received tramadol (trabar*) at doses of 15 mg/kg body weight. One hour after each treatment, 0.1 mL of formalin solution (2, 5 %) was injected in hind paw of rats. The length of time the animal licked the paw was measured during the first 5 minutes, then between the 15th and 30th minute after formalin injection. The analgesic activity was expressed as percentage using the following relation: (Tc – Tt) /Tc * 100%. Tc and Tt are mean licking time for control group and treated groups respectively.
Tissue preparation and histopathology
At the end of the sub-acute toxicity study, the animals were fasted and euthanized after deep anesthesia, using chlorehydrate at a dose of 400mg/kg body weight intra peritoneal (IP) then rat were perfused intra-cardiac with normal saline followed the 8% Para formal aldehyde slow intra cardiac perfusion and the kidney was removed and fixed in formalin and subjected to routine processing (dehydration, embedding in paraffin, preparing 5 μm sections) for staining with Haematoxylin and eosin subsequently the histopathological examination was carried out as described previously [14,15] where we evaluated for the presence or absence of lesions as indication of toxicity.
Statistical analysis
Data obtained from the experiments was analyzed and expressed as means (±SEM). The differences between the LC extract treated rats means, and normal saline-treated ‘control' was analyzed statistically by student’s t-test, followed by one-way analysis of variance (ANOVA; 95% confidence interval). Values of p ≤ 0.05 was taken to imply statistical significant
Results
1 Percentage Yield of methanolic Extract of L. cornuta
After the extraction process, a light brown extract, of LC brown yelowish extract with a yield of 33% was obtained
Phytochemical analysis
The phytochemical screening of methanolic extract of LC showed the presence of glycosides, phenols tannins, alkaloids and flavonoids as shown in table1 below.
Table 1: Phytochemical contents of LC extract
|
Constituent |
Inference LC Leaves |
|
Cardiac Glycosides |
+ |
|
Glycosides |
+ |
|
Phenols |
+ |
|
Steroids |
- |
|
Flavonoids |
+ |
|
Tannins |
+ |
|
Alkaloids |
+ |
|
Saponin |
- |
Keys: + Presence, - Absence
Acute Toxicity of methanol extracts of Lettuce cornuta
The extract of Lettuce. cornuta did not exhibit any observable signs of acute oral toxicity in experimental rats at the tested doses (10mg/Kg BW, 100mg/Kg BW, and 1000 mg/Kg BW 2,900 up to 5000 mk/kg , respectively), throughout the 2 days experiment and no mortality was recorded. Therefore, the LC50 of the extract was considered to be>5000mg/KgBW.
Sub-acute toxicity study
No mortality was observed in the extract treated rats which survived up to 28 days after treatment with LC extract at dose level of 250 mg/kg and 500mg/kg body weight.
Histo-pathological analysis
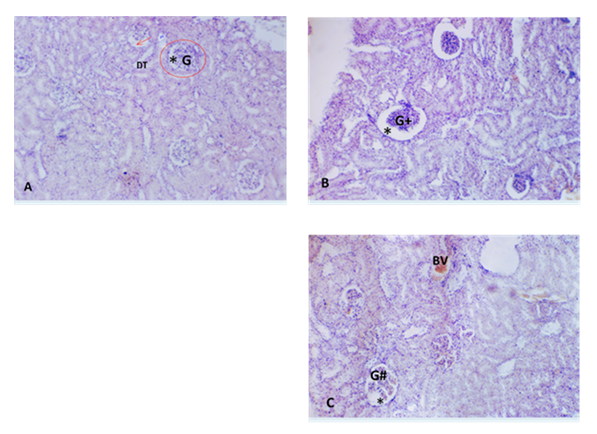
On exposure of targeted organs for histo-architectural studies, none of the kidneys revealed any toxicity related histopathological alterations. However, few sections of kidneys showed minimal degeneration and congestion respectively as incidental changes following treatment with LC with 500mg/kg of extract. Rat’s renal cortex of the treated group with LC 250mg/kg shows mild pathological lesions, shrunken glomerulus (G +), C 500mg/kg LC group showed segmented glomerulus (G#), large thick wall congested blood vessel (BV), and dilated Bowman’s space (asterisk) Figure 2.
Figure 2: Histo-pathological changes in treated rats versus control. A The rat renal cortex of the normal control presented a typical histological construction of glomeruli with narrow renal glomerular capsular space and Proximal distal convoluted tubular B Rat’s renal cortex of the treated group with LC 250mg/kg shows mild pathological lesions, shrunken glomerulus (G +), C 500mg/kg LC group showed segmented glomerulus (G#), large thick wall congested blood vessel (BV),and dilated Bowman’s space (asterisk).
Analgesic and anti-inflammatory activities of L. cornuta
In this study using formalin test we assess the analgesic property, it was found that the extract LC was most effective in suppressing the pain at a dose of 500mg/kg versus tramadol .The data summary appears in Table 2 below
Table 2: Formalin test in rats
|
Groups |
Dose(mg/kg) |
First phase 5 min |
Second phase 15 min-30min |
||||
|
|
|
Mean Licking time+SEM |
Percentage inhibition |
P value |
Mean Licking time, SEM |
Percentage inhibition |
P value |
|
(Normal Saline) |
74.00 ± 2.309 |
69.67 ± 0.88 |
|||||
|
LC |
250 |
64.33 ± 2.333 |
13.51% |
0.042 a |
28.33 ± 3.28 |
58% |
.004a |
|
0.004 b |
0.34 b |
||||||
|
LC |
500 |
55.00 ± 2.08 |
26% |
0.003 a |
28.67 ± 0.6 |
59% |
|
|
0.0001a |
|||||||
|
0.006 b |
|||||||
|
0.01 b |
|||||||
|
Tramadol |
15 |
31.33 ± 0.33 |
58% |
0.002 a |
24.33 ± 0.88 |
65% |
0.0001a |
Key: a treatment groups versus control, b, and all groups versus tramadol
Discussion
The leaves of Launaea cornuta have been locally used in traditional medicine for inflammatory conditions a; however, their pharmacologic efficacy and toxicity has not been scientifically fully investigated and validated. Various studies revealed that plants could modulate disease conditions and achieves their pharmacological effect through their phytochemicals. In this present study the phytochemical screening of LC leaves methanolic extract revealed the presence of glycosides, phenols flavonoids tannins and alkaloids which were reported to have various pharmacological bioactivity and this is line with a study conducted by Akimat EK et al. 2021 using Aqueous Root Extract of Launaea cornuta[16] . Hence both the root and leaves of LC used in this current study revealed the presence of similar phyto-compounds. Natural products derived from plants are seen , especially in developing countries, as safe and are consumed without any consideration to their potential toxic effects; hence investigating their safety is crucial[17]. Lettuce cornuta is used in Zimbabwe communities to treat ailments such as arthritis, analgesic and wound healing; however, its safety and toxicological profile data are lacking specially regarding the arial part of the plant. Therefore, we evaluated the acute oral toxicity effects of the methanolic extract of L. cornuta leaves in experimental rats. In acute toxicity using Lork methods Methanolic extract of LC sowed no sign of toxicity since no mortality, abnormal behaviour, or any acute oral toxicity associated signs were recorded up to 5000mg/Kg hence indicating its relatively safe Therefore, the methanol extract of Lettuce cornuta was safe as its LD50 was greater than 5000 mg/Kg BW, this corroborate with the study done by Akimat et al [16] which showed the aqueous root extract of Lettuce. cornuta was safe as its LD50 was greater than 2000 mg/Kg BW using the OECD guidelines[18]
Our finding are also in line with a study done by Maina M et al 2023 which used alternative methods namely the up-and-down procedure described by the OECD[18] . In this method, a LC extract is considered safe if it does not elicit clinical signs of acute toxicity such as coma, tremors, discoloration of the mucosa, excessive salivation, diarrhea, morbidity, among others, and death upon oral administration into experimental animals at doses ≤2000 mg/kg BW (LD50 > 2000 mg/kg BW) [19]. This is the most preferred method for evaluating the safety of plant extracts and chemicals in experimental animals, as it is easy to follow and yields precise and reproducible results, which are easily extrapolatable to humans
In a study using the brine shrimp lethality test represents a rapid, inexpensive and simple bioassay for testing the L. cornuta extracts for activity which in most cases correlates reasonably well with cytotoxic, anti-tumor and insecticidal activity while the methanolic extract had LC50 values of between 500-1000 μg/ml and this suggests low toxicity. L. cornuta and the results for brine shrimp lethality test confirms that this plant is safe to use as medicine and can be a potent source for complementary/alternative medicines and modern medicine too. The preliminary phytochemical screening bioassay studies using brine shrimp paves way for ethno botanical and pharmacological investigations for the future discovery of new sources of drugs. [20]. In subacute toxicity study lasting 28 days Rat’s renal cortex of the treated group with LC 250mg/kg shows mild pathological lesions, shrunken glomerulus (G +), C 500mg/kg LC group showed segmented glomerulus (G#), large thick wall congested blood vessel (BV),and dilated Bowman’s space (asterisk) stipulating mild lesion . This could be correlate with previous in-vivo toxicity studies of LC where a daily administration of LC for 28 days specially the aqueous extract significantly increased enzymes such as AST and Creatinine Kinase ( CK) and significantly lowered ALT and ALP and had no effect to Blood Urea Nitrogen (BUN ) compared to the normal control mice. Oral administration of ethyl acetate extract significantly lowered BUN, AST, ALP, and CK; and had no effect on ALT compared to normal control Mice .Injury of organs resulting from tissue hypoxia may also partly account for the altered serum levels of alkaline phosphatase from liver, kidney and spleen; alanine from liver and aspartate aminotransferase from liver, kidneys, heart and pancreas, and creatinine kinase from the heart and skeletal muscle in mice orally administered daily with extracts of L. cornuta at 1g/kg body weight daily for 28 days [21].
In the formalin test, LC showed significant analgesic properties and anti-inflammatory activities. In the first phase of formalin test (early, acute phase) which begins immediately (0–5 min) after administration of formalin and coincide with neurogenic pain, and the second phase (late, tonic phase) usually beginning 15 min after formalin administration and it indicates inflammatory pain, LC inhibited pain comparably to tramadol in the early phase which has been proved to be sensitive to reversal by analgesics such as opioids. Formalin nociception is associated with tissue injury and is therefore believed to more closely resemble clinical pain when compared to other tests that use mechanical or thermal stimuli[10] [Tanko Mahamane Salissou, Maibouge, et al11].In this test, the nociceptive response was in two distinct phases involving different mechanisms. The first phase (neurogenic pain) which began immediately after formalin injection resulted from the direct chemical stimulation of myelinated and unmyelinated nociceptive afferent fibers, mainly C fibers [12]. The second phase noted 20 - 30 min after formalin injection resulted from the release of inflammatory mediators in the peripheral tissues as well as facilitation of synaptic transmission at the spinal level[13] which stipulates the ability of LC to combat neurogenic and inflammatory pain. In this study, LC inhibited the licking response of mice in both phases of the formalin test, suggesting that its anti-nociceptive effects could be through central mechanisms as compared to the standard control drugs tramadol. The present results suggest that the management of pain by this plant maybe by both peripheral and central pain inhibition or by inhibition of inflammatory mediators. and this may be attributed to some phytochemicals in that fraction like flavonoids, which have been shown to reduce the number of paw licking in the formalin test in rats[22].
Conclusion
The following conclusions were drawn from this study;
- The methanol extract of lettuce cornuta leaves possesses anti-inflammatory activity due to its ability inhibit the second phase of formalin induced edema,
- Methanol extract of lettuce cornuta leaves has no oral acute toxicity effects how ever showed mild toxicity in sub-acute toxicity study.
- Extract of Lettuce cornuta possess tannins, cardiac glycosides, alkaloids, steroids, phenols and flavonoids. These phytochemicals could be responsible for analgesic anti-inflammatory activities
Recommendations
- The extract of cornuta can be a good candidate to be subjected to higher tests to elucidate if the plant could be used to develop affordable and safe novel analgesic anti-inflammatory agents.
- The extract dose of 250 mg/kg bw up to 500mg/kg are appropriate for analgesic anti-inflammatory activity in rats models.
- The extract appears to be safe, however more, histo-pathological and biochemical effects need to be assessed before validating its traditional use.
Declaration
Ethical Considerations
This study was ethically approved by Africa University ethical commitee (AUREC) Approval Number (AUREC 4105/25) and was conducted in accordance to ethical standard regulating animals research . Key guidelines include the "3Rs" principle—Replacement, Reduction, and Refinement—which are used to minimize harm to animals in research, and a cost-benefit analysis that weighs potential harm against scientific or societal benefits. Our protocols justify using animals, evidence of no viable alternatives, and detailed plans for care and euthanasia
Consent to publication
All Authors contribute mutually and consents for publication
Availability of data and materials
Data and materials are available upon request
Funding
This work was funded through Africa university research grant: Grant Number: AUORI-003-2025: Identification Number: AUORI-003-2025-02
Authors’ contributions
Prof M.T.M design the research and supervised the work and Prof MTMS and Dr SC wrote the paper
Competing interests
All authors declare no competing interests.
Acknowledgements
Authors wish to acknowledge the Africa University Research and Innovation office through its Director Professor Eltony Mugomeri in facilitating this research including the all laboratory technicians in CHANS colleges at Africa University
References
- World Health, O. The world health report 2002: reducing risks, promoting healthy life; World Health Organization (2002).
- Maina M, Mbaria J, Kamanja I, et al. Acute oral toxicity, cognitive-enhancing and anti-lipid peroxidation efficacy, and qualitative phytochemistry of the aqueous aerial part extract of Launaea cornuta (Hochst. ex. Oliv. &Hiern) C. Jeffrey. Heliyon 9 (2023).
- Lorke D. A new approach to practical acute toxicity testing. Archives of toxicology 54 (1983): 275-287.
- Egwaikhide P, Gimba C. Analysis of the phytochemical content and anti-microbial activity of Plectranthus glandulosis whole plant. Middle-East Journal of Scientific Research 2 (2007): 135-138.
- Narasimhan R, Mohan A. Phytochemical screening of Sesamum indicum seed extract. World journal of pharmacy and pharmaceutical sciences 1 (2012): 1298-1308.
- Sawant RS, Godghate AG. Preliminary phytochemical analysis of leaves of Tridax procumbens Linn. International Journal of Science, Environment and Technology 2 (2013): 388-394.
- Mir MA, Sawhney S, Jassal M. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker Journal of Pharmacy and Pharmocology 2 (2013): 001-005.
- Chhetri HP, Yogol NS, Sherchan J, et al. Phytochemical and antimicrobial evaluations of some medicinal plants of Nepal. Kathmandu university journal of science, engineering and technology 4 (2008): 49-54.
- Talukdar AD, Choudhury MD, Chakraborty M, et al. Phytochemical screening and TLC profiling of plant extracts of Cyathea gigantea (Wall. Ex. Hook.) Haltt. and Cyathea brunoniana. Wall. ex. Hook (Cl. & Bak.). Assam University Journal of Science and Technology 5 (2010): 70-74.
- Dos Santos MD, Almeida MC, Lopes NP, et al. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biological and Pharmaceutical Bulletin 29 (2006): 2236-2240.
- Tanko Mahamane Salissou M, et al. Analgesic and anti-inflammatory activities of N-buthanol portion of Acacia nilotica pods in mice and Wistar rats. International Journal of Scientific Research and Management 10 (2022): 519-530.
- Viegas Jr C, Alexandre-Moreira MS, Fraga CAM, et al. Antinociceptive profile of 2, 3, 6-trisubstituted piperidine alkaloids: 3-O-acetyl-spectaline and semi-synthetic derivatives of (−)-spectaline. Chemical and Pharmaceutical Bulletin 56 (2008): 407-412.
- Hao S, Takahata O, Iwasaki H. Antinociceptive interaction between spinal clonidine and lidocaine in the rat formalin test: an isobolographic analysis. Anesthesia & Analgesia 92 (2001): 733-738.
- Shukla S, Bhaskaran N, Babcook MA, et al. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 35 (2014): 452-460.
- Shukla S, MacLennan GT, Flask CA, et al. Blockade of β-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer research 67 (2007): 6925-6935.
- Akimat EK, Omwenga GI, Moriasi GA, et alP. Antioxidant, Anti-Inflammatory, Acute Oral Toxicity, and Qualitative Phytochemistry of The Aqueous Root Extract of Launaea cornuta (Hochst. Ex Oliv. & Hiern.). Journal of evidence-based integrative medicine 26 (2021).
- Jothy SL, Zakaria Z, Chen Y, et al. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules 16 (2011): 5268-5282.
- Organisation for Economic, C.-o.; Development. Test No. 425: acute oral toxicity: up-and-down procedure; OECD publishing (2008).
- Maina M, Mbaria J, Kamanja I, Moriasi G. Acute oral toxicity, cognitive-enhancing and anti-lipid peroxidation efficacy, and qualitative phytochemistry of the aqueous aerial part extract of Launaea cornuta (Hochst. ex. Oliv. &Hiern) C. Jeffrey. Heliyon 9 (2023): e15487.
- Misonge JO, Kinyanjui G, Kingori WM, et al. Phytochemical screening and cytotoxicity evaluation of Launaea Cornuta H (Asteraceae) using brine shrimp (2015).
- Njagi ENM, Karau GM, Machocho AK, et al. Efficacy and safety assessment of Launaea cornuta extracts in the management of diabetes mellitus (2014).
- Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazilian Journal of medical and Biological research 33 (2000): 179-189.